Abstract
Background:
The objective of this study was to assess the epidemiological characteristics of a new emerging focus of cutaneous leishmaniasis (CL) in southern villages of Bam District, southeastern Iran, 2010.
Methods:
A house-to- house census survey of 5544 individuals were interviewed and physically examined for the presence of active lesions or scars. Diagnosis was confirmed by direct smears, cultures and identification by PCR. The data were entered into a computer and SPSS ver. 15.
Results:
Overall, 1.2% of the inhabitants were infected, 0.5% active and 0.7% scars and females were more significantly infected (1.7%) than males (0.8%), (P= 0.003). All age groups were equally affected. Most of the lesions were on the face and majority had single lesion. Most of the cases appeared from 2006 to 2008 during the CL epidemic in the city of Bam. PCR indicated L. tropica as the causative agent.
Conclusion:
The presence of non-immune individuals along with suitable ecological conditions could induce a new emerging focus of ACL in villages.
Keywords: Anthroponotic cutaneous leishmaniasis, Leishmania tropica, Emergence, Iran
Introduction
Leishmaniasis consists of a spectrum of clinical manifestation ranging from simple self-limiting cutaneous lesions to disfiguring and fatal visceral forms (Postigo 2010). The disease endemicity extends to over 88 countries, but its public health impact remains grossly neglected. The overall prevalence is estimated 12 million, with 2 million new cases occur annually and the population at risk is 350 million. Cutaneous leishmaniasis (CL) comprises 1.5 million new cases each year and 90% are limited to 8 countries including Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil and Peru (WHO 2009).
Two epidemiological forms are present in Iran, anthroponotic cutaneous leishmaniasis (ACL) due to Leishmania tropica is a well-known disease in Bam (Nadim and Aflatoonian 1995) where field trials of killed L. major against L. tropica have recently been conducted (Sharifi et al. 1998b). In Bam district, most of the cases are confined to the city, the reservoir is human and the main vector is Phlebotomus sergenti. Zoonotic CL (ZCL) due to L. major presents in a frequency of 5 to 10% where gerbils are the main reservoir and P. papatasi, the vector (Nadim and Aflatoonian 1995, Sharifi et al. 1997, Aghasi and Sharifi, 2003, Oshaghi et al. 2008), although, ZCL cases have been reported predominantly from southern and central Iran (Akhavan et al. 2007, Rassi et al. 2008, Parvizi et al. 2010, Yaghoobi-Ershadi et al. 2010).
Epidemics of CL have been occurred after the earth quake of December 2003, where the number of recorded cases increased to almost 4- folds two years following the earth quake (Sharifi et al. 2011). Several risk factors such as human behaviors, ecological disturbances, environmental changes, and population displacement, due to natural disasters and human intervention influence on the emergence and spread of ACL (Ashford 2000, Patz et al. 2000, Desjeux 2001).
The incidence of CL cases has been significantly increased. Simultaneously, local health authorities detected CL cases in these villages of Bam district. The selection of theses villages was based on the newly reported CL cases by passive case detection in early 2010. There has not previously been report of any case of CL from these areas. During June to August in 2010 the CL cases have been occurred and a survey team was appointed to carry out an epidemiological investigation and to identify the causative agent.
The objective of this study was to assess the magnitude of a newly emerged focus in southern villages of Bam District, Kerman Province, southeastern Iran. Due to different strategic measures between ACL and ZCL this study is highly needed for planning a suitable future control method.
Materials and Methods
Study area
This study was carried out in Nezamshahr County, 50 km south to the city of Bam, the center of Bam District, Kerman Province, southeastern Iran. The altitude is 1032 meters above sea level and the main crops are palm trees, orchard of oranges and alfalfa. The county is hot in summer (40–45 °C) and rather moderate in winter (15–25 °C). The yearly rainfall is about 40mm with relative humidity of 25%.
