Abstract
The mechanisms that regulate the acidification of intracellular compartments are key to host defense against pathogens. In this paper, we demonstrate that Abl tyrosine kinase, a master switch for cell growth and trafficking of intracellular organelles, controls the acidification of lysosomes in human macrophages. Pharmacological inhibition by imatinib and gene silencing of Abelson (Abl) tyrosine kinase reduced the lysosomal pH in human macrophages by increasing the transcription and expression of the proton pumping enzyme vacuolar-type H+-adenosine triphosphatase. Because lysosomal acidification is required for antimicrobial activity against intracellular bacteria, we determined the effect of imatinib on the growth of the major human pathogen Mycobacterium tuberculosis. Imatinib limited the multiplication of M. tuberculosis. and growth restriction was dependent on acidification of the mycobacterial compartment. The effects of imatinib were also active in vivo because circulating monocytes from imatinibtreated leukemia patients were more acidic than monocytes from control donors. Importantly, sera from imatinib-treated patients triggered acidification and growth restriction of M. tuberculosis in macrophages. In summary, our results identify the control of phagosomal acidification as a novel function of Abl tyrosine kinase and provide evidence that the regulation occurs on the level of the vacuolar-type H+-adenosine triphosphatase. Given the efficacy of imatinib in a mouse model of tuberculosis and our finding that orally administered imatinib increased the ability of human serum to trigger growth reduction of intracellular M. tuberculosis, clinical evaluation of imatinib as a complementary therapy of tuberculosis, in particular multidrug or extremely drugresistant disease, is warranted.
Lysosomes are subcellular organelles that function to digest cellular debris and aid in the destruction of microbial pathogens. These functions in cell homeostasis and host defense are dependent on the acidification of lysosomes, providing the optimal environment for the activation of degradative enzymes. Definition of the mechanisms that regulate the acidification of intracellular compartments will provide new insights into host defense against microbial pathogens.
Recent studies indicate that lysosome function is regulated by the Abelson (Abl) tyrosine kinase (1). The Abl kinase gene family consists of the Abl tyrosine kinase (Abl1), its paralog Arg, and the oncogenic fusion protein Bcr-Abl (2). Abl tyrosine kinase is activated in response to extracellular or intracellular stimuli. Activation triggers ATP-dependent interactions with multiple cellular targets including cytoskeletal proteins that coordinate actin dynamics and cell migration (2). More specifically, Abl tyrosine kinase positively regulates autophagy by orchestrating the localization and activity of glycosidases, cathepsins, and lysosomes, suggesting that Abl tyrosine kinase is involved in digestion and removal of self- and foreign material (1, 3).
Chromosomal translocation of the breakpoint cluster region gene to the ABL gene produces the Bcr-Abl fusion protein resulting in constitutive Abl tyrosine kinase activity and chronic myeloid leukemia (CML) (4). This sentinel finding has been translated into clinical guidelines, and pharmacological inhibition of Abl tyrosine kinase by imatinib (STI571) is the current standard treatment for early-stage CML (5). Imatinib neutralizes Abl tyrosine kinase activity by competitive displacement of ATP from the binding pocket. Despite the broad functional activity of Abl tyrosine kinase, the treatment is generally well tolerated. As opposed to many other cancer treatments, imatinib does not increase the risk of infections raising the intriguing possibility that it supports immune effector mechanisms.
Mycobacterium tuberculosis, the causative agent of tuberculosis, exploits the host cell machinery by manipulating the trafficking of intracellular organelles and avoiding phagosome acidification (6). Because Abl tyrosine kinase is involved in the movement of lysosomes, it is conceivable that M. tuberculosis and the host cell kinase interact and affect the outcome of infection. Recently, it was demonstrated that silencing of ABL1 affects the growth of the in-tracellular pathogen M. tuberculosis (7) and that inhibition of Abl tyrosine kinase reduces the bacillary load in a mouse model of tuberculosis (8). Because restriction of mycobacterial growth requires the acidification of phagosomes, we hypothesized that Abl tyrosine kinase regulates the acidity in lysosomes and modulates the growth of M. tuberculosis and human macrophages.
In this study, we demonstrate that Abl tyrosine kinase controls phagosomal acidification by modulating the expression of the proton pumping enzyme vacuolar-type H+-adenosine triphosphatase (vATPase). Imatinib—added in vitro or after oral administration— strengthens the antimicrobial activity of human macrophages against M. tuberculosis and should be evaluated as an adjuvant therapy against drug-resistant tuberculosis.
Materials and Methods
Cell culture reagents
Cells were cultured in RPMI 1640 medium (Biochrom) supplemented with glutamine (2 mM; Sigma-Aldrich), 10 mM HEPES, 13 mM NaHCO3, 100 µg/ml streptomycin, 60 µg/ml penicillin (all from Biochrom), and 5% heat-inactivated human AB serum (Cambrex) (= complete medium [CM]). For the culture of bronchoalveolar lavage (BAL) cells, streptomycin was replaced by amphotericin B (5.6 µg/ml) (Sigma-Aldrich). Ten percent non–heat-inactivated human AB serum was used to optimize the uptake of the bacilli (= BAL medium).
Abs and reagents
The following Abs were used for immunofluorescence, flow cytometry, or Western blot analysis: anti-CD1a (HI149; BD Biosciences), anti–CD14-APC (clone Tu¨K4), Systems, and anti–CD68-FITC (clone Y1/82A; BD Biosciences), anti–CD83-APC (clone HB15e; BD Biosciences), anti–HLA-DR-PerCP (clone L243; BD Biosciences), rabbit polyclonal anti–c-Abl, anti-rabbit IgG F(ab′)2 fragment 488 conjugate, anti-mouse IgG F(ab′)2 fragment 488 conjugate (all from Cell Signaling Technology), anti–EEA1-FITC (clone14/EEA1; BD Biosciences), rabbit polyclonal anti-vATPase, subunit c (9), mouse monoclonal anti-vATPase, subunit a3 (TCIRG1; Abcam), mouse monoclonal anti–β-actin (AC-15; Abcam), anti-CD63 (clone CLB Gran/12; Immunotech), and AnnexinVFITC (Responsif, Erlangen, Germany).
