Abstract
Objective
This investigation aims to evaluate the validity of a Persian Tanner Stages Self-Assessment Questionnaire.
Methods
In this cross sectional study, 190 male students aged 8-16 years selected from three layers of different regions of Tehran (North, Central and South) were enrolled. A Persian questionnaire illustrated with Tanner stages of puberty (genital development and pubic hair distribution) was prepared. Children were asked to select the illustration that best described their pubertal development. Tanner status of the children was also estimated by an independent physician using physical examination. The degree of agreement between subjects’ judgments with assessments made by the rater was compared through the calculation of the weighted kappa statistic coefficient.
Findings
We found a substantial agreement between self-assessment of pubertal development made by the children and doctor's assessment of genital development (kappa=0.63, P<0.0001) and also the pubic hair distribution (kappa= 0.74, P<0.0001). Although a large proportion of subjects in G4 (89.2%) and G5 (85.7%) were capable of accurately or almost accurately identifying their own Tanner sexual stages, some degree of disagreement was observed in G3 Tanner stage (%46.9).
Conclusion
Self-assessment of puberty should be used very cautiously and may not be a substitute method for routine evaluation of pubertal state especially for early and mid pubertal groups.
Keywords: Adolescence, Persian, Sexual Maturation, Self-Assessment Questionnaire, Late Puberty
Introduction
Puberty starts when the level of sex hormones in the body rises and is marked by the development of secondary sexual characteristics, accelerated growth, behavioral changes, and eventual attainment of reproductive capacity[1]. The onset of puberty is different in various ethnic groups. Besides the numerous intrinsic physiological and genetic factors[2, 3], environmental factors including migration, chemical materials, diet habit, and even the day length[4] can affect the onset of puberty. However by large, mechanisms underlying the onset of a normal puberty and also its pathological variant, known as precocious puberty, are still poorly understood.
Although assessment of sexual development and maturation is essential for the appropriate assessment of growth in children and adolescents, the information about the stages of sexual development have been assessed only in some limited communities[5]. Chronological age is not a reliable parameter for determining the biological characterization of individuals, therefore to assess the sexual maturity in growing children, most clinicians relay on the physical examination. In many cultures however, it is not possible to perform the physical examination in many young people, due to privacy concerns and cultural restrictions. Although the physical examination is an appropriate method for estimating the developmental stages of puberty in adolescents, it has often been observed that children, especially teenagers, do not like to get naked and be examined in front of strangers. To overcome this problem, and to ease the situation that prevents the examination of sex organ in clinical practice, many research studies have been carried out to correlate and evaluate the validity of self- assessment and objective-assessment methods.
The self-assessment method, performed by providing a questionnaire to the subjects, is a suitable method for evaluation of sexual maturation and has been used in several epidemiological studies[6–8].
Based on the above explanations, in this study we aimed to determine the correlation between self-assessment and objective-assessment of pubertal maturation in a sample of Iranian male students. To do so, a questionnaire was designed with illustrations identifying various stages of pubertal development. Using this questionnaire, the teenager was asked to specify his own level of puberty.
Subjects and Methods
Study population
This cross-sectional study was performed in Tehran (Iran) from 2011 to 2012; 190 male students at two different levels (clusters of “elementary school” (age 7-11 years) and “secondary school” (age 11-14)) in three layers of different regions of Tehran (North, Central and South) were enrolled in the study.
Study protocol
The Tanner stage questionnaire was first translated by three different individuals to Persian language (the native language in our country). The translated questionnaires were then compared across and revised to have the best compatibility with the original version. The illustrations of Tanner stages, printed on separate flash cards were added to the final questionnaire (Fig 1). At the bottom of flash cards, simple sentences describing specific steps of Tanner were written. Male students, who were enrolled in the study, were individually taken to the examination room, where the proper facility for examination was provided. The subject was then asked to undress and by looking at himself, select the card which he thinks is most appropriate to the physical conditions of his own genital and growth of pubic hair. Therefore, the self-assessment was done through a comparison of self-image and the printed illustrations of Tanner stage. When the self-assessment was done, all the boys were examined by a trained physician who determined the sexual maturity of the subjects according to the Tanner stages. The results of questionnaire was not known to the physician.
