Abstract
Background:
Wolbachia pipientis is maternally inherited endoparasitic bacterium belonging to the α-proteobacteria, infecting 20–75% of all insect species including sand flies. The Wolbachia surface protein (wsp) was employed as an appropriate marker for strain typing. The objective of our research was to find the possibility of detection of W. pipientis in Phlebotomus perfiliewi transcaucasicus.
Methods:
Individual sand flies were screened for the presence of W. pipientis. The obtained sequences were edited and aligned with database sequences to identify W. pipientis haplotypes.
Results:
Two haplotypes of W. pipientis were found in P. perfiliewi transcaucasicus. The common haplotype of W. pipientis was found to be identical to the sequences of those submitted in GenBank. New strain (haplotype) of W. pipientis was found novel. The sequence of new strain of W. pipientis occurs in P. perfiliewi transcaucasicus is very different from those already submitted in GenBank.
Conclusion:
Finding one genetically modified new strain of W. pipientis in P. perfiliewi transcaucasicus, now we can conclude that further documents and studies need to reach the role of cytoplasmic incompatibility of W. pipientis through wild sand fly populations to drive a deleterious gene into and to reduce the density of natural populations of sand flies.
Keywords: Wolbachia pipientis, Phlebotomus perfiliewi transcaucasicus, Leishmania infantum, Kala-azar, Iran
Introduction
Phlebotomus perfiliewi transcaucasicus transmits ‘infantile visceral leishmaniasis’ (IVL) in northwest of Iran to the east of Turkey which has a Mediterranean climate (Nadim et al. 1978, Parvizi et al. 2008, Franco et al. 2011, Mahamdallie et al. 2011). The intracellular Rickettsia-like bacterium Wolbachia pipientis Hertig has been detected in phlebotomine sand flies (Diptera: Psychodidae, Phlebotominae) using PCR to amplify a fragment of the major Wolbachia surface protein (wsp) gene (Zhou et al. 1998, Cui et al. 1999, Ono et al. 2001, Benlarbi and Ready 2003, Kassem et al. 2003, Parvizi et al. 2003).
Wolbachia, are gram negative, polymorphic and wide spread bacteria belonging to the family Anaplasmataceae within the order Rickettsiaceae, related to α-proteobacteria that infect reproductive tissues of many arthropods and nematodes. In addition, these bacteria have been found in approximately 80% of insect species all over the world (Zhou et al. 1998, Cui et al. 1999, Benlarbi and Ready 2003, Kassem et al. 2003, Parvizi et al. 2003).
Wolbachia are unique endosymbionts that is maternally inherited, intracellular Rickettsia like bacteria which spread themselves to next generation, by transovarian transmission (Weeks et al. 2002, Benlarbi and Ready 2003).
This transmission is known as vertical transmission. Wolbachia has been implicated in causing reproductive manipulations on its hosts. They induce a number of reproductive abnormalities that appear in their host phenotypes including cytoplasmic incompatibility (CI), parthenogenesis, feminization, male killing (Curtis and Sinkins 1998, Cui et al. 1999, Ono et al. 2001, Weeks et al. 2002).
Cytoplasmic incompatibility is a common observed phenotype of Wolbachia, when infected populations of same species cross to each other, results may appear to be signs of incompatibility, unidirectional incompatibility or bidirectional incompatibility that can be completed or partial (Curtis and Sinkins 1998, Kassem et al. 2003).
In case of an infected male mates with an uninfected female (or infected with another strain of Wolbachia, this cross is incompatible and no offspring will be produced (Unidirectional CI). In case of male and female populations are infected with more than one strain of Wolbachia, Bidirectional incompatibility will be observed (Braig et al. 1998, Werren 1998, Cui et al. 1999, Weeks et al. 2002, Kassem et al. 2003).
