Abstract
Background:
Malaria still remains a public health problem in Iran. There are different vector control interventions such as insecticide spraying. The present study was carried out to determine the susceptibility status of Anopheles stephensi larvae to temephos as a national plan for monitoring and mapping of insecticide resistance
Methods:
Eight different localities in two main malarious provinces were determined as field collecting sites. Mosquitoes were collected from the field and reared in an insectray. Susceptibility assays were carried out according to the WHO method. The laboratory reared susceptible Beech-Lab strain was used for comparison. Data were analyzed using Probit analysis to determine LC50 and LC90 values.
Results:
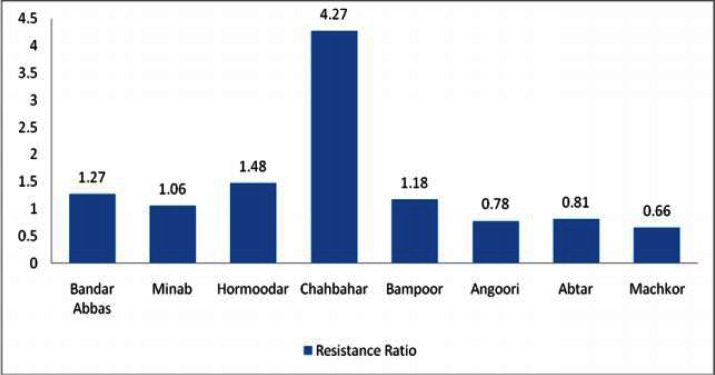
Susceptibility of An. stephensi to temephos indicated that the LC50 ranged from 0.0022 mg/l to 0.0141 mg/l. Although all field strains were susceptible to temephos, considerable variations in temephos resistance ratios of field strains were noticed from all the localities studied in comparison with the susceptible strain. A low level of resistance ratio was noticed in An. stephensi populations except for the Chabahar strain (RR= 4.27 fold). All field-collected An. stephensi populations exhibited homogeneity to the larvicide except for Bandar Abbas and Hormoodar village strains (P> 0.05%).
Conclusion:
Due to intensive use of temephos in the neighboring countries and occurrence of resistant to this insecticide in the main malaria vector in the region, insecticide resistance gene may evolve in the populations of An. stephensi. If temephos be applied as a larvicide it should be used judiciously for resistance management, as rotation strategy.
Keywords: Anopheles stephensi, Temephos, Susceptibility, Iran, Larvicide resistance
Introduction
Before implementing the national malaria control program in Iran in 1957, about 60% of population of the country was living in endemic areas with 30% to 40% malaria morbidity (Edrissian 2006). Despite the relatively successful implementation of malaria control programs in Iran in recent years, malaria still remains a main health problem especially in southeastern regions including Hormozgan Province, Sistan and Baluchistan Province and southern parts of Kerman Province with a population of 4.8 million people where more than 90% of all cases are reported from. About 68% of all malaria cases have been reported from these provinces in 2002, whereas it increased to 95% in 2007 (Raeisi et al. 2008, Vatandoost et al. 2010). The presence of vector species in these regions beside tropical climate and socio-economic conditions make appropriate situation for occurrence and persistent transmission of malaria in these regions. Malaria in malarious areas of Iran is unstable with two seasonal peaks mainly in spring and autumn. Outbreaks due to Plasmodium vivax usually occur after rainy season (Hanafi-Bojd et al. 2012).
In Iran several species and biological forms of Anopheles were recorded, but only 7 Anopheline mosquitoes including Anopheles stephensi, An. dthali, An. culicifacies s.l., An. fluviatilis s.l., An. superpictus, An. sacharovi and An. maculipennis s.l., have been confirmed as the main vectors and An. pulcherrimus reported as a suspected vector (Naddaf et al. 2003, Oshaghi et al. 2003a, Sedaghat et al. 2003a, 2003b, Vatandoost et al. 2006a, Doosti et al. 2006, Vatandoost et al. 2007, Vatandoost et al. 2010).