Fig. 1.
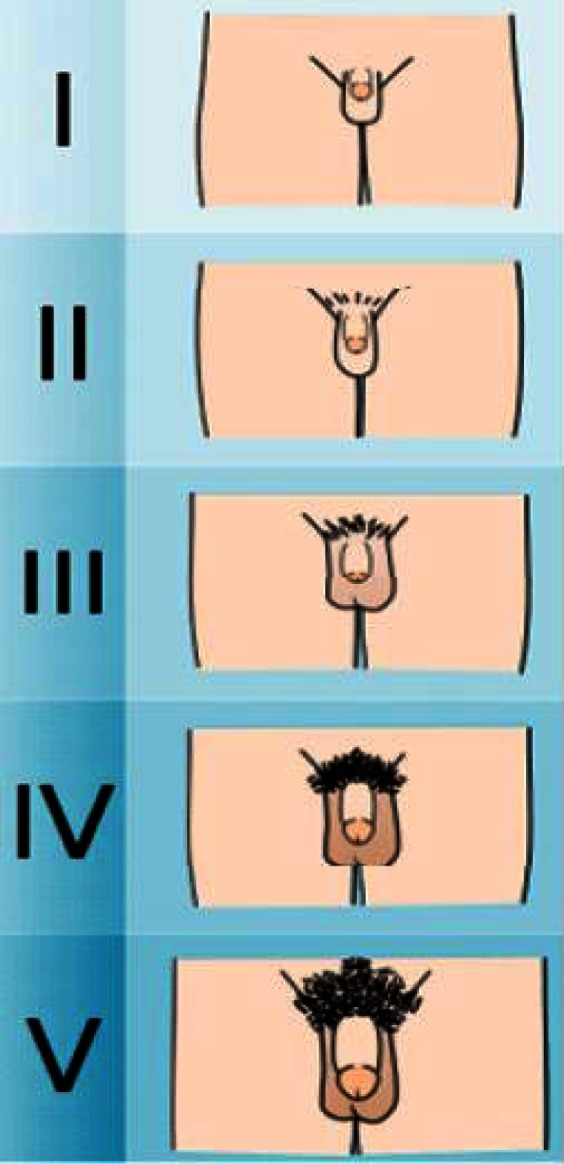
The illustrations of Tanner stages, printed on flash cards
The protocol of study was approved by the ethics committee of Tehran University of Medical Sciences and the participants signed informed consent at the time of recruitment.
At the end of examination, the weight and height of each individual was measured. Body mass index (BMI) was calculated using following equation:
BMI= weight (kg)/height2 (m)
Statistical analysis
Data was analyzed using Statistical Package for the Social Sciences (SPSS) software version 19. Because Tanner stage was reported by both student self-assessment and physician, there was a categorical variable with five levels (stages 1-5). The Reliability Assessment Index (weighted K statistic) was used in this study. Given that the study aimed also at comparing the entire agreement between the student and the physician's diagnosis (student-rater agreement), the kappa statistic was used in analyzing the results for the final agreement. The quadratic standard weighting (rather than the linear weighting) was used to determine the weighted kappa. This is because the weighted kappa was closer to the inter-class correlation coefficient (ICC) than the linear weighting method. In addition, the kappa deviations and error rates with the variables grouped into three sets or more, were minimized. The threshold levels of agreement (in the test-post-test and the final assay) were calculated by Kappa ‘Benchmarks’ of the strength of agreement and defined as follows: poor (<0.001); slight (0.00–0.20); fair (0.21-0.40); moderate (0.41-0.60); substantial (0.61-0.80) and almost perfect (>0.8).
Findings
Characteristics of study population
The average age of participants was 12.46±0.11 years with a minimum of 9 years and the maximum of 16 years. The majority of subjects were in age group 12-13 years (n=30, 15.8%) and age group 14-15 years had the least number of participants (n=24, 12.6%) (Table 1).
Table 1.