There are four known forms of parthenogenesis that have been observed in different types of insect species (Thelytoky, Pseudogamy, Automixis, Apomixis). They have different aspects but there was the same one in which female individual produces offspring without participation of male partner in mating. Parthenogenesis in insects can cover a wide range of mechanisms. Wolbachia induces a particular type of parthenogenesis in some species, called Thelytoky Parthenogenesis. By this action, Wolbachia causes duplication in gametes in some insects therefore the resulting offspring would be all female, they also carry the Wolbachia infection (Anonymous 1997).
In addition, Wolbachia causes embryonic mortality of male zygotes in some arthropods. In addition, Wolbachia has horizontal transmission between different species of arthropods (O’ Neill et al. 1992, Breeuwer and Jacobs 1996, Braig et al. 1998). This transmission has been observed in groups of parasitic wasps that they were not infected with Wolbachia naturally, due to predating and feeding from a species of Drosophila, after a short period of time within the host cells of mentioned wasps, they have become infected with the same strain that was present in Drosophila species (Rousset and Solignac 1995).
In addition, Wolbachia was found in Isopods, mites and nematodes. The following reasons can describe research on Wolbachia: the wide spread of bacteria, manipulations on its hosts and its role to make speciation, the affect of bacteria of host’s fertility and being a potential natural enemy or a vector. Useful genes can be synthesized by genetic engineering and then transferring both into Wolbachia and insect populations for biological control which might cause decreasing arthropod transmitted diseases in human as a secondary reservoir (Curtis and Sinkins 1998, Turelli and Hoffmann 1999).
The selection of Wolbachia surface protein (wsp) gene has been due to free availability of this protein at the surface of the bacteria, ease of its identification, diversity of this gene in various genus and the possibility of usage of this gene for the purpose of studying the evolutional relationship and phylogenetic proximity of these groups of bacteria (Werren 1997, Bandi et al. 1998, Werren 1998, Sinkins and O’Neill 2000).
The Wolbachia surface protein (wsp) gene is useful marker for strain typing (Stouthamer et al. 1999, Baldo et al. 2006). There are no reports of the bacterial wsp gene being isolated and being sequenced from the same individual specimens of Phlebotomus perfiliewi transcaucasicus, in order to investigate the number of strains of W. pipientis infecting wild populations of this insect. We have reached to beneficial consequences. This is now reported of P. perfiliewi transcaucasicus from an endemic of (IVL) in Iran, and it is an essential piece of information for detecting W. pipientis through the wild populations of this sand fly (BenIsmail et al. 1987, Seccombe et al. 1993, Benlarbi and Ready 2003, Parvizi et al. 2009).
Materials and Methods
Sand flies were collected from three locations of Sarab and Kaleybar in the northwest of Iran in Azerbaijan Province as well as Meshkin Shahr in Ardabil Province using CDC traps and sticky papers. Sand flies were dissected, head and genital termination were kept for identifying of species based on morphological characters, thorax and abdomen were stored at −80 °C until further operations for DNA extraction followed PCR assays (Parvizi et al. 2003, Parvizi and Ready 2008).
About 550 base-pairs (bp) (minus primers) of the wsp fragment of Wolbachia Surface Protein were amplified by PCR using the primer pair wsp 81F (Forward) (5′TGGTCCAATAAGTGATGAAGAAAC 3′) and wsp 691R (Reverse) (5′AAAAA TTAAACGCTACTCCA3′), PCR amplification was carried out according to the protocol of Benlarbi and Ready (2003).
A 20μl PCR reaction mixture consisted of 2μl 1x Promega buffer, 2μl MgCl2, 0.5μl of each dNTP, 1μl of each primer, 0.2μl Taq DNA polymerase (Promega) and 2μl of sand fly genomic DNA. The PCR amplification was carried out with the following thermal profile using a GeneAmp® PCR System 9700 thermal cycler (PE Applied Biosystems): 2 min. denaturation at 94 °C, 35 cycles of denaturation at 94 °C for 30 sec., annealing at 50 °C for 30 sec, extension at 72 °C for 1.5 min, and a final extension at 72 °C for 10 min (Benlarbi and Ready 2003, Parvizi et al. 2003).