Sampling and population
This work was carried out as descriptive cross-sectional study from June to August 2010. A census survey of 5544 individuals was carried out by house-to-house visits.
Diagnosis and culturing
A list of households was obtained from the Bam Health Center. A team including an experienced physician, two health assistants and a driver performed interviewing and physical examination. A whole body was examined for the presence of active lesions or scars. Suspected active lesions were scraped with a sterile blade. A questionnaire was completed for each individual, recording age, sex, the location and number of the CL lesions, place of residence and the year of contraction. The samples smeared on to glass slides, fixed in methanol, stained using Giemsa and examined by a light microscope for presence of amastigotes. At the same time, samples from 15 patients were inoculated into Novy–MacNeal–Nicolle (NNN) culture media, transported on ice to Kerman and Leishmaniasis Research Centre for further characterization of the isolates by PCR. The culture media were maintained at 24±1 °C for 7days, then transferred into RPMI 1640 medium (Gibco, UK) containing 15% heat inactivated fetal calf serum (FCS), penicillin (200 units/ml) and streptomycin (200 mg/ml) and incubated at 24±1 °C. Cultures were checked weekly for the growth of promastigotes for a period of 4 weeks.
Of 26 samples we could extract DNA from 15 isolates. The remaining 11 samples were either culture negative or lost their viability through being transferred from the rural health clinic to School of Medicine in Kerman. Although, the direct smear preparations were available, no attempt was made to scrape and extract DNA from these smears, since we intended to identify only a representative sample.
Oral consent of the inhabitants was obtained. The infected subjects received proper medication free of charge. The data were entered into a computer and SPSS ver. 15 and χ2- test were used to determine any significant difference between disease prevalence and demographic characteristics. Statistical significance was at P< 0.05.
Molecular characterization
DNA was prepared from 15 randomly selected clinical isolates. The extraction protocol followed relatively the method described elsewhere (Mahboudi et al. 2001). A pair of primers, upstream (5′TCGCAGAACGCCCCTAC C3′) and downstream (5′AGGGGTTGGTGT AAAATAGGC3′) specific for conserved sequences of kDNA of Leishmania species was used. The differentiation was based on the size of the products. Two Leishmania species provided amplified fragments of about 800 bp for L. tropica and 620 bp for L. major. L. tropica (MHOM/IR/02/Mash2) and L. major (MRHO/IR/75/ER), kindly provided from Tehran University of Medical Sciences were used as positive and distilled water as negative controls.
The PCR was carried out applying a step-up program as follows, initial denaturation for 3 min at 95 °C, followed by 30 cycles of 94 °C for 1 min, 62 °C for 1 min at 72 °C, with a final extension for 7 min at 72 °C.
Results
A total of 5544 inhabitants aged 1–96 years (mean, 26.3 years, SD±18.3), comprising 2647 females (47.8%) and 2897 males (52.2%) were interviewed and physically examined for presence of active lesions or scars (Table1). In both sexes most of the individuals (24.4%) were in the age group 21–30 years and the lowest (12.5%) in 31–40 years.
Table 1.