Age distribution of the subjects
| Frequency | Age group (year) | ||||||
|---|---|---|---|---|---|---|---|
| 9-10 | 10-11 | 11-12 | 12-13 | 13-14 | 14-15 | 15-16 | |
| Number | 27 | 27 | 28 | 30 | 27 | 24 | 27 |
| Percent | 14.2 | 14.2 | 14.7 | 15.8 | 14.2 | 12.6 | 14.2 |
The average BMI of the subjects was 20.05±0.26 kg/m2 (range=13.9-34.5 kg/m2). 68.9% of participants (n=131) had normal BMI (BMI<%85), 20% (n=38) were at risk (BMI=%85-%95) and 11.1% (n=21) were overweight (BMI>%95).
Self-assessment of genital and pubic hair development
The average age for onset of puberty among our participants was 11.43 years. Table 2 shows the distribution of Tanner score for male genitalia and pubic hair distribution comparing self-assessment and physician assessment (objective assessment). Regarding the male genitalia, we observed a complete agreement between self-assessment and objective assessment for 138 (72.6%) subjects.
Table 2.
Distribution of Tanner score for male genitalia and pubic hair growth, comparing self-assessment and objective- assessment
| Objective assessment of Tanner stage | self-assessment of Tanner stage (genital development) | Total | self-assessment of Tanner stage (pubic hair distribution) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| 1 | 18 | 7 | 1 | 0 | 0 | 26 | 20 | 8 | 0 | 0 | 0 | 28 |
| 2 | 14 | 58 | 9 | 0 | 0 | 81 | 12 | 56 | 4 | 0 | 0 | 72 |
| 3 | 2 | 5 | 17 | 7 | 1 | 32 | 2 | 3 | 28 | 4 | 1 | 38 |
| 4 | 0 | 0 | 2 | 33 | 2 | 37 | 0 | 0 | 0 | 36 | 2 | 38 |
| 5 | 0 | 0 | 0 | 2 | 12 | 14 | 0 | 0 | 0 | 2 | 12 | 14 |
| Total | 34 | 70 | 29 | 42 | 15 | 190 | 34 | 67 | 32 | 42 | 15 | 190 |
The overall results indicated a substantial agreement with a coefficient κ=0.63 (P<0.001).
The highest percentage of agreement and disagreement was among G4 (89.2%) and G3 (46.0%), respectively. 14.2% of the subjects overestimated by one or two stages, and 13.2% of them underestimated their conditions.
Evaluation of agreement for Tanner staging of pubic hair distribution as reported by subjects or doctors revealed that the highest and lowest agreement was observed in P4 (94.7%) and P1 (71.4%) groups respectively (Table 2). The overall kappa for Tanner staging of pubic hair distribution reported by subjects and doctors was 0.74 (P<0.001). About 10% of the students had an overestimation of 1 to 2 stages and a similar percent of them underestimated themselves. 80% of them did not properly evaluate themselves.
Self-assessment of genital development and pubic hair distribution in overweight boys
Twenty-one boys (11.05%) in this study were overweight (BMI>%95) and 66.7% of the overweight subjects correctly assessed their pubertal development, when compared with the doctor's estimates (Table 3). The highest percentage of agreement was found in G1 (80%) and the lowest in G3 (0%). The agreement between self assessment of genital development and physician's report was 0.50±0.15 (P<0.001). 76.2% of the subjects correctly determined their pubertal development based on Tanner stages of pubic hair distribution when compared with doctor's estimates (Table 3). The highest percentage of agreement among obese students was in P4 (100%), and the lowest agreement was observed in P3 (66.7%) (Table 3).
Table 3.