After amplification, the samples were fractionated by horizontal submerged gel electrophoresis, using 1.5% agarose gels and DNA size markers (Promega PCR markers G316A, or Bioline Hyper ladder IV). DNA fragments were visualized by ethidium bromide staining, then excised and purified using a Geneclean II Kit (BIO 101 Inc) before cycle sequencing each strand. The sequences obtained were edited and aligned with database sequences using Sequencher TM v. 3.1 software (Gene Codes Corp.) to identify unique sequences (=haplotypes), which were analyzed phylogenetically using PAUP* software (Swofford 2002).
Results
Five species of Larrossius subgenus and six species of Adlerius subgenus identified from three locations of Sarab, Kaleybar in north eastern of Azerbaijan province and Meshkin Shahr in Ardabil Province in northwest of Iran. Majority of sand fly species identified in these locations belonged to Phlebotomus and Paraphlebotomus subgenera and Sergentomyia genus. Abundance of some species of Larrossius subgenus and Adlerius subgenus were very low but due to their importance in IVL transmission we tried to detect wsp gene of Wolbachia to all sand fly species (Table 1).
Table 1.
Wolbachia infections in two subgenus species using wsp gene in three endemic visceral leishmaniasis locations in North West of Iran
| Province / Region | Subgenus | Larrossius | Adlerius | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region Species | P. kandelakii | P. tobbi | P.perfiliewi transcaucasicus | P. keshishiani | P. major | P. simici | P. brevis | P. halepensis | P. longidoctus | P. balcanicus | Chinensis Group | Total | ||
| East Azarbayejan Province | Sarab | Ghalajough | 5 | 2 | 2 | 1 | 3 | 13 | ||||||
| Razligh | 2 | 2 | 6 | 10 | ||||||||||
| Sahzab | 1 | 6 (2+ve) | 7 (2+ve) | |||||||||||
| Sanzigh | 2 | 3 | 1 | 6 | ||||||||||
| Agh miun | 1 | 3 | 4 | |||||||||||
| Arzanagh | 1 | 5 | 5 | 6 | 17 | |||||||||
| Hasan jan | 2 | 2 | 4 | 8 | ||||||||||
| Dowlat abad | 4 | 2 | 7 | 1 | 1 | 15 | ||||||||
| Total | 14 | 5 | 14 (2+ve) | 0 | 2 | 7 | 1 | 21 | 0 | 1 | 15 | 80 (2+ve) | ||
| Kaleybar | Sheghlan | 2 | 1 | 1 | 1 | 5 | ||||||||
| Bastam lou | 1 | 1 | ||||||||||||
| Safar lou | 1 | 1 | ||||||||||||
| Aslanbagh lou | 3 | 4 | 7 | |||||||||||
| Sarma lou | 1 | 1 | 2 | |||||||||||
| Aylily | 6 (4+ve) | 6 (4+ve) | ||||||||||||
| Shekhm lou | 1 (1+ve) | 3 | 4 (1+ve) | |||||||||||
| Olou gheshlagh | 4 (2+ve) | 4 (2+ve) | ||||||||||||
| Oliurdy | 2 | 1 | 3 | |||||||||||
| Abdolrazagh | 4 (2+ve) | 2 | 6 (2+ve) | |||||||||||
| Aghamir lou | 1 | 1 | ||||||||||||
| Molan | 1 | 2 | 1 | 4 | ||||||||||
| Jou aghaj | 1 | 1 | ||||||||||||
| Ajoudan abad | 1 | 1 | ||||||||||||
| Total | 10 | 0 | 24 (7+ve) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 11 (2+ve) | 46 (9+ve) | ||
| Ardabil Province | Meshkin shahr | Alni | 1 | 3 | 2 | 6 | ||||||||
| Ourkandi | 2 | 3 | 5 | 10 | ||||||||||
| Mizan | 3 | 5 | 3 | 11 | ||||||||||
| Mouyil | 9 | 9 | ||||||||||||
| Ghassabeh | 2 | 1 | 3 | |||||||||||
| Aghbolagh | 4 (1+ve) | 2 | 6 (1+ve) | |||||||||||
| Ghourt tappeh | 6 | 3 | 3 | 12 | ||||||||||
| Total | 26 (1+ve) | 0 | 3 | 0 | 5 | 0 | 0 | 7 | 6 | 0 | 10 | 57 (1+ve) | ||
| Total | 29 Village | 50 (1+ve) | 5 | 41 (9+ve) | 0 | 7 | 7 | 1 | 28 | 7 | 1 | 36 (2+ve) | 183 (12+ve) | |
In total 12 out of 183 sand flies were found infected with Wolbachia including 11 out of 41 P. perfiliewi transcaucasicus and one P. kandelakii.