The tyrosine kinase inhibitors imatinib (Gleevec; Novartis) and dasatinib (Tasigna; Bristol-MyersSquibb) were dissolved inDMSO(Sigma-Aldrich) at 1 mg/ml and stored at –70°C. The MAPK p38 inhibitor (SB203580) and the Src family tyrosine kinase inhibitor PP2 were from Calbiochem. The vATPase inhibitor concanamycin A and unlabeled pepstatin were from Sigma-Aldrich. The fluorescent probes were all purchased from Sigma-Aldrich (Hoechst 33258), pepstatin A bodipy-FL conjugated (BPF), Lyso-Sensor Green DND189, and LysoTracker Red DND99. IFN-γ was purchased from R&D Systems, and L-N6-(1-iminoethyl)lysine was from Calbiochem.
Preparation of macrophages
PBMC were isolated by density gradient centrifugation of buffy coat preparations from the blood of healthy donors. Monocytes were isolated by adherence on plastic and cultured in the presence of GM-CSF (10 ng/ml; Berlex). Monocyte-derived macrophages (MDM) were detached with EDTA (1 mM; Sigma-Aldrich) after 4–6 d of culture. Phenotypic characterization confirmed that the purity of macrophages exceeded 97% (CD68-positive, HLA-DR–positive) and were not significantly contaminated by dendritic cells (CD1a–negative and CD83-negative) or T cells (CD3-negative).
Alveolar macrophages (AM) were obtained from the BAL of patients who underwent bronchoscopy for diagnostic purposes. Patients given a diagnosis of an infectious lung disease or a disease afflicting the alveolar space were excluded. Lavage fluid was filtered through a tea strainer (WMF) and centrifuged (1300 rpm, 10 min) at 4°C. Cells were plated in 6-well plates (2 × 106/ml), and after 1 h, nonadherent cells were removed by vigorous washing.
LysoSensor staining
Macrophages (0.25 × 106/ml in 250 µl) were plated in 8-chamber slides (Nalge Nunc International) and incubated with imatinib (5 µM) for 24 h. LysoSensor (4 µM) and the nucleic acid stain Hoechst 33258 (1 µg/ml) were added for 2 h. Cells were analyzed by confocal laser (Zeiss 510) or fluorescence (Zeiss Axioskop-2) microscopy. For flow cytometry, macro-phages or whole blood (250 µl) were incubated with LysoSensor (4 µM) as above and immediately analyzed by flow cytometry using WinMDI 2.8 software (J. Trotter, Salk Institute, La Jolla, CA).
LysoTracker staining
In contrast to LysoSensor, LysoTracker red DND99 is not washed out by Triton X-100 and was therefore used for fluorescence microscopy (10). MDM were incubated with LysoTracker (100 nM) for 1 h at 37°C. Para-formaldehyde (4%)-fixed cells were treated with Triton X-100 (0.3%, 10 min on ice; Sigma-Aldrich) and anti-EEA1 (1:100) or anti-CD63 (1:100) for 2 h at 4°C. After incubation with anti-mouse IgG F(ab′)2 fragment, Alexa 488 cells were analyzed by fluorescence microscopy.
Immunostaining for vATPase
For the detection of vATPase, subunit a3 macrophages were adhered to 8-chamber slides and treated with imatinib (5 µM) for 24 h. Macrophages were fixed (4% paraformaldehyde), permeabilized (0.5% Triton X-100), and stained with a polyclonal anti-vATPase Ab (1:500, 1 h). Labeling was detected using anti-rabbit Alexa 488 (1:800). Hoechst 33258 (1 µg/ml) was added for nuclear staining during the last 10 min. Cells were evaluated by confocal laser or fluorescence microscopy. For analysis by flow cytometry, cells were detached and stained exactly as described for immunofluorescence.
Silencing of Abl tyrosine kinase RNA
MDM were transfected with a nontargeted control small interfering RNA (siRNA) or on-target–specific siRNA directed to Abl sequences (11) (Santa Cruz Biotechnology): 5′-UCAACAGUCUGGAGAAACA-3′; 5′-CUUC-AUCCCUCUCAUAUCA-3′; and 5′-UGUGAAUCCUGGCAAGAAA-3′. MDM were suspended in human macrophage nucleofector solution (100 µl; Amaxa Biosystems) and siRNA (300 nM). Cells were electroporated using preoptimized conditions as specified by the manufacturer (program Y-10).
Immunoprecipitation and Western blot analysis
Modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl [Roth] [pH 7.4], 1% Nonidet P-40 [Fluka], 0.25% sodium deoxycholate [Fluka], 150 mM NaCl [Roth], 1 mM EDTA, and protease inhibitor mixture tablets [Roche]) was added in each well. Cell debris was removed by centrifugation (14,000 rpm, 10 min), and protein content in the supernatant was determined (bicinchoninic acid protein assay; Pierce). Lysates were incubated with anti–c-Abl Abs (1:50) Abs overnight at 4°C and immunoprecipitated with 10 µl Sepharose 4B (Invitrogen) for 2 h at 4°C to increase the sensitivity and specificity of detection. Immunoprecipitates were boiled for 10 min in Laemmli sample buffer (1% [w/v] SDS [Sigma-Aldrich], 4 mM urea [Roth], 80 mM Tris-HCl [pH 6.8], and 0.1% [w/v] bromphenol blue [Sigma-Aldrich]) and analyzed by SDS-PAGE (16%) and Western blot. The membranes were incubated with an anti–c-Abl Ab (1:2000) or an anti-vATPase subunit a3 Ab (1:1000) and incubated with donkey anti-rabbit IgG-HRP (1:1000) (Jackson ImmunoResearch Laboratories) or anti-mouse IgG-HRP (1:5000) (Invitrogen). Proteins were detected by chem-iluminiscence (Amersham Biosciences) following the manufacturer’s protocol.