Distribution of Tanner score for genital development and pubic hair distribution comparing self-assessment and objective assessment in overweight students
| Objective assessment of Tanner stages | Self-assessment of Tanner stages (pubic hair distribution) | Total | Self-assessment of Tanner stages (genital development) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| 1 | 2 | 1 | 0 | 0 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 3 |
| 2 | 1 | 13 | 0 | 0 | 0 | 14 | 1 | 13 | 0 | 0 | 0 | 14 |
| 3 | 1 | 1 | 2 | 0 | 1 | 5 | 1 | 1 | 2 | 0 | 1 | 5 |
| 4 | 0 | 0 | 0 | 9 | 0 | 9 | 0 | 0 | 0 | 9 | 0 | 9 |
| 5 | 0 | 0 | 0 | 1 | 6 | 7 | 0 | 0 | 0 | 1 | 6 | 7 |
| Total | 4 | 15 | 2 | 10 | 7 | 38 | 4 | 15 | 2 | 10 | 7 | 38 |
Discussion
Normal pubertal development happens across a wide range of ages and at different rates[1]. Recent investigations of pubertal onset in US girls suggest earlier maturation, however the situation for boys is unknown[1]. In our country the accurate data are missing, and existing investigations are outdated. The traditional method of determining pubertal stages as achieved through physical examination is unpleasant to children and their parents. Data on male puberty are even more difficult to obtain than female data because of the absence of an easily determined marker, such as menarche. In our country, due to cultural and religious beliefs, this problem is even more prominent, makes it a real challenge for both children and physicians.
Invasive methods such as hormonal assay may be expensive and unaffordable and even not very useful in early stages of puberty[1, 2]. Therefore in this study we evaluated the validity and efficacy of self-assessment of pubertal development in 190 Iranian male students with an age range of 9-16 years. This range was obtained based on the minimum and maximum age onset of puberty in white boys as was explained by NHANES III[9]. Interestingly, we observed that the average age of onset of puberty in our subjects was similar to what has been reported in the United States[1].
We observed that overall there is an acceptable rate of agreement between self-assessment of pubertal development and physician estimates of pubertal stage. To our knowledge this study is among the first studies which are done in our region in this matter.
Our results showed that most of the students could determine correctly or almost correctly their own Tanner stage with assistance of a self-assessment questionnaire. The agreement coefficient between the self-assessment results and the results reported by a doctor, with regards to the pubic hair stages, was greater than the agreement coefficient obtained for the genital stages. Similar results had previously been reported by other researchers[10–13].
Desmangles et al used the Tanner staging of pubic hair distribution in self-assessment of pubertal development in boy students[14]. They reported that higher rate of underestimation was seen in late puberty, whereas patients in earlier pubertal stages were more prone to overestimate themselves. The authors assumed that perhaps this discrepancy is caused by the notion of self-image as the older children view themselves as younger than what they are and therefore hoped to have more growth. Whereas younger children view themselves as older than they actually are[14]. Although we found similar findings in this study, but also high degrees of consensus were seen in groups G4 and G5 and P4 and P5. We believe that good agreement observed in our study is due to alertness of adolescents about their puberty and sexual maturation. One reason for this statement would be the result of study performed by Stephen in which self-assessment of diabetic children was more reliable in mid- to late-pubertal youth, while it does not appear to be very helpful in identifying the early stages of puberty[15].
In our study, the greatest disagreement was seen in mid pubertal subjects. Two thirds of children in prepubertal and early stages of puberty, correctly choose the pubertal stage as compared with physician estimates. While in children with mid pubertal state, this agreement was much lower. Based on these observations, one might assume that the element of luck may have been involved in choosing the correct response of prepubertal group. In mid pubertal group, though we cannot clearly justify our observations, one explanation would be that since the questionnaire was originally designed for clinician, its content may not be very clear for the children. This may put more emphasis on the fact that factors such as selected methods certainly account for discrepant results. Since the self-image that obese kids have from themselves could be different from normal weight kids, in this study we intended also to evaluate the efficacy of our method in assessment of pubertal stages in overweight and obese children. We found that the agreement rate between the self-assessment staging and the physician estimate of pubertal status in both Tanner stagings of genital development and pubic hair distribution intends to be lower in overweight kids than in normal weight boys. Our results are in agreement with the results from other studies in which the agreement in overweight group was found to be lower than in non-overweight group[2, 3, 16]. Overweight children and adolescents tend to assess less accurately their sexual maturity than normal weight children and adolescents. A great percent of overweight students underestimate their pubertal status. This might be due to excessive fat tissue that exists in the pubic area, which is misleading in the diagnosis process. However, due to the low percentage of obese students in this study further studies should be done for determination of the difference between overweight and normal weight boys.