Two out of seven P. perfiliewi transcaucasicus were found infected with wsp gene in Sarab region in north east of Azerbaijan Province. Seventy three sandflies species belonged to other Larrossius and Adlerius species were examined for Wolbachia but no positive specimen was found for wsp gene in this focus.
Nine out of 24 P. perfiliewi transcaucasicus were found infected with wsp gene in Kaleybar region in north east of Azerbaijan Province.
Twenty two sandflies species were belonged to other Larrossius and Adlerius species which were tried to detect Wolbachia but no positive wsp gene was found in this region.
Three of P. perfiliewi transcaucasicus were examined but no infection found with wsp gene in Meshkin Shahr in Ardabil Province in North West of Iran. One out 26 P. kandelakii was found infected with wsp gene in Meshkin Shahr in Ardabil Province but the band of PCR product in agarose gel was too weak to sequence.
In total (12 /183) 6.5% of all screened sand flies were positive with Wolbachia wsp gene that (2/81) 2.5% of these specimens were male and (10/102) 9.8% were female (Table 2).
Table 2.
Wolbachia detection in sand flies screened by PCR using wsp gene in North West of Iran (+ve = Wolbachia positive)
| Location | No. female sand flies screened by PCR | No. +ve female sand flies | +ve female sand flies% | No. male sand flies screened | +ve male sand flies | +ve male sand flies% | Total sand flies | Total +ve sand flies | Total +ve sand flies % |
|---|---|---|---|---|---|---|---|---|---|
| Kaleybar | 39 | 9 | 23.1 | 7 | 0 | 0 | 46 | 9 | 19.6 |
| Sarab | 29 | 0 | 0 | 51 | 2 | 3.9 | 80 | 2 | 2.5 |
| Meshkin Shahr | 34 | 1 | 2.9 | 23 | 0 | 0 | 57 | 1 | 1.7 |
| Total | 102 | 10 | 9.8 | 81 | 2 | 2.5 | 183 | 12 | 6.5 |
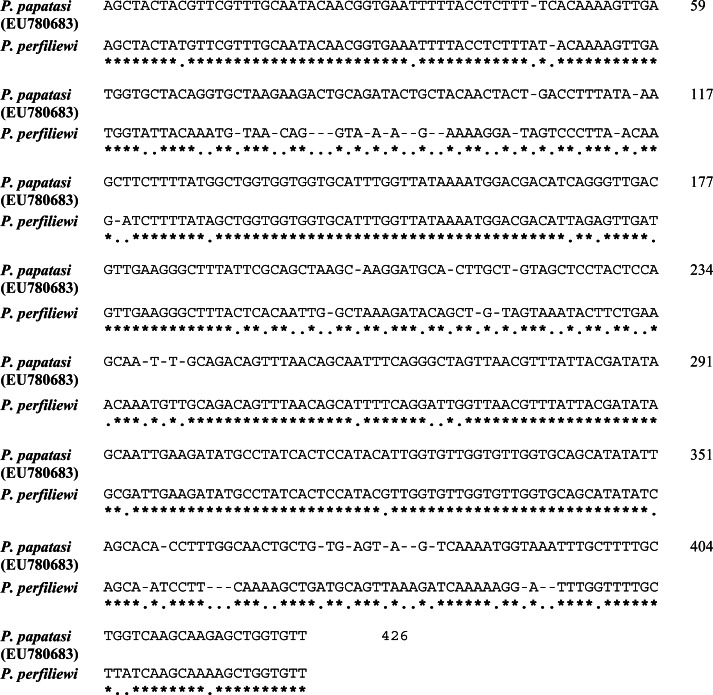
Only 9/11 (80%) positive PCR products of Wolbachia wsp gene in P. perfiliewi transcaucasicus contained enough DNA for direct sequencing. One haplotype of the wsp gene were recognized by aligning of new sequences comparison with homologous ones from GenBank. The common haplotype of W. pipientis was found to be identical to the sequences of those submitted in GenBank (GenBank accession number EU780684), and it predominated in Iranian sand flies infected with this species (7/11 infections). New strain of W. pipientis (GenBank accession number (JX 488735) was found novel (2/11 infections). This new strain differs pairwise by 36 to 120 bp nucleotide positions from those haplotypes of W. pipientis submitted in GenBank (GenBank Accession Numbers AF237882, AY 288297, HM563686, HM775090) (Fig. 2).