Real-time quantitative PCR
MDM were treated with imatinib (5 µm, 24 h), and mRNA was prepared following the instruction of the manufacturer (RNeasy; Qiagen). Contaminating DNA was removed by DNase I treatment. cDNA was prepared by Random Decamers (Applied Biosystems) as suggested by the manufacturer. cDNA was analyzed by quantitative real-time quantitative PCR (qPCR) (ABI step one) using the following primers (selected by Pearlprimer; synthesized by Metabion): vATPase, subunit c, 5′-ATGTCCGA-GTCCAAGAGC-3′ (forward) and 5′-CTACTTTGTGGAGAG-GATGAG-3′ (reverse); vATPase subunit a3, 5′-ATCTGGCAGACTTTCT TCAG-3′ (forward) and 5′-AAGATGCTGGTGGCGCGACT-3′ (reverse); and 18S rRNA, 5′-ACCGATT-GGATGGTTTAGTGAG-3′ (forward) and 5′-CCT-ACGGAAACCTTG TTA CGAC-3′ (reverse). Threshold cycle values were quantified using appropriate software (ABI).
Culture of M. tuberculosis
M. tuberculosis (virulent strain H37Rv) was grown in suspension with constant gentle rotation in roller bottles containing Middlebrook 7H9 broth (BD Biosciences) supplemented with 1% glycerol (Roth), 0.05% Tween 80 (Sigma-Aldrich), and 10% Middlebrook oleic acid, albumin, dextrose, and catalase enrichment (BD Biosciences). Aliquots from logarithmically growing cultures were frozen in PBS/10% glycerol, and representative vials were thawed and enumerated for viable CFU on Middlebrook 7H11 plates. Staining of bacterial suspensions with fluorochromic substrates differentiating between live and dead bacteria (BacLight; Invitrogen) revealed a viability of the bacteria .>90% and confirmed the absence of large bacterial aggregates.
Succinimidyl ester labeling of mycobacteria
To detect intracellular mycobacteria, bacilli were labeled with the fluorescein succinimidyl ester Alexa 647 or Alexa 568 (red) prior to infection (12). Mycobacteria were washed twice with PBS, 0.5% Tween 80, and 0.2 M sodium bicarbonate (Roth) (pH 8.8) and resuspended in 1 mM Alexa 647. After 1 h, bacteria were washed three times with PBS/0.5% Tween 80. Bacterial viability following Alexa 647 labeling was typically >95% as assessed by comparing the number of CFU between labeled and unlabeled bacilli.
Quantification of mycobacterial growth
Macrophages were infected with single-cell suspensions of M. tuberculosis (multiplicity of infection [MOI] 5). After 18 h, macrophages were washed three times to remove extracellular bacteria. The efficacy of infection was determined in each experiment by an acid fast stain (auramin rhodamine; Merck) and was 45 ± 17%. The number of bacilli per infected MDM ranged between 2 and 7, and the viability remained >60% at the end of the incubation period as determined by Annexin V staining. Importantly, imatinib treatment had no effect on the viability of the macrophages (data not shown). Even though imatinib had no effect on the uptake of the bacilli (data not shown), MDM were infected as bulk cultures in 6-well plates, harvested, and redistributed before addition of imatinib. To enumerate the number of viable bacilli, infected macrophages were lysed with 0.3% saponin (Sigma-Aldrich) to release intracellular bacteria. Cell lysates were resuspended vigorously, transferred into screw caps, and sonicated in a preheated (37°C) water bath for 10 min. Aliquots of the sonicate were serially diluted (1:10, 1:100, and 1:1000) in BAL medium. Four dilutions of each sample were plated in duplicates on 7H11 agar plates and incubated for 14 d before determining the number of CFU.
CML patients
Patients with CML were recruited at the University Hospital of Ulm. The study was approved by the ethical committee of the University of Ulm (number 192/07), and written informed consent was given by all patients. Patients (n = 19; 11 male, 8 female; mean age, 53 y; range, 25–75 y) presented for routine checkup of their disease between February and September 2009. Inclusion criteria were as follows: 1) chronic stage of the disease; 2) imatinib treatment (400 mg) with a once daily dosing regimen; and 3) good treatment response defined by the presence of complete hephagolysosomes, matologic and cytogenetic remission.
Statistical analysis
The results are presented as mean ± SD. A Student t test was used to determine statistical significance between differentially treated cultures. Differences were considered significant if p < 0.05.