Anopheles stephensi is a sub-tropical species and also an important vector of human malaria throughout the Middle East and South Asian region, including the Indo-Pakistan subcontinent (Dash 2007, Hanafi-Bojd et al. 2012), with a westward extension through Iran and Iraq into the Middle East and Arabian peninsula. This species is considered to be the main malaria vector in the Persian Gulf area. Sporozoite rates of samples from the south of Iran were reported from 0.2 to 1.8% (Raeisi et al. 2008).
Previous studies have shown An. stephensi to be the most prevalent anopheline species in the malarious areas of southern Iran (Hanafi-Bojd et al. 2012). A wide range of anthropophilic indices has been reported from different geographical regions of India (Tyagi and Yadav 2001).
The first report of resistance of this species to DDT was in 1958 from southern Iran. Anopheles stephensi is resistant to DDT, dieldrin, and malathion at the adult stage (Edrissian 2006), although there is some indications of tolerance to pyrethroids (Hanafi-Bojd et al. 2012). There are different studies for evaluation of different control methods for this species in Iran (Enayati et al. 2003, Vatandoost and Hanafi-Bojd 2005, Davari et al. 2007, Abai et al. 2008, Rafinejad et al. 2008, Soltani et al. 2008, Vatandoost et al. 2008, Vatandoost and Hanafi-Bojd 2008, Vatandoost et al. 2009a, Vatandoost et al. 2009b, Omrani et al. 2010)
Resistance to DDT, dieldrin and malathion mainly in the adults of An. stephensi, have been widely distributed in Persian Gulf, Middle-East and Indian subcontinent causing operational problems for control programs (Vatandoost et al. 2005).
In 2006 for the first time in the Middle East, resistance to the organophophate larvicide, temephos, was confirmed in the Al-Dhahira region (Oman) in the malaria vector An. stephensi breeding in water storage tanks. The level of resistance was 2.5 times higher than WHO diagnostic dose (Anderasen 2006). Low level of larval resistance was found in Pakistan (Omer et al. 1980). Despite of development of DDT resistance in adults of An. stephensi, the larvae showed susceptibility to DDT in South of Iran. In Hormozgan Province, An. stephensi larvae showed susceptibility to malathion, temephos and chlorpyrifos, but resistance to fenitrothion in Bandar Abbas (Vatandoost et al. 2004) and tolerance to fenthion in Bashagard area (Hanafi-Bojd et al. 2012).
Larval control in the past had been dependent mainly on the use of chemicals such as Paris green and larvicidal oils (Ansari et al. 2004). At present, biological control methods using larvivorous fish and Bacillus thuringiensis in addition to chemical control using organophosphorus insecticides are being used for larviciding in south of Iran (Soltani et al. 2008). Environmental concerns shifted researches to find natural larvicides originated from plants in recent years (Vatandoost and Vaziri 2004, Hadjiakhoondi et al. 2005, Sadat Ebrahimi et al. 2005, Hadjiakhoondi et al. 2006, Shahi et al. 2010, Sedaghat et al. 2011).
Temephos, an organophosphorus insecticide, has been included in the list of WHO as a suitable and safe mosquito larvicide that can be used in drinking water. The toxicity of this insecticide is low and unlikely present acute hazard (Chavasse and Yap 1997).
Center for Disease Control section of Ministry of Health and Medical Education of Iran recently decided to reuse the temephos as larvicide in malaria control program. Considering the incidence of resistance in An. stephensi to temephos in Oman (Anderasen 2006), the southern neighbors of Iran, the present study was undertaken to determine the susceptibility status of An. stephensi larval stages to the temephos before the reuse of temephos in the field.