Fig. 2.
Alignment of the single wsp gene sequence of W. pipientis isolated from Iranian P. papatasi with the GenBank sequence EU780683 reported by Parvizi et al. (2009). Nucleotide differences are marked by a point, not a star
Discussion
Recently, wsp gene has been used to improve phylogenetic resolution within the species clade of W. pipientis, which was divided into four groups (A–D) and 12 subgroups (Zhou et al. 1998, Ono et al. 2001). The groups A and B are concordant with those identified by 16S rDNA for the strains of W. pipientis from insects, mites and crustaceans, whereas groups C and D harbor the strains from filarial nematodes. Populations of P. perfiliewi transcaucasicus from Iran were screened and for the first time, W. pipientis were found in this sand fly. Two haplotypes including, one new haplotype were obtained from wsp gene (Fig. 2).
The common widespread strain (GenBank ID: EU780683, AY288297) was the A-group strain of W. pipientis (wPap) but the new haplotype was indistinguishable from that of the A-group strain of W. pipientis (wPap) previously isolated from P. papatasi originating from Israel/West Bank (AF237883 (Ono et al. 2001) and India (GenBank accession number AF237882 (Ono et al. 2001), as well as from Spain and Iran (Benlarbi and Ready 2003, Parvizi et al. 2003).
From the three regions under study, 183 sand flies were selected in order of their genus and diversities of types and regions. Wolbachia infection was detected in 1 case out of 57 samples in Meshkin Shahr (1.7%), 9 cases out of 46 samples in Kaleybar (19.6%), and 2 cases out of 80 samples in Sarab (2.5%) out of subgenus of Larroussius “Wolbachia Surface Protein genes”.
Larroussius and Adlerius subgenus species were first identified as male because only males have good morphological characters of the head and abdominal terminalia for differentiation of species but females do not have this advantage. So it is obvious that this females are mates of that detected male geneses that were taken from this area (the males of these three species were taken from the given three regions, same as females) (Parvizi et al. 2003, Akhondi et al. 2012).
Out of Adlerius subgenus, only two cases in female sand flies were infected by Wolbachia which had no morphological characters for identification of genus (as mentioned above), but the males had good morphological characteristics for identification. As in these regions, male sand flies of Adlerius subgenus “P. longiductus, P. halepensis, P. brevis, P. balcanicus, P. simici” have been found, the two cases of detected Wolbachia in female sand flies of Adlerius subgenus in Kaleybar region, could be of any of the above five mentioned types. We would like to mention that all collected sand flies of above 5 mentioned species from Kaleybar were not examined because it was not the purpose of this paper.
The selection of Wolbachia surface protein gene has been due to free availability of this protein at the surface of the bacteria, ease of its identification, diversity of this gene in various genus and the possibility of usage of this gene for the purpose of studying the evolutional relationship and phylogenetic proximity of these groups of bacteria (Werren 1997, Bandi et al. 1998, Werren 1998, Sinkins and O’Neill 2000).