Distribution by sex and age of 5544 inhabitants examined for cutaneous leishmaniasis in southern villages of Bam, southeastern Iran, 2010
| Age (yr) | Female | Male | Total | |||
|---|---|---|---|---|---|---|
|
| ||||||
| No. | Percent | No. | Percent | No. | Percent | |
| ≤10 | 565 | 21.3 | 592 | 20.4 | 1157 | 20.9 |
| 11–20 | 586 | 22.1 | 630 | 21.7 | 1216 | 21.9 |
| 21–30 | 615 | 23.2 | 737 | 25.5 | 1352 | 24.4 |
| 31–40 | 325 | 12.3 | 370 | 12.8 | 695 | 12.5 |
| >40 | 556 | 21.1 | 568 | 19.6 | 1124 | 20.3 |
| Total | 2647 | 100.0 | 2897 | 100.0 | 5544 | 100.0 |
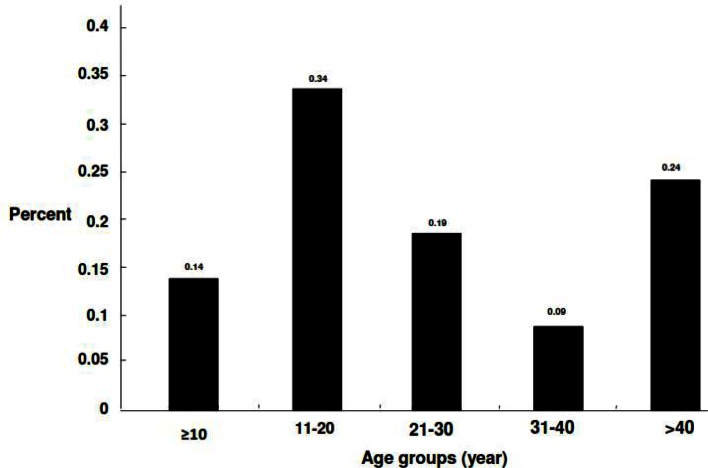
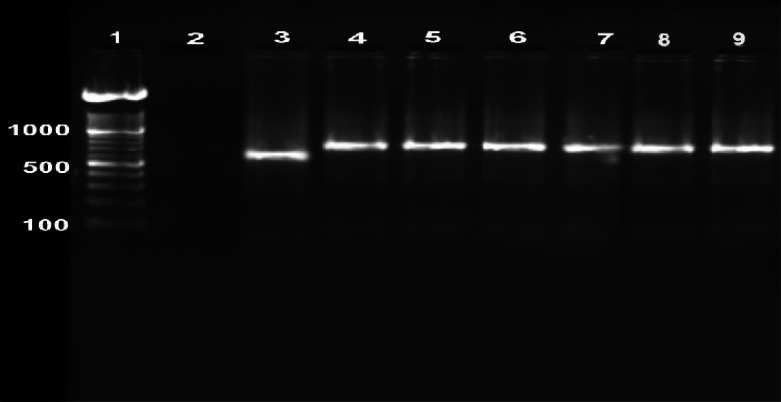
Overall, 1.2% of the population had lesions, 0.5% active and 0.7% scars (Table 2). There was a significant difference between females (1.7%) and males (0.8%) in terms of the prevalence of active lesions and scars (P< 0.005). The age distribution of the CL infection is presented in Fig. 1. All age groups were affected, although the number of affected subjects was generally low and some increase was observed in the age group 11–20 years, though with no significant difference. Most of the lesions were on the face (37%) followed by legs (31%), hands (27%) and others (5.0%). The majority had one lesion (88%), and 6% had 2 or ≥3 lesions each. The average number of lesions was 1.2, equally distributed among the sex. The households had frequent history of travelling to the city of Bam and the lesions were merely restricted to the endogenous inhabitants in this locality. There are 4 main villages in this county; Taraz was the most infected (29.3%), followed by Nezamabad (28.4%), Bagh-e-Balla (25.5%) and Momenabad (16.8%). The first case started as sporadic during the winter of 2005, however, the cases increased significantly, there after. Random selection of 15 isolates by PCR method indicated L. tropica the sole causative species (Fig. 2).
Table 2.
Distribution of anthroponotic cutaneous leishmaniasis by sex and type of lesion in southern villages of Bam, southeastern Iran, 2010
| Sex | No. / Percent | Active lesion | Scar |
|---|---|---|---|
|
| |||
| No. (Percent) | No. (Percent) | ||
| Female | 44/1.7 | 16(0.6) | 28(1.1) |
| Male | 23/0.8 | 10(0.3) | 13(0.5) |
| Total | 67/1.2 | 26(0.5) | 41(0.7) |
Fig. 1.
Distribution of anthroponotic cutaneous leishmaniasis by age in southern villages of Bam, southeastern Iran, 2010
Fig. 2.