Materials and Methods
Study area
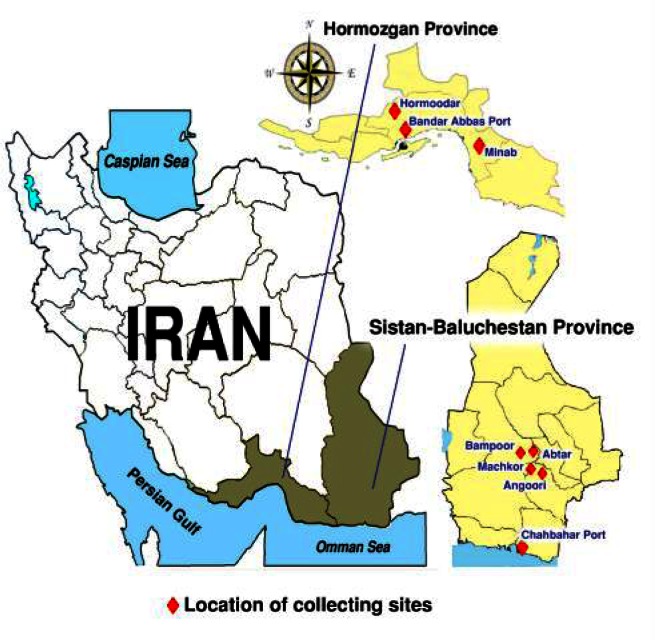
Eight different areas in two important malarious provinces were considered as field collecting sites including: Bandar Abbas Port, Minab County and Hormoodar Village (near the Bandar Abbas) in Hormozgan Province, and Chabahar Sea Port, two villages of Iranshahr County (Bampoor and Abtar) and two villages of Sarbaz County (Angoori and Machkor) in Sistan and Baluchistan Province (Fig. 1).
Fig. 1.
Location of Anopheles stephensi collection sites from malarious areas of Iran, 2011
Bandar Abbas Port (54°53′–56°03′E, 26°53′–27°31′N) is the capital of Hormozgan Province, a plain area with an average altitude of 9 m above sea level. The city has a hot and humid climate. Maximum summer temperature can reach up to 49 °C, whereas minimum winter temperature drops to about 5 °C. Average annual rainfall in 2004–2008 was 118.44 mm and mean annual relative humidity was 63.4% (www.weather.ir). In 2010 total population of Bandar Abbas City was 572584. About 77% of this population is living in urban area, 23% in rural area.
Minab (27°11′53″N 54°22′7″E) is a county in Hormozgan Province that located in eastern part of the province with climatic conditions similar to the Bandar Abbas (Fig. 1). According to the 2010 census, the county’s population was 243055 in 50478 families.
Chabahar Port (25°33′N 60° 41′E) is a county in Sistan and Baluchistan Province, with a hot and humid climatic conditions in the vicinity of Oman Sea and Pakistan border. Based on the 2011 census, the county's population was 246175 in 41532 families. The urban population is 77128, of which a majority resides in Chabahar City.
Iranshahr (27°34′N 59°53′E) is another tropical county in western part of Sistan and Baluchistan Province. According to the 2010 census, the county's population was 244779 in 49443 families.
Sarbaz (26°26′N 61°29′E) is another county in Sistan and Baluchistan Province in Iran. According to the 2006 census, the county's population was 162960 in 31449 families.
Mosquito strains
The field collected strains of An. stephensi were reared in the insectaries of Bandar Abbas and Iranshahr Health Research stations for further tests.
After the establishment of the field strains in the laboratory, the first generation of mosquitoes was used for susceptibility tests. A susceptible laboratory strain of An. stephensi (Beech-Lab from insectarium of department of medical entomology, Tehran University of Medical Sciences) was used to compare the susceptibility status of the field strains with. This strain has been maintained in the laboratory without exposure to insecticides for 28 years.
Insecticide
Technical grade insecticide used in the present study was temephos 90% (Batch No: TEM/136-229) provided by Levant Overseas Development Ltd., Argenteuil, France.
Based on pre-tests, five concentrations of the larvicide (0.25 mg/l, 0.0625 mg/l, 0.0156 mg/l, 0.0039 mg/l and 0.00195 mg/l) were considered for susceptibility assays. Bioassay consisted of five concentrations resulting 10–90% mortality. Butanone 2% in absolute ethanol was used as a control.
Susceptibility tests
Susceptibility assay was carried out according to the method described by WHO (WHO 2012). The toxicity of temephos to An. stephensi, from field-collected population was determined and compared with laboratory reared susceptible Beech-Lab strain.