Results
Inhibition of Abl tyrosine kinase triggers acidification in MDM
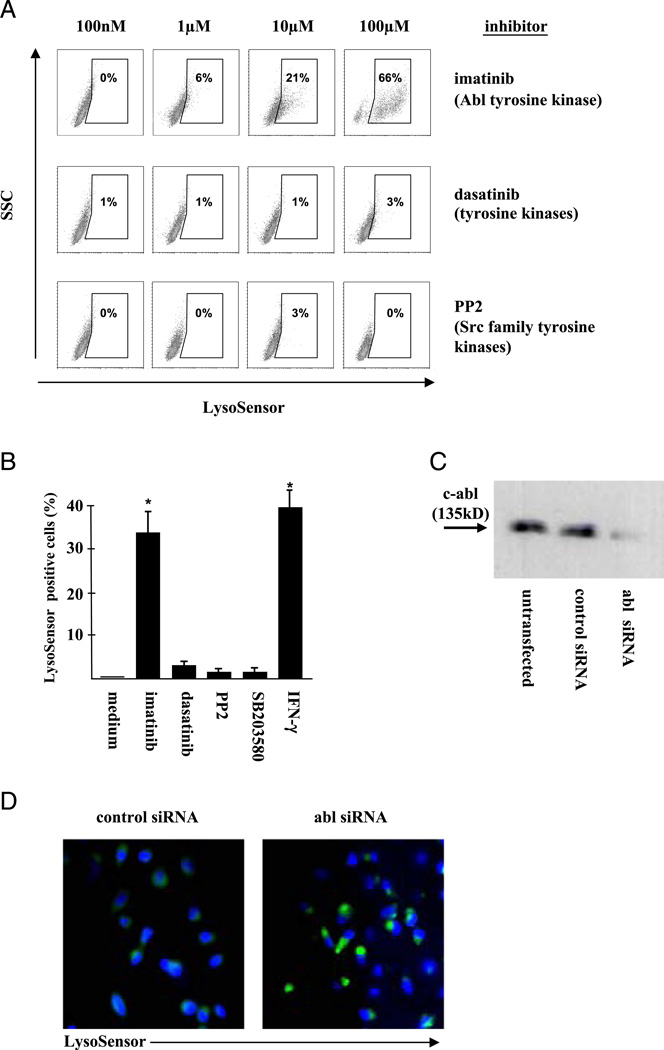
The fusion of lysosomes and endosomes is a key mechanism of cell homeostasis and is required for the formation of acidic vesicles. Because Abl tyrosine kinase regulates the mobility and distribution of lysosomes 1, we hypothesized that Abl tyrosine kinase controls the pH in human macrophages. To test this possibility, MDM were treated with the Abl tyrosine kinase inhibitor imatinib (100 nm–100 µm) and stained with LysoSensor, a dye that emits green fluorescence when the surrounding pH is lower than 6 (Fig. 1A). In untreated MDM, LysoSensor staining was weak (data not shown), and this was set as the baseline for further experiments. Imatinib increased the frequency and fluorescence intensity of LysoSensor-positive cells in MDM in a dose-dependent manner to 66% at 100 µM, indicating a decreased pH. In contrast, nonselective inhibition of tyrosine kinases by dasatinib or the broad Src family tyrosine kinase inhibitor PP2 had no effect on the frequency of LysoSensor-positive cells (Fig. 1A). The inhibition of other classes of kinases (MAPKs and PI3Ks) had no effect on the pH (Fig. 1B). At concentrations reached in CML patients after oral administration (5 µm), imatinib was as efficient in inducing acidification as pretreatment of MDM with IFN-γ, which was used as a positive control (Fig. 1B) (13). These data, showing that imatinib promotes acidification in human MDM, suggest that Abl tyrosine kinase is responsible for maintaining a neutral pH under steady-state conditions.
FIGURE 1.
Inhibition of Abl tyrosine kinase triggers acidification in MDM. (A) MDM were treated with increasing concentrations of kinase inhibitors for 24 h. LysoSensor (4 µM) was added for 2 h, and cells were analyzed by flow cytometry. The figure shows the result of one of two donors analyzed. (B) MDM were incubated with imatinib (5 µM), dasatinib (100 nM), PP2 (10 µM), SB203580 (10 µM), wortmannin (100 nM), or IFN-γ (10 ng/ml) for 24 h and incubated with LysoSensor (4 µM) for 2 h. Lyso-Sensor-positive cells were enumerated by flow cytometry. The graph shows the average result of six experiments with different donors. Error bars show the SD. *p < 0.05, as compared with the medium control. (C) MDM were transfected with control siRNA or c-Abl siRNA. After 24 h, lysates were immunoprecipitated, standardized for protein concentration, and evaluated by Western blot for the expression of Abl tyrosine kinase protein levels using a c-Abl Ab. The blot shows a representative result of three independent experiments. (D) MDM were transfected with a non targeted control siRNA (left panel) or Abl-specific siRNA (right panel). Transfected MDM were incubated with LysoSensor (4 µM, green) and Hoechst 33258 (1 µg/ml, blue) for 2 h at 37°C and analyzed by fluorescence microscopy (original magnification ×630). The images show representative sections from three independent experiments with different donors.
Besides neutralization of Abl tyrosine kinase, imatinib also affects platelet-derived growth factor, c-kit, and non–kinase-associated enzymes (14). To directly assess whether Abl tyro-sine kinase controls the pH in MDM, we inhibited the transcription of Abl tyrosine kinase by siRNA (Fig. 1C) and assessed the intracellular pH by LysoSensor labeling. Silencing of Abl tyrosine kinase mRNA resulted in significant acidification in MDM as compared with control cells transfected with scrambled siRNA (Fig. 1D). Taken together, these results demonstrated that Abl tyrosine kinase is required for maintaining the pH above 6 in human MDM.