Gel electrophoresis of the Leishmania species- specific PCR products using kDNA marker extracted from promastigotes grown in culture media. Lane 1: 100bp ladder marker, Lane2: negative control, Lane3: Leishmania major positive control, Lane4: Leishmania tropica positive control, Lane 5, 6, 7, 8, 9: Leishmania tropica isolates obtained from the cases with anthroponotic cutaneous leishmaniasis in southern villages of Bam, southeastern Iran
Discussion
Cutaneous leishmaniasis is still a major health problem in Iran, where mainly non-healing forms of leishmaniasis recidivans and non-responsive clinical forms to pentavalent animony drugs are present (Esfandiarpour et al. 2007, Sharifi et al. 2010). The disease has a dynamic epidemiology with a complex life cycle and diverse transmission pattern. It can change under the influence of climate, ecology, human behavior, presence of suitable vectors and reservoirs (Ashford 2000, Patz et al. 2000, Desjeux 2001). These factors singly or in combination have been accompanied by global increases in morbidity and mortality from emergent Leishmania species (Patz et al. 2000, Desjeux 2001).
The prevalence data showed that all age groups were affected, which indicated that this emergence was a new occurrence among a non-immune population. In addition the data suggest that females were more than twice susceptible (odd ratio= 2.1) to the infection than males. The households, frequently travel to the city of Bam, where they contract the infection, however, there is no clear explanation for such a sex distribution. It might be due to individual risk factors and more exposure of females to the source of infection (Desjeux 2001, Fazaeli et al. 2009).
The clinical features of the CL cases in terms of the number and the location of the lesions observed in this study are consistent with those of ACL reported previously from the city of Bam (Nadim and Aflatoonian 1995, Sharifi et al. 1998a, Aflatoonian and Sharifi 2006) or elsewhere (Zahraei-Ramazani et al. 2007). More over molecular characterization of the PCR of a number of isolates proved that L. tropica was responsible for this emergence.
The devastating earthquake of Bam in December 2003 demolished almost 90% of the health infrastructures, sacrificed 30000 and left million tons of construction and raw bricks around the houses. Although, the health conditions and infrastructures have been considerably improved, but various risk factors are still around, creating a suitable condition for vector breeding and transmission of the causative agent. In spite of remarkable efforts and implementation of various approaches to reduce transmission and control the disease unfortunately, the CL cases have been increased to epidemic proportion after the earthquake especially during 2006–2008 in the city of Bam.
In Bam district, CL control has been integrated with that of other infectious diseases. The disease has not been reported from this county before. Occupation is mainly restricted to farming and agricultural activities. Movement of the villagers, both men and women back and forth to the city of Bam, more frequently after the earth quake, where the disease is highly endemic, for various purposes is common. Therefore, due to presence of a suitable anthropophyllic vector (Aghasi and Sharifi 2003), the villagers become a highly sensitive reservoir for transmission of ACL endemic to their former home grounds.
The current expansion of villages and urbanization constitute other contributory factors (Patz et al. 2000, Desjeux 2001). Similar risk factors have contributed to the emergence of new ACL foci in Sudan (El-Safi and Peters 1991), Morrocco (Guessous-Idrissi et al. 1997, Ramaoui et al. 2008), Israel (Jacobson et al. 2003), Pakistan (Kolczinski et al. 2004) and new ZCL foci in Iran and abroad (Ashford et al. 2000, Yaghoobi- Ershadi et al. 2001, Fazaeli et al. 2009, Razmjou et al. 2009, Sharifi et al. 2011).
In conclusion, the presence of non-immune population along with suitable ecological conditions could induce a new emerging focus of ACL for human, beyond the original focus. Further epidemiological studies are required to identify the main vectors and strains of the Leishmania species involved in this focus.