Conclusion
Approximately half of the children in mid puberty and one third of them in prepubertal and early stage of puberty were not able to correctly estimate their pubertal development. Self-assessment of puberty should be used very cautiously and may not be a substitute method for routine evaluation of pubertal state especially for early and mid pubertal groups. In adolescent subjects (ages between 14-16 years), however, the self-assessment of pubertal stages might be appropriate when there is limitation for physical examination.
Acknowledgment
This study was part of a fellowship thesis (of Dr. Shahab Noorian) supported by Growth and Development Research Center, Tehran University of Medical Sciences. The authors wish to acknowledge the efforts by Dr. Alireza Ahmadvand, for his efforts for analyzing data of this study. The authors would like to thank Dr. Vahid Ziaee for his kind and helpful contribution in designing of this study and reviewing the manuscript.
Conflict of Interest
None
References
- 1.Herman-Giddens ME, Steffes J, Harris D. Secondary sexual characteristics in boys: Data from the pediatric research in office settings network. Neo Reviews. 2012;130(5):e1058–68. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE. Genetics of puberty. Horm Res. 2007;68(Suppl 5):75–9. doi: 10.1159/000110583. [DOI] [PubMed] [Google Scholar]
- 3.Gajdos ZK, Henderson KD, Hirschhorn JN, et al. Genetic determinants of pubertal timing in the general population. Mol Cell Endocrinol. 2010;324(1-2):21–9. doi: 10.1016/j.mce.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 5.Boyne MS, Thame M, Osmond C, et al. Growth, body composition, and the onset of puberty: longitudinal observations in Afro-Caribbean children. J Clin Endocrinol Metab. 2010;95(7):3194–200. doi: 10.1210/jc.2010-0080. [DOI] [PubMed] [Google Scholar]
- 6.Azevedo JC, Brasil LM, Macedo TB, et al. Comparison between objective assessment and self-assessment of sexual maturation in children and adolescents. J Pediatr (Rio J) 2009;85(2):135–42. doi: 10.2223/JPED.1875. [DOI] [PubMed] [Google Scholar]
- 7.Raman A, Lustig RH, Fitch M, Fleming SE. Accuracy of self-assessed Tanner staging against hormonal assessment of sexual maturation in overweight African-American children. J Pediatr Endocrinol Metab. 2009;22(7):609–22. doi: 10.1515/jpem.2009.22.7.609. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SJ, Whincup PH, Hindmarsh PC, et al. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee PA, Houk CP. Puberty and its disorders. In: Lifshitz F, editor. Pediatric Endocrinology. New York: Informa Healthcare; 2007. pp. 273–303. [Google Scholar]
- 10.Chan NP, Sung RY, Kong AP, et al. Reliability of pubertal self-assessment in Hong Kong Chinese children. J Paediatr Child Health. 2008;44(6):353–8. doi: 10.1111/j.1440-1754.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- 11.Leone M, Comtois AS. Validity and reliability of self-assessment of sexual maturity in elite adolescent athletes. J Sports Med Phys Fitness. 2007;47(3):361–5. [PubMed] [Google Scholar]
- 12.Stephen MD, Bryant WP, Wilson DP. Self-assessment of sexual maturation in children and adolescents with diabetes mellitus. Endocr Pract. 2008;14(7):840–5. doi: 10.4158/EP.14.7.840. [DOI] [PubMed] [Google Scholar]
- 13.Wacharasindhu S, Pri-Ngam P, Kongchonrak T. Self-assessment of sexual maturation in Thai children by Tanner photograph. J Med Assoc Thai. 2002;85(3):308–19. [PubMed] [Google Scholar]
- 14.Desmangles JC, Lappe JM, Lipaczewski G, et al. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab. 2006;19(3):213–21. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- 15.Stephen MD, Bryant WP, Wilson DP. Self-assessment of sexual maturation in children and adolescents with diabetes mellitus. Endocr Pract. 2008;14(7):840–5. doi: 10.4158/EP.14.7.840. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Kaciroti N, Appugliese D, et al. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc. 2010;164(2):139–44. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]