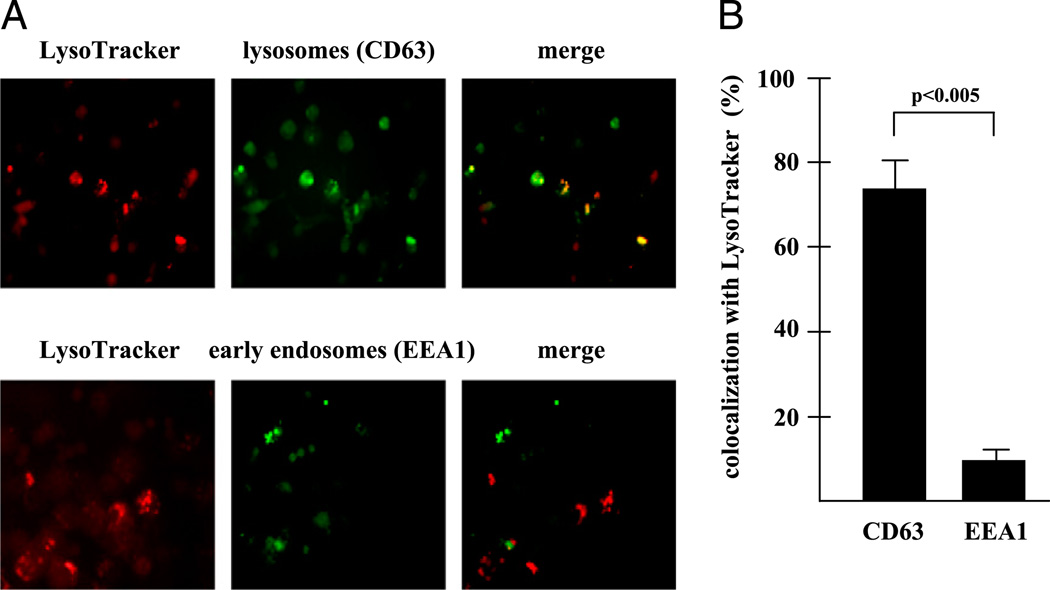
To define the intracellular compartment in which imatinib mediates acidification, we stained imatinib-treated MDM with specific markers for early endosomes (early endosomeAg 1[EEA1]) (15) or lysosomes (CD63) (16). Analysis by fluorescence microscopy demonstrated that 74 ± 8% of LysoTracker-positive cells were CD63+, but only 8 ± 5% EEA1+ (Fig. 2) demonstrating that imatinib preferentially decreases the pH in lysosomes of MDM.
FIGURE 2.
Inhibition of Abl tyrosine kinase induces acidification in lysosomes. (A) MDM were treated with imatinib (5 µM) for 24 h and labeled with CD63 (green, upper panel) or EEA1 (green, lower panel) and LysoTracker (red). The photographs show representative areas of three independent experiments. (B) MDM were treated and stained as in (A). At least 100 LysoTracker-positive MDM were analyzed at a original magnification of ×400 by fluorescence microscopy. The graph shows the percentage of MDM coexpressing CD63/ EEA1 and LysoTracker. Error bars present the SD calculated from the results of three independent experiments.
Mechanism of imatinib-induced acidification
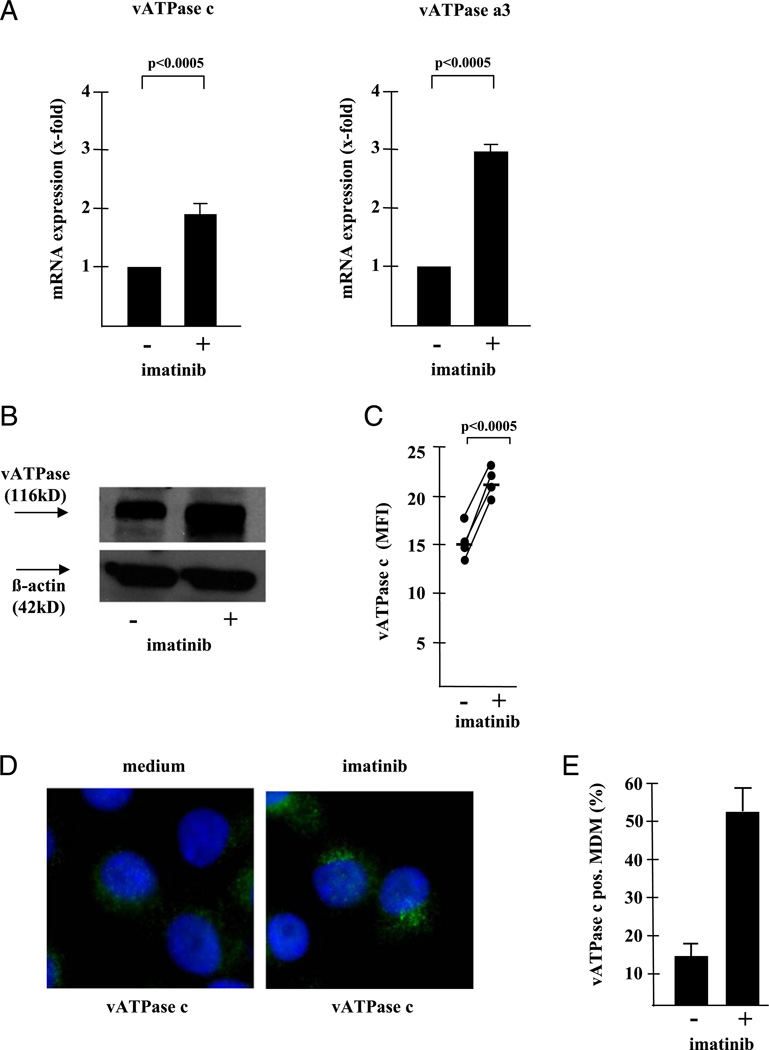
There are at least two mechanisms by which imatinib could induce intracellular acidification. First, inhibition of Abl tyrosine kinase could increase the frequency of lysosomes per cell. We excluded this possibility by demonstrating an essentially equal frequency of CD63-positive vesicles per cell in control- and imatinib-treated MDM (28 ± 3 versus 28 ± 5%) (data not shown). Alternatively, acidification could be mediated by enhanced expression of the vATPase, an enzyme designated for translocating protons across biomembranes, thereby regulating the pH of vesicles (17). To test this, we compared the transcription, expression, and cellular distribution of the vATPase in imatinib-treated and control MDM. Because the subunits a3, c, and d are critical for the function of the enzyme (18), we analyzed their transcription by real-time qPCR. mRNA transcripts of the vATPase subunits a3 and c were significantly increased by imatinib treatment (Fig. 3A), whereas the mRNA levels for subunit d were only moderately affected (data not shown). Next, we measured the protein expression by Western blot analysis and flow cytometry and found that protein levels were consistently higher in imatinib-treated MDM (Fig. 3B, 3C). Finally, fluorescence microscopy demonstrated a prominent punctate staining pattern in imatinib-treated MDM indicative of vacuolar staining (51 ± 4 versus 14 ± 2% in control cultures) (Fig. 3D, 3E). Given the well-known function of vATPase in acidifying intracellular compartments, this set of experiments provides evidence that Abl tyrosine kinase controls the phagosomal pH by upregulating the transcription and expression of the vATPase.