Acknowledgments
This work was approved by the Vice Chancellor for Research and received financial support from the Kerman University of Medical Sciences and Health Services (Project no. 89/24). We are grateful to the health-worker team in Bam Health System for their help in carrying out this study. The authors declare that there is no conflict of interest.
References
- Aflatoonian MR, Sharifi I. Prevalence of cutaneous leishmaniasis in primary school children in Bam District, 2005. J Kerman Univ Med Sci. 2006;14:82–84. [Google Scholar]
- Aghasi M, Sharifi I. Survey of the fauna and monthly activity of the sand fly as the vector of the cutaneous leishmaniasis in the city of Bam. J Kerman Univ Med Sci. 2003;10(2):85–91. [Google Scholar]
- Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, Jafari R, Abdoli H, Arandian MH, Soleimani H, Zahraei-Ramazani AR, Mohebali M, Hajjaran H. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. Iran J Arthropod-Borne Dis. 2007;1:1–8. [Google Scholar]
- Ashford RW. The leishmaniasis as emerging and reemerging zoonoses. Int J Parasit. 2000;30:1269–1281. doi: 10.1016/s0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Desjeux P. The increase in risk factors for the leishmaniasis worldwide. Tran Roy Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- El-Safi SH, Peters W. Studies on the leishmaniasis in the Sudan. 1. Epidemic of cutaneous leishmaniasis in Khartoum. Tran Roy Soc Trop Med Hyg. 1991;85:44–47. doi: 10.1016/0035-9203(91)90151-n. [DOI] [PubMed] [Google Scholar]
- Esfandiarpour I, Dabiri SH. Treatment of cutaneous leishmaniasis recidivans with a combination of allopurinol and meglumine antimoniate: a clinical and histologic study. Int J Dermatol. 2007;46:848–852. doi: 10.1111/j.1365-4632.2007.03086.x. [DOI] [PubMed] [Google Scholar]
- Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector Borne Dis. 2009;46:36–42. [PubMed] [Google Scholar]
- Guessous-Idrissi N, Hamdani A, Rhalem A, Riyad M, Sahibi H, Dehbi F, Bichichi M, Essari A, Berrag B. Epidemiology of human visceral leishmaniasis in Taounate, a northern province of Morocco. Parasite. 1997;4(2):181–185. doi: 10.1051/parasite/1997042181. [DOI] [PubMed] [Google Scholar]
- Jacobson RL, Eisenberger CL, Svobodova M, Banesh G, Sztern J, Carvalho J, Nasereddin A, El Fari M, Shalom U, Volf P, Votypka J, Dedet JP, Pratlong F, Schonian G, Schnur LF, Jaff CL, Warburg A. Outbreak of cutaneous leishmaniasis in northern Israel. J Infect Dis. 2003;188:1065–1074. doi: 10.1086/378204. [DOI] [PubMed] [Google Scholar]
- Kolaczinski J, Brooker S, Reyburn H, Rowland M. Epidemiology of anthroponotic cutaneous leishmaniasis in Afghan refugee camps in northwest Pakistan. Tran Roy Soc Trop Med Hyg. 2004;98:373–378. doi: 10.1016/j.trstmh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Mahboudi F, Abolhassan M, Yaran M, Mobtaker H, Azizi M. Identification and differentiation of Iranianian Leishmaniaspecies by PCR amplification of kDNA. Scand J Infect Dis. 2001;33:596–598. doi: 10.1080/00365540110026746. [DOI] [PubMed] [Google Scholar]
- Nadim A, Aflatoonian MR. Anthroponotic cutaneous leishmaniasis in Bam, southeast Iran. Iranian J Publ Health. 1995;24:15–24. [Google Scholar]
- Oshaghi MA, Yaghoobi-Ershadi MR, Abbassi M, Parvizi P, Akhavan AA, Rahimi Foroshani A, Zahraei AR, Rassi Y, Mohtarami F. Detection of Leishmania majorin naturally infected sand flies using semi nested-PCR. Iranian J Publ Health. 2008;37(4):59–64. [Google Scholar]