There is a natural Wolbachia infection in sand flies. Cytoplasmic incompatibility is the recognized phenotype in sand flies which have been shown to be infected with Wolbachia (McGraw et al. 2002, Rasgon 2003). Moreover, it has been suggested that Wolbachia can prevent the carriage and transferring of parasites and viruses via infected insects. In addition, it is a unique unknown manner which allows Wolbachia to be a good selection and used as a transgene of the target genes and controlling the leishmaniasis which has been recently adapted among the population of sand flies (Breeuwer and Jacobs 1996, Werren 1998, Sinkins and O’Neill 2000, Brownstein et al. 2003).
As the existence of Wolbachia in many sand flies which carry cutaneous leishmaniasis agents of urban and rural types is not known, it is suggested that the existence of this bacteria in other types of carriers of leishmaniasis is surveyed (Dobson et al. 2002).
Wolbachia can be used as a transferring gene or transgene in insects. This can be done by designing and synthesizing of target genes by genetic engineering techniques and then to transfer them into Wolbachia genome the point at which proper genes of Wolbachia can be released to the mass of insects. It appears that the use of this technology would be very useful for the purpose of biologically control and combat against varieties of parasites and viruses of the region by employing arthropods (Werren 1998, Dobson et al. 2002, Mitsuhashi et al. 2002, Rasgon 2003).
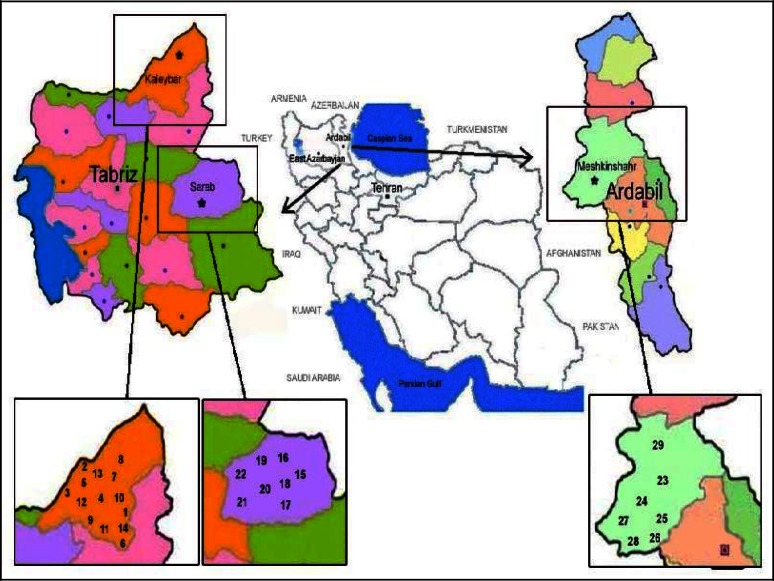
Fig. 1.
Locations of Iranian provinces, cities and villages where Larrossius and Adlerius group sand fly species were sampled. (Villages: 1- Sheghlan, 2- Bastam lou, 3- Safar lou, 4- Aslanbagh lou, 5- Sarma lou, 6- Aylily, 7- Shekhm lou, 8- Olou gheshlagh, 9- Oliurdy, 10- Abdolrazagh, 11- Aghamir lou, 12- Molan, 13- Jou aghaj, 14- Ajoudan abad, 15- Agh miun, 16- Sahzab, 17- Hasan jan, 18- Sanzigh, 19- Razligh, 20- Ghalajough, 21- Dowlat abad, 22- Arzanagh, 23- Alni, 24- Ourkandi, 25- Mizan, 26- Mouyil, 27- Ghassabeh, 28- Aghbolagh, 29- Ghourt tappeh)
Acknowledgments
The work was supported by the Pasteur Institute of Iran, grant 463 awarded to Dr Parviz Parvizi. The collections of sand flies were made possible by the assistance of the Centre of Health Service in Kaleybar and Sarab, Azerbaijan Province as well as Meshkin Shahr in Ardabil Province. We thank Mehdi Baghban for help with the field work and Elnaz AlaeeNovin for help in Molecular Systematics Laboratory. This research was MSc studentships to Farzaneh Fardid and Somaieh Soleimani, based at the Pasteur Institute of Iran, Tehran, and registered for Islamic Azad University, Qom, Iran. The authors declare that there is no conflict of interest.