FIGURE 3.
Inhibition of Abl tyrosine ki-nase induces upregulation of vATPase. (A) MDM were treated with imatinib for 16 h. mRNA was prepared and real-time qPCR for the vATPase, domain V0, subunits a3 and c and the housekeeping gene (18S rRNA) was performed. The crossing points for the vATPase curve of the untreated control was standardized to the housekeeping gene and defined as “1.” The graph shows the average x-fold increase of imatinib-treated samples compared with the untreated control calculated from 11 independent donors ± SEM. (B) Lysates were prepared from imatinib-treated (24 h) and control MDM and analyzed by Western blot using a mAb specific for vATPase, subunit a3 (1:1000) or, as a loading control, β-Aktin (1:1000) and a secondary anti-mouse IgG, HRP-linked secondary Ab (1:5000). The panel shows a typical result of four Western blots with different donors. (C) MDM were treated with imatinib for 24 h and analyzed by intracellular flow cytometry using a polyclonal rabbit anti vATPase subunit c Ab (1:500) and a secondary anti rabbit Alexa 488 Ab (1:800). The figure shows the individual results of four donors expressed as MFI of untreated and imatinib-treated MDM. (D) MDM were cultured in 8-chamber slides and stained as described for the flow cytometry. Hoechst 33258 (1 µg/ml) was added for nuclear staining for the final 10 min of incubation. At least 200 cells per donor were analyzed by fluorescence microscopy at an original magnification of ×630. The panel shows three representative cells and (E) the average frequency of MDM containing prominent, punctate vATPase clusters calculated from at least 200 cells in five different donors.
Functional relevance of imatinib-mediated lysosomal acidification
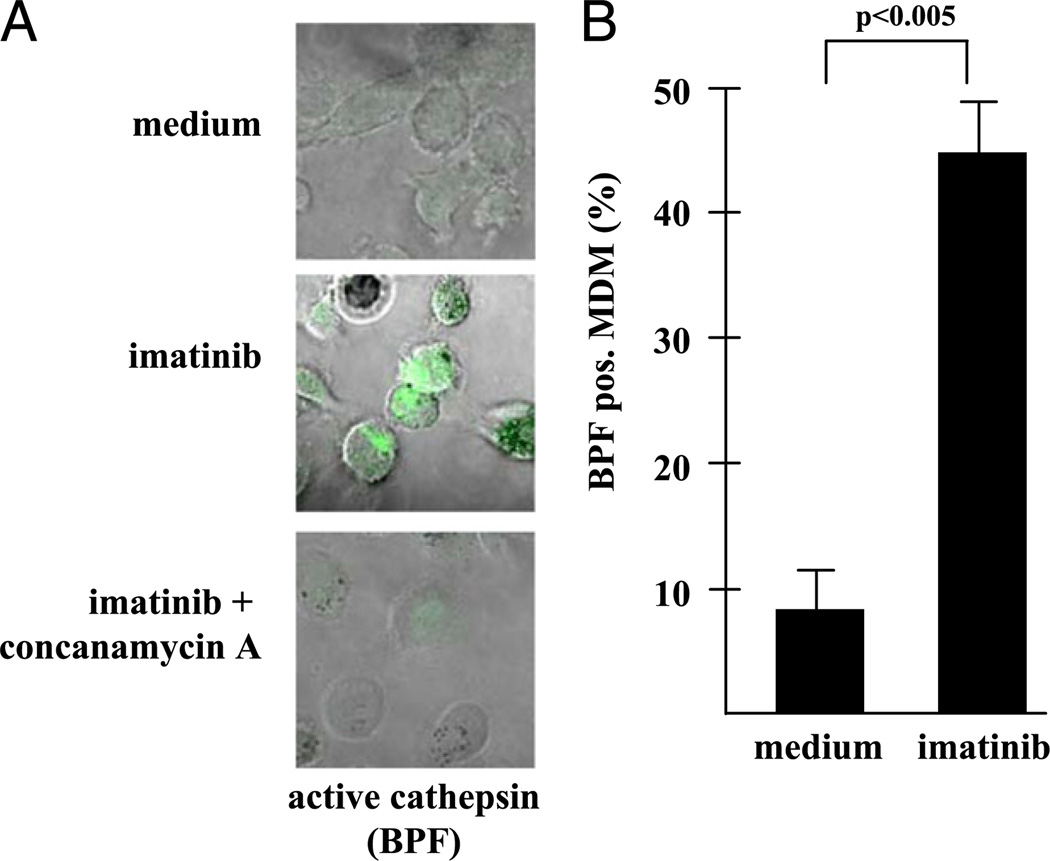
To assess whether imatinib-mediated lysosomal acidification is functionally relevant, we measured 1) the activation of cathepsin D and 2) the induction of antimicrobial activity, both of which are dependent on an acidic environment. First, we determined the expression of cathepsin D, an aspartic protease that is targeted to late endosomes where it remains inactive until it is cleaved to its active form upon endosomal/lysosomal fusion in an acidic environment (19). Active cathepsin D was detected by the fluorescent substrate pepstatin-bodipy (.BPF), which selectively binds to proteolytically cleaved cathepsin and is therefore a biomarker for an acidic environment (20). BPF was readily detected in imatinib-treated cells (45 ± 4%) but much less in untreated control cultures (9 ± 3%) (Fig. 4). Binding of BPF was specific because it was displaced by unlabeled substrate (data not shown) and was virtually undetectable when imatinib-mediated acidification was inhibited by concanamycin, an inhibitor of the vATPase (Fig. 4) (21). Therefore, the effect of imatinib on the lysosomal pH is reflected by an enhanced activity of digestive proteases.