Late 3rd instar larvae were exposed to five doses of the larvicide. At each concentration, a total of 100 larvae in four replicates of 25 larvae were tested. Two replicates of 25 larvae were used as control in each test. The larvae were fed with Bemax® and fish food, and mortality counts were made 24 h after exposure. Moribund larvae (presenting tremors, rigidity or mobility to reach water surface on touch) were considered as dead. Abbott’s formula was used to correct the observed mortality of larvae. All the data were corrected if the control mortality is between 5 and 20% (Abbott 1965).
Data analysis
Data were analyzed using probit analysis (Finney 1971) to determine the 50% lethal concentration values (LC50) and 90% lethal concentration values (LC90) of the field and Beech-Lab strains. Control mortality was corrected using Abbotts’ formula (Finney 1971). A statistical analysis of LC50 and LC90 was based on overlap of 95% confidence intervals. Resistance ratio was defined as LC50 of field strains to LC50 of lab strain.
Results
Susceptibility of An. stephensi to temephos (Table 1, Fig. 1) indicated that the LC50 ranged from 0.0022 mg/l in Machkor population (Sarbaz County, Sistan and Baluchistan Province) to 0.0141 mg/l in Chabahar Port population (south of Sistan and Baluchistan Province). The lowest LC90 was from the Beech-Lab strain and the highest was 0.0338 mg/l from Chabahar Port population.
Table 1.
Probit regression analysis of the temephos mortality data of field collected larvae of An. stephensi, 2011
| Location | Lethal Concentration(a.i.)/ppm | Chi-Square Heterogeneity (D.F.) | Regression Coefficient (Slope) | Resistance Ratio (RR)* | |
|---|---|---|---|---|---|
| LC50 | LC90 | ||||
|
| |||||
| (95% confidence limit) | (95% confidence limit) | ||||
| Bandar Abbas | 0.0042(0.0037–0.0048) | 0.0091(0.0075–0.0120) | 29.061 (D.F.= 2) | 3.8078 | 1.27 |
| Minab | 0.0035(0.0028–0.0042 | 0.0159(0.0122–0.0230) | 4.780 (D.F.= 3) | 1.9464 | 1.06 |
| Hormoodar | 0.0049(0.0043–0.0057) | 0.0129(0.0105–0.0170) | 27.846 (D.F.= 3) | 3.0519 | 1.48 |
| Chabahar | 0.0141(0.0122–0.0163) | 0.0338(0.0279–0.0435) | 4.025 (D.F.= 3) | 3.3709 | 4.27 |
| Bampoor | 0.0039(0.0035–0.0044) | 0.0084(0.0069–0.0111) | 2.740 (D.F.= 2) | 3.8727 | 1.18 |
| Angoori | 0.0026(0.0022–0.0030) | 0.0082(0.0065–0.0115) | 0.161 (D.F.= 3) | 2.5853 | 0.78 |
| Abtar | 0.0027(0.0022–0.0032) | 0.0097(0.0076–0.0138) | 1.746 (D.F.= 3) | 2.2949 | 0.81 |
| Machkor | 0.0022(0.0017–0.0025) | 0.0068(0.0055–0.0097) | 4.445 (D.F.= 3) | 2.5657 | 0.66 |
| Beech-Lab | 0.0033(0.0030–0.0036) | 0.0055(0.0048–0.0067) | 962.660 (D.F.= 1) | 5.8092 | - |
RR50, Resistance Ratio at LC50 (RR50= LC50 of field population/ LC50 of Beech-Lab)
According to WHO criteria, a 98–100% mortality rate indicates susceptibility, 80–97% mortality rate indicates tolerance (requires confirmation of resistance with other methods) and <80% mortality suggests resistance (WHO 1998).
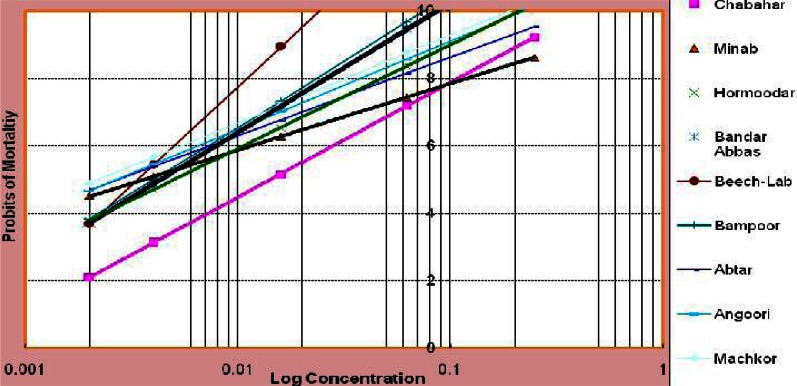
Results of susceptibility tests on laboratory and field strains of An. stephensi showed that the larvae were susceptible to temephos at the diagnostic dose (0.25 mg/l). Although all field strains were susceptible to temephos, considerable variation in temephos resistance ratio of filed strains in comparison with susceptible strain was noticed from all the locations studied. A low level of resistance ratio was observed in the populations of An. stephensi except in that of the Chabahar Sea Port (RR= 4.27 folds) compared to Beech-Lab strain (P< 0.05) (Table 1, Fig. 2, 3).
Fig. 2.
temephos resistance ratio pattern in An. stephensi field strains from southern Iran, 2011
Fig. 3.
Regression lines of eight strains of An. stephensi and susceptible Beech-Lab strain, 2011
A comparatively low degree of resistance ratio to temephos (Table 1, Fig. 2) was obtained in the An. stephensi from all the localities studied (RR= 0.66–1.48) whereas from Chabahar Port, more than 4-folds resistance was noticed, compared to Beech-Lab strain. Almost all the field-collected An. stephensi populations exhibited homogeneity to insecticide bioassay except for the population from Bandar Abbas Port and Hormoodar Village (Hormozgan Province), where Chi-square value exceeded table value at 0.05% (Table 1, Fig. 3).
Discussion
Temephos (EC 50%) has been used for years for larval control program of malaria in Southern Iran (Edrissian 2006). Many studies on the susceptibility level of An. stephensi to pesticides such as DDT and temephos has been done in Iran and other countries. An. stephensi larvae from Pakistan and United Arab Emirate were reported to be resistant to DDT (Vatandoost 1996). Different levels of resistance to larvicides were reported in anopheline malaria vectors worldwide. Anopheles stephensi has an extensive resistance comparing to other species and is resistant or tolerant to fenitrothion, temephos and fenthion in India, fenitrothion and pirimiphos-methyl in Iraq, fenitrothion, temephos, pirimiphos-methyl, chlorfoxim and foxim in Iran and finally fenitrothion in Pakistan (Vatandoost and Hanafi-Bojd 2005). Resistance of An. dthali to temephos also was reported (Hanafi-Bojd et al. 2006). In 2006 for the first time in the Middle East, resistance to temephos was confirmed in An. stephensi breeding in water storage tanks in the Al-Dhahira region of Oman (Anderasen 2006), there is not confirmed report of temephos resistance of An. stephensi in Iran.
The results of other investigations in Iran showed that this species was completely susceptible to all tested larvicides including temephos at the WHO diagnostic dose (Vatandoost et al. 2004, Vatandoost and Hanafi-Bojd 2005, Vatandoost et al. 2005, Vatandoost 2006a).
In our study, a variation in toxicity levels of temephos to An. stephensi was noticed. This may be justified by a wide scope of sampling locations. Different slopes of insecticide bioassay regression lines to An. stephensi from different locations shows different degree of developing insecticide resistance. Table 1 show that the toxicity of temephos to An. stephensi from Angoori, Abtar and Machkor was lower than that of susceptible Beech-Lab strain. Similar results were reported by other researcher that some filed populations were more susceptible to insecticide than laboratory strain (Ponlawat 2005, Tikar et al. 2008). Although the exact reason for this phenomenon is not known, overcrowding in breeding places leading to insufficient food could result in a weaker progeny. Our data provided baseline information on insecticide susceptibility of An. stephensi from geographically different locations in Iran. An. stephensi is still susceptible to temephos from all studied localities in Iran and it can be used as an effective insecticide in malaria control program despite the fact that resistance to temephos has been reported from other malarious countries such as India and Oman. Chabahar strain of An. stephensi exhibited the highest resistance ratio to temephos compared with all other collection sites. The most probable explanation for this is that Chabahar Port is the nearest area to Oman that resistance of An. stephensi to Temephos was confirmed by Anderasen (2006). Therefore it could be an important alarm for developing of resistance to temephos in Iranian An. stephensi.