- Parvizi P, Baghban N, Novin EA, Absavaran A. Detection, identification and molecular typing of Leishmania major in Phlebotomus papatasi from a focus of zoonotic cutaneous leishmaniasis in Central of Iran. Exp Parasitol. 2010;124(2):232–237. doi: 10.1016/j.exppara.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Inter J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010;36(1):62–65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Ramaoui K, Guernaoui S, Boumezzough A. Entomological and epidemiological study of a new focus of cutaneous leishmaniasis in Morocco. Parasitol Res. 2008;103:859–863. doi: 10.1007/s00436-008-1068-3. [DOI] [PubMed] [Google Scholar]
- Rassi Y, Abai MR, Javadian E, Rafizadeh S, Imamian H, Mohebali M, Fateh M, Hajjaran H, Ismaili K. Molecular data on vectors and reservoir hosts of zoonotic cutaneous leishmaniasis in central Iran. Bull Soc Path Exot. 2008;101:425–428. [PubMed] [Google Scholar]
- Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emami M, Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Tran Roy Soc Trop Med Hyg. 2009;103:727–730. doi: 10.1016/j.trstmh.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Ardehali S, Motazadian H, Aflatoonian MR. Identification and characterization of Leishmaniaisolates in school children in Bam, southeastern Iran. Iran J Med Sci. 1997;22:82–88. [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Nadim A, Nikian Y, Khamesipour A. Cutaneous leishmaniasis in primary school children in the southeastern Iranian city of Bam, 1994–1995. Bull WHO. 1998a;76:289–293. [PMC free article] [PubMed] [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Nadim A, Ahmadi Mousavi MR, Momeni A, Dowlati Y, Godal T, Zicker F, Smith GP. Randomized vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998b;351:1540–1544. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Mahboudi F, Dowlati Y, Nadim A, Modabber F. Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994–2006. Intern J Dermatol. 2010;49:557–561. doi: 10.1111/j.1365-4632.2010.04419.x. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Nakhaei N, Aflatoonian MR, Hakimi Parizi M, Fekri AR, Safizadeh H, Shirzadi MR, Goya MM, Khamesipour A. Cutaneous leishmaniasis in Bam: A comparative evaluation of pre- and post- earthquake years (1999–2008) Iranian J Publ Health. 2011;40:49–56. [PMC free article] [PubMed] [Google Scholar]
- Sharifi I, Poursmaelian S, Aflatoonian MR, Fotouhi Ardakani R, Mirzaei M, Fekri AR, Khamesipour A, Hakimi Parizi M, Fasihi Harandi M. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropicain rural communities of Bam district after the earthquake, Iran. Trop Med Intern Health. 2011;16(4):510–513. doi: 10.1111/j.1365-3156.2011.02729.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2009. Leishmaniasis: background information. A brief history of the disease. Available at: www.who.int/leishmaniasis/en/ [Google Scholar]
- Yaghoobi-Erashadi MR, Hanafi-Bojd AA, Akhavan AA, Zahrai Ramazani AR, Mohebali M. Epidemiological study in a new focus of cutaneous leishmaniasis due to Leishmaniasis majorin Ardestan town, central Iran. Acta Trop. 2001;79:115–121. doi: 10.1016/s0001-706x(01)00085-7. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Hakimiparizi M, Zahraei-Ramazani AR, Abdoli H, Akhavan AA, Aghasi M, Arandian MH, Ranjbar AA. Sand fly surveillance within an emerging epidemic focus of cutaneous leishmaniasis in southeastern Iran. Iran J Arthropod-Borne Dis. 2010;4:17–23. [PMC free article] [PubMed] [Google Scholar]
- Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Mokhtari AR, Akhavan AA, Abdoli H, Arandian MH. Anthroponotic cutaneous Leishmaniasis in nonendemic quarters of a central city in Iran. Iranian J Publ Health. 2007;36:7–11. [Google Scholar]