FIGURE 4.
Inhibition of Abl tyrosine kinase promotes the expression of active cathepsin D. (A) MDM were treated with imatinib (5 µM) and concanamycin (50 nM) where indicated. To detect active cathepsin D, cultures were labeled with BPF (1 µ,M, green) and analyzed by confocal laser microscopy. The pictures present representative areas (original magnification ×630) as an overlay phase contrast/green fluorescence. (B) The figure gives the percentage of BPF-positive cells that was determined by analyzing at least 100 MDM in three independent experiments. Error bars show the SD of the results from the different donors.
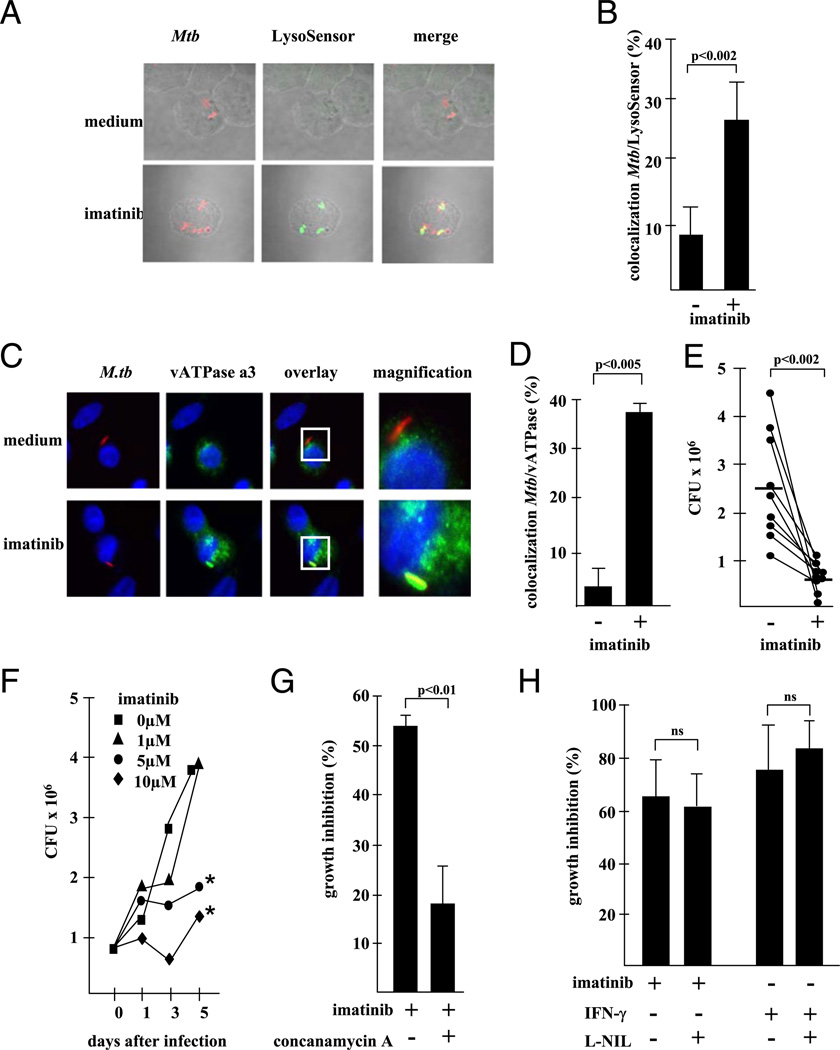
As a second approach to determine the functional impact of imatinib-mediated acidification, we measured antibacterial activity, because the acidification of lysosomes is required for antimicrobial effector functions against intracellular pathogens that reside in phagosomes. As a model organism, we used the intra-cellular bacterium M. tuberculosis, a major human pathogen that survives and even multiplies within phagosomes by arresting maturation before acidification occurs (6). Initially, we sought to establish whether the pharmacological inhibition of the Abl tyrosine kinase induces acidification of the compartments in which mycobacteria reside. By infection of MDM with prestained M. tuberculosis (red) and colabeling with LysoSensor (green), we demonstrated that imatinib mediates acidification of M. tubercu- losis-containing vacuoles (Fig. 5A, 5B). The colocalization of M. tuberculosis and LysoSensor increased from 9 ± 7 to 24 ± 9% (p < 0.002) by imatinib treatment, suggesting that inhibition of Abl tyrosine kinase overcomes the pathogen-driven suppression of acidification. To understand the mechanism of acidification of the mycobacterial niche, we capitalized on our earlier finding demonstrating imatinib-driven upregulation of vATPase (Fig. 3) and investigated the level of colocalization of M. tuberculosis and vATPase (Fig. 5C, 5D). Although only a minority of bacilli stained positively for vATPase in control cultures (3 ± 2%), 38 ± 2% of M. tuberculosis were colocalized with the vATPase in the presence of imatinib.