Based on the results of this research, temephos can be used as a larvicide in Integrated Vector Management in malaria control programs in the region warily. Some important issues including continuous insecticide resistance status monitoring in the vectors should be considered to ensure judicious use of pesticide and implementing insecticide resistance management strategies e.g. rotation. In addition it is essential to focus on regular surveillance of malaria vectors as a routine practice in high risk malaria areas.
Acknowledgments
This article is a part of the first author’s dissertation for fulfillment of a PhD degree in Medical Entomology and Vector Control from Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. The authors are very grateful to Mr M Yarian from Hormozgan University of Medical Sciences and Mr A Pakari and Mr Shahbakhsh technicians of the National Institute of Health Research, Bandar Abbas and Iranshahr Research Stations, for their kind collaboration during this study. This study was financially supported by the Deputy for Research, Tehran University of Medical Sciences. The authors declare that there is no conflict of interest.
References
- Abai MR, Mehravaran A, Vatandoost H, Oshaghi MA, Javadian E, Mashayekhi M, Mosleminia A, Piyazak N, Edallat H, Mohtarami F, Jabbari H, Rafi F. Comparative performance of imagicides on Anopheles stephensi, main malaria vector in a malarious area, southern Iran. J Vector Borne Dis. 2008;45(4):307–312. [PubMed] [Google Scholar]
- Abbott WS. A method of comparing the effectiveness of an insecticide. J Econ Entomol. 1965;18:265–267. [Google Scholar]
- Anderasen MH. Emerging resistance to temephos in Anopheles stephensi in the Al-Dhahira Region of Oman. World Health Organization; Geneva: 2006. pp. 1–13. [Google Scholar]
- Ansari MA, Mittal PK, Razdan RK, Dhiman RC, Kumar A. Evaluation of pirimiphos-methyl (50% EC) against the immatures of Anopheles stephensi/An. culicifacies (malaria vectors) and Culex quinquefasciatus (vector of bancroftian filariasis) J Vector Borne Dis. 2004;41(1–2):10–16. [PubMed] [Google Scholar]
- Chavasse DC, Yap HH. Chemical methods for the control of vectors and pests of public health importance. WHO/CTD/WHOPES/97.2, World Health Organization; Geneva: 1997. [Google Scholar]
- Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Current Sci. 2007;92:1571–1578. [Google Scholar]
- Davari B, Vatandoost H, Oshaghi MA, Ladonni H, Enayati AA, Shaeghi M, Basseri HR. Selection of Anopheles stephensi with DDT and dieldrin and cross-resistance spectrum to pyrethroids and fipronil. Pestic Biochem Physiol. 2007;89(2):97–103. [Google Scholar]
- Doosti S, Azari-Hamidian S, Vatandoost H, Oshaghi MA, Hosseini M. Taxonomic differentiation of Anopheles sacharovi and An.maculipennis s.l. (Diptera: Culicidae) by seta 2 (antepalmate hair) of larvae in Iran with a historical review and an annotated bibliography of Iranian An. maculipennsi complex. Acta Med Iran. 2006;44(1):21–27. [Google Scholar]
- Edrissian G. Malaria past and present situation in Iran. Iran J Parasitol. 2006;1(1):1–14. [Google Scholar]
- Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17(2):138–144. doi: 10.1046/j.1365-2915.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. III edn. Cambridge University Press; Cambridge: 1971. [Google Scholar]
- Hadjiakhoondi A, Vatandoost H, Khanavi M, Abaee MR. Biochemical investigation of different extracts and larvicidal activity of Tagetes minuta L on Anopheles stephensi larvae. Iran J Pharm Sci. 2005;1:81–84. [Google Scholar]