References
- Akhoundi M, Parvizi P, Baghaei A, Depaquit J. The subgenus Adlerius Nitzulescu (Diptera, Psychodidae, Phlebotomus) in Iran. Acta Trop. 2012;122:7–15. doi: 10.1016/j.actatropica.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Anonymous 1997. Wolbachia pipientis overview. National Science, Available at: http://www.wolbachia.sols.uq.edu.au.
- Baldo L, Bordenstein S, Wernegreen JJ, Werren JH. Widespread recombination throughout Wolbachia genomes. Mol Biol Evol. 2006;23:437–449. doi: 10.1093/molbev/msj049. [DOI] [PubMed] [Google Scholar]
- Bandi C, Anderson TC, Genchi C, Blaxter ML. Phylogeny of Wolbachia in filarial nematodes. Proc R Soc Lond B. 1998;265:2407–2413. doi: 10.1098/rspb.1998.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ismail R, Gramiccia M, Gradoni L, Helal H, Ben Rachid MS. Isolation of Leishmania major from Phlebotomus papatasi in Tunisia. Trans R Soc Trop Med Hyg. 1987;81:749. doi: 10.1016/0035-9203(87)90018-6. [DOI] [PubMed] [Google Scholar]
- Benlarbi M, Ready PD. Host-specific Wolbachia strains in widespread populations of Phlebotomus perniciosus and P. papatasi (Diptera: Psychodidae), and prospects for driving genes into these vectors of Leishmania. Bull Entomol Res. 2003;93:383–391. doi: 10.1079/ber2003251. [DOI] [PubMed] [Google Scholar]
- Braig HK, Zhou W, Dobson SL, O’neill SL. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer JAJ, Jacobs G. Wolbachia: intracellular manipulators of mite reproduction. Exp Appl Acarol. 1996;20:421–434. doi: 10.1007/BF00053306. [DOI] [PubMed] [Google Scholar]
- Brownstein JS, Hett E, O’Neill SL. The potential of virulent Wolbachia to modulate disease transmission by insects. J Invert Pathog. 2003;84:24–29. doi: 10.1016/s0022-2011(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Curtis CF, Sinkins SP. Wolbachia as a possible means of driving genes into populations. Parasitology. 1998;116:111–115. doi: 10.1017/s0031182000084997. [DOI] [PubMed] [Google Scholar]
- Cui L, Chang SH, Stickman D, Rowton E. Frequency of Wolbachia infection in laboratory and field sand fly (Diptera: Psychodidae) populations. J Am Mosq Control Assoc. 1999;15:571–572. [PubMed] [Google Scholar]
- Dobson SL, Fox CW, Jiggins FM. The Effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc Lon (Biol) 2002;269:437–445. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AO, Davies CR, Mylne A, Dedet JP, Gállego M, Ballart C, Gramiccia M, Gradoni L, Molina R, Galvez R, Morillas-Marquez F, Baron-Lopez S, Pires CA, Afonso MO, Ready PD, Cox J. Predicting the distribution of canine leishmaniasis in western Europe based on environmental variables. Parasitol. 2011;14:1–14. doi: 10.1017/S003118201100148X. [DOI] [PubMed] [Google Scholar]
- Kassem HA, Hassan AN, Abdel-Hamed I, Osman G, El Khalab EM, Madkour MA. Wolbachia infection and the expression of cytoplasmic incompatibility in sand flies (Diptera: Psychodidae) from Egypt. Ann Trop Med Parasitol. 2003;97:639–644. doi: 10.1179/000349803225001391. [DOI] [PubMed] [Google Scholar]
- Mahamdallie SS, Pesson B, Ready PD. Multiple genetic divergences and population expansions of a Mediterranean sand fly, Phlebotomus ariasi, in Europe during the Pleistocene glacial cycles. Heredity. 2011;106:714–726. doi: 10.1038/hdy.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw EA, Merritt DJ, Droller JN, O’ Neill SL. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Nat Acad Sci USA. 2002;99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W, Saiki T, Wei W, Kawakita H, Sato M. Two novel strains of Wolbachia coexisting in both species of mulberry leafhoppers. Insect Mol Biol. 2002;11:577–584. doi: 10.1046/j.1365-2583.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- Nadim A, Navid-Hamidi A, Javadian E, Tahvildari Bidruni GH, Amini H. Present status of kala-azar in Iran. Am J Trop Med Hyg. 1978;27:25–28. doi: 10.4269/ajtmh.1978.27.25. [DOI] [PubMed] [Google Scholar]