FIGURE 5.
Inhibition of Abl tyrosine kinase induces acidification of the mycobacterial compartment and antimicrobial activity. (A) MDM were infected with Alexa 647-labeled mycobacteria (red, MOI 5) and treated with imatinib (5 µM) for 24 h. Infected MDM were stained with LysoSensor (4 µM) and analyzed by confocal laser microscopy. The photographs present representative areas (original magnification 3630) from one experiment of five performed with different donors. (B) The figure gives the average percentage of LysoSensor-positive mycobacteria of five independent experiments. Error bars show the SD of the results from the five different donors. (C) MDM were infected with Alexa 647-labeled M. tuberculosis (red) overnight and treated with imatinib for an additional 24 h. Infected cells were fixed, permeabilized, and stained with anti-vATPase subunit c (1:500), anti-rabbit Alexa 488 (1:800) Abs, and Hoechst 33258 (1 µg/ml). Slides were analyzed by confocal laser microscopy or conventional fluorescence microscopy and evaluated for colocalization of vATPase and M. tuberculosis. The panels show representative areas (original magnification ×600, inset ×1000) from experiments performed with nine donors. (D) gives the average percentage ± SEM of M. tuberculosis/vATPase double-labeled MDM calculated from at least 200 cells from all nine donors. (E) Infected MDM (24 h, MOI 5) were cultured with imatinib (5 µM), and the number of viable bacilli was determined by plating the cell lysates after 72 h. The graph gives the individual results of all nine donors investigated. The horizontal line shows the mean values. (F) AM were infected with M. tuberculosis (MOI 5, 24 h) and treated with imatinib at the concentrations indicated. The number of viable bacilli was determined by plating the cell lysates directly after removal of extracellular bacilli and before addition of imatinib (time point 0) and 1, 3, and 5 d after initiation of imatinib treatment. The figure shows a representative result of three with comparable results. *p < 0.05, as compared with the untreated controls. (G) Infected MDM were treated with concanamycin A (50 nM) and incubated with imatinib (5 µM, 72 h), and lysates were plated in 10-fold dilutions on 7H9 agar plates. The graph shows the growth inhibition (percentage) as calculated by comparison with the untreated control (n = 5). Error bars show the SD of the results from the different donors. (H) MDM were treated with imatinib (5 µM) or IFN-γ (10 ng/ml) for 3 d in the presence or absence of L-N6-(1-iminoethyl)lysine (1 mM). The number of viable bacilli was determined by plating cell lysates in serial dilutions on 7H9 agar plates. The figure shows the average reduction of CFU (three different donors tested in independent experiments) as compared with untreated control cultures.
Because acidification of phagosomes correlates with increased antimycobacterial activity 6, we hypothesized that inhibition of Abl tyrosine kinase would induce killing of intracellular M. tuberculosis. To test this possibility, we infected MDM with virulent M. tuberculosis, added imatinib, and determined mycobacterial viability after 3 d of incubation. Imatinib reduced the number of viable M. tuberculosis from 2.5 3 106 to 0.6 3 106 on average in nine independent experiments (p , 0.002; Fig. 5E). This was not due to a direct effect of imatinib on M. tuberculosis because metabolic activity and viability were not affected by incubation of extracellular bacilli with imatinib for 5 d (data not shown). Because tuberculosis is primarily a disease of the lung, we next examined whether imatinib also affects the antimicrobial activity of freshly isolated human AM. Imatinib increased the number of acidic AM as efficiently as in MDM (data not shown) and consequently enhanced killing of virulent M. tuberculosis in a time-and dose-dependent manner (Fig. 5F). Antimicrobial activity becomes evident after 3 d of incubation, demonstrating that acidification (detectable after 18 h) (Fig. 1) precedes killing of the bacilli. To provide a direct link between the low pH and myco-bacterial growth inhibition, we neutralized imatinib-induced acidification with concanamycin A. Concanamycin A prevented acidification by imatinib (data not shown) and significantly reduced mycobacterial growth inhibition from 53 ± 3 to 18 ± 9% (p , 0.01; Fig. 5G).
Besides phagosomal acidification, the production of NO is an important defense mechanism against M. tuberculosis (22). To determine whether nitrogen radicals contribute to imatinib-mediated antimicrobial activity, we measured the release of NO and more importantly the effect of the specific inducible NO syn-thase inhibitor L-N6-(1-iminoethyl)-lysine on imatinib-mediated onstrating imatinib-driven upregulation of vATPase (Fig. 3) and investigated the level of colocalization of M. tuberculosis and vATPase (Fig. 5C, 5D). Although only a minority of bacilli stained positively for vATPase in control cultures (3 ± 2%), 38 ± 2% of M. tuberculosis were colocalized with the vATPase in the presence of imatinib. antimycobacterial activity. In three different donors, imatinib neither induced detectable levels of NO in infected MDM (data not shown) nor did inhibition of inducible NO synthase by L-N6-(1-iminoethyl)lysine affect antimicrobial activity (Fig. 5H). Similarly, IFN-γ, which also triggered lysosomal acidification (Fig. 1B), restricted mycobacterial growth independently of NO confirming our recent findings (Fig. 5H) (23).
Even though apoptotic macrophages as determined by Annexin VFITC staining were detectable after 72 h of incubation, the frequency did not differ between imatinib-treated and untreated macrophages (40 ± 5 versus 41 ± 4%).
Taken together, we show that Abl tyrosine kinase controls the acidity of intracellular compartments in human macrophages, thereby regulating the activity of proteases (e.g., cathepsin D) and antimicrobial activity.
Effect of imatinib therapy on antimicrobial activity and the pH of circulating monocytes