- Hadjiakhoondi A, Sadeghipour-Roodsari HR, Vatandoost H, Khanavi M, Abaee MR, Vosoughi M. Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iran J Pharm Sci. 2006;2:97–102. [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Jafari R. Susceptibility status of Anopheles dthali and An. fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. J Vector Borne Dis. 2006;43(1):34–38. [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM, Yeryan M, Pakari A. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 2012;121:85–92. doi: 10.1016/j.actatropica.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Naddaf SR, Oshaghi MA, Vatandoost H, Asmar M. Molecular characterization of the Anopheles fluviatilis species complex in Iran. East Mediterr Health J. 2003;9(3):257–265. [PubMed] [Google Scholar]
- Omer SM, Georghiou GP, Irving SN. DDT/pyrethroid resistance interrelationships in Anopheles stephensi. Mosq News. 1980;40:200–209. [Google Scholar]
- Omrani S-M, Vatandoost H, Oshaghi MA, Shokri F, Guerin PM, Ershadi MRY, Rassi Y, Tirgari S. Fabrication of an olfactometer for mosquito behavioural studies. J Vector Borne Dis. 2010;47(1):17–25. [PubMed] [Google Scholar]
- Oshaghi MA, Sedaghat MM, Vatandoost H. Molecular characterization of the Anopheles maculipennis complex in the Islamic Republic of Iran. East Mediterr Health J. 2003a;9(4):659–666. [PubMed] [Google Scholar]
- Oshaghi MA, Yaghoobi F, Vatandoost H, Abai MR, Akbarzadeh K. Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pak J Biol Sci. 2006;9(2):294–298. [Google Scholar]
- Ponlawat A, Scott JG, Harrington LC. Insecticide Susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 2005;42(5):821–825. doi: 10.1603/0022-2585(2005)042[0821:ISOAAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Raeisi A, Ranjbar M, Shoghli A, Vatandoost H, Faraji L. National strategy plan for malaria control (IR Iran, 2004–2008) Ministry of Health and Medical Education of Iran Publication; Tehran: 2008. [Google Scholar]
- Rafinejad J, Vatandoost H, Nikpoor F, Abai MR, Shaeghi M, Duchen S, Rafi F. Effect of washing on the bioefficacy of insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) against main malaria vector Anopheles stephensi by three bioassay methods. J Vector Borne Dis. 2008;45(2):143–150. [PubMed] [Google Scholar]
- Sadat Ebrahimi SE HA, Rezazadeh Sh, Fereidunian N, Vatandoost H, Abaei MR. The components of Tagetes minuta L. and its biological activities against malaria vector, Anopheles stephensi in Iran. J Med Plants. 2005;4:43–47. [Google Scholar]
- Sedaghat MM, Linton YM, Nicolescu G, Smith L, Koliopoulos G, Zounos AK, Oshaghi MA, Vatandoost H, Harbach RE. Morphological and molecular characterization of Anopheles (Anopheles) sacharovi Favre, aprimary vector of malaria in the Middle East. Syst Entomol. 2003a;28:241–256. [Google Scholar]
- Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterizations and recognition of a new species. Bull Entomol Res. 2003b;93:527–535. doi: 10.1079/ber2003272. [DOI] [PubMed] [Google Scholar]
- Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Mohtarami F, Vatandoost H. Chemical composition and larvicidal activity of essential oil of Cupressus arizonica EL Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Pharmacognosy Res. 2011;3(2):135–139. doi: 10.4103/0974-8490.81962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi M, Hanafi-Bojd AA, Iranshahi M, Vatandoost H, Hanafi-Bojd AA. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae) J Vector Borne Dis. 2010;47(3):185–188. [PubMed] [Google Scholar]
- Soltani A, Vatandoost H, Jabbari H, Mesdaghinia AR, Mahvi AH, Younesian M, Hanafi-Bojd AA, Bozorgzadeh S, Abai MR, Pakari A, Shabkhiz H. Use of Expanded Polystyrene (EPS) and Shredded Waste Polystyrene (SWAP) Beads for Control of Mosquitoes. Iran J Arthropod-Borne Dis. 2008;2(2):12–20. [Google Scholar]
- Tikar SN, Mendki MJ, Chandel K, Parashar BD, Prakash S. Susceptibility of immature stages of Aedes (Stegomyia) aegypti, vector of dengue and chikungunya to insecticides from India. Parasitol Res. 2008;102(5):907–913. doi: 10.1007/s00436-007-0848-5. [DOI] [PubMed] [Google Scholar]
- Tyagi BK, Yadav SP. Bionomics of malaria vectors in two physiographically different areas of the epidemic-prone Thar Desert, north-western Rajasthan (India) J Arid Environ. 2001;47(2):161–172. [Google Scholar]
- Vatandoost H. The functional Basis of pyrethroids resistant in the malaria vector Anopheles stephensi[PhD dissertation] University of Liverpool; UK: 1996. [Google Scholar]
- Vatandoost H, Vaziri VM. Larvicidal activity of a neem tree extract (Neemarin) against mosquito larvae in the Islamic Republic of Iran. East Mediterr Health J. 2004;10(4–5):573–581. [PubMed] [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. Larval habitats of main malaria vectors in Hormozgan Province and their susceptibility to different larvicides. South Asian J Trop Med Pub Hlth. 2004;35(2):22–25. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Current Resistant Status of Anopheles stephensi Liston to Different Larvicides in Hormozgan Province, Southeastern Iran, 2004. Pak J Biol Sci. 2005;8:1568–1570. [Google Scholar]
- Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Kerman Province, southeastern Iran. J Vector Borne Dis. 2005;42(3):100–108. [PubMed] [Google Scholar]
- Vatandoost H, Oshaghi M, Abai MR, Shahi M, Yaghoobi F, Baghai M, Hanafi-Bojd AA, Zamani G, Townson H. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, southern Iran. Acta Trop. 2006a;97(2):196–205. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Shahi M, Hanafi-Bojd AA, Abai MR, Oshaghi MA, Rafii F. Ecology of Anopheles dthali Patton in BandarAbbas. Iran J Arthropod-Borne Dis. 2007;1(1):21–27. [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Laboratory evaluation of 3 repellents against Anopheles stephensi in the Islamic Republic of Iran. East Mediterr Health J. 2008;14(2):260–267. [PubMed] [Google Scholar]
- Vatandoost H, Khazani A, Rafinejad J, Khoobdel M, Kebriai-Zadeh A, Abai MR. Comparative efficacy of neem and dimethylphthalate (DMP) against malaria vector, Anopheles stephensi (Diptera: Culicidae) Asian Pac J Trop Med. 2008;1(3):1–6. [Google Scholar]
- Vatandoost H, Abai MR, Abbasi M, Shaeghi M, Abtahi M, Rafie F. Designing of a laboratory model for evaluation of the residual effects of deltamethrin (K-othrine WP 5%) on different surfaces against malaria vector, Anopheles stephensi (Diptera: Culicidae) J Vector Borne Dis. 2009a;46(4):261–267. [PubMed] [Google Scholar]
- Vatandoost H, Ramin E, Rassi Y, Abai MR. Stability and Wash Resistance of Local Made Mosquito Bednets and Detergents Treated with Pyrethroids against Susceptible Strain of Malaria Vector Anopheles stephensi. Iran J Arthropod-Borne Dis. 2009b;3(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Vatandoost H, Akbarzadeh K, Hanafi-Bojd AA, Mashayekhi M, Saffari M, Elfatih MM. Malaria stratification in a malarious area, a field exercise. Asian Pac J Trop Med. 2010;3(10):807–811. [Google Scholar]
- WHO . Test procedure for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces, WHO/CDS/CPC/MAL/98.12. World Health Organization; Geneva: 1998. [Google Scholar]
- WHO . Malaria Entomology and Vector Control, Participant, s Guide. WHO; Geneva: 2012. [Google Scholar]