- O’ Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplamic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Braig HR, Munstermann LE, Ferro C, O’Neill SL. Wolbachia infections of phlebotomine sand flies (Diptera: Psychodidae) J Med Entomol. 2001;38:237–241. doi: 10.1603/0022-2585-38.2.237. [DOI] [PubMed] [Google Scholar]
- Parvizi P, Benlarbi M, Ready PD. Mitochondrial and Wolbachia markers for the sand fly Phlebotomus papatasi: little population differentiation between peridomestic sites and gerbil burrows in Isfahan Province, Iran. Med Vet Entomol. 2003;17:351–362. doi: 10.1111/j.1365-2915.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Parvizi P, Fardid F, Amirkhani A. Isolation process of two genes of wsp and 16s rRNA in the intracellular bacterium, Wolbachia pipientis in Phlebotomus papatasi sand fly vector of Zoonotic Cutaneous leishmaniasis in Iran. Iran J Med Microb. 2009;4:53–60. (Persian) [Google Scholar]
- Parvizi P, Moradi Gh, Akbari Gh, Farahmand M, Ready PD, Piazak N, Assmar M, Amirkhani A. PCR detection and sequencing of parasite ITS-rDNA gene from reservoirs host of zoonotic cutaneous leishmaniasis in central Iran. Parasitol Res. 2008;103:1273–1278. doi: 10.1007/s00436-008-1124-z. [DOI] [PubMed] [Google Scholar]
- Parvizi P, Ready PD. Nested PCRs of nuclear ITS-rDNA fragments detect three Leishmania species of gerbils in sanflies from Iranian Foci of zoonotic cutaneous leishmaniasis. Trop Med Int Health. 2008;13:1159–1171. doi: 10.1111/j.1365-3156.2008.02121.x. [DOI] [PubMed] [Google Scholar]
- Rasgon JL, Styer LM, Scott TW. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J Med Entomol. 2003;40:125–132. doi: 10.1603/0022-2585-40.2.125. [DOI] [PubMed] [Google Scholar]
- Rousset F, Solignac M. Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc Natl Acad Sci USA. 1995;92:6389–6393. doi: 10.1073/pnas.92.14.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seccombe AK, Ready PD, Huddleston LM. A catalogue of Old World Phlebotomine sand flies (Diptera: Psychodidae, Phlebotominae) Occ Pap Syst Ent. 1993;8:1–57. [Google Scholar]
- Sinkins SP, O’ Neill SL. In: Wolbachia as a vehicle to modify insect populations. In Insect Transgenesis: Methods and Applications. Handler AM, James AA, editors. CRC Press; Boca Raton: 2000. pp. 271–288. [Google Scholar]
- Stouthamer R, Breeuwer JAJ, Hurst GDD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods) version 4.0. Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Turelli M, Hoffmann AA. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- Weeks AR, Reynolds KT, Hoffmann AA. Wolbachia dynamics: what has (and has not) been demonstrated? Trends Ecol Evol. 2002;17:257–262. [Google Scholar]
- Werren JH. Biology of Wolbachia. Ann Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH. Wolbachia and speciation. In: Harvard D, Berlocher S, editors. Endless Forms: Species and Speciation. Oxford University Press; Oxford: 1998. pp. 245–260. [Google Scholar]
- Zhou W, Rousset F, O’neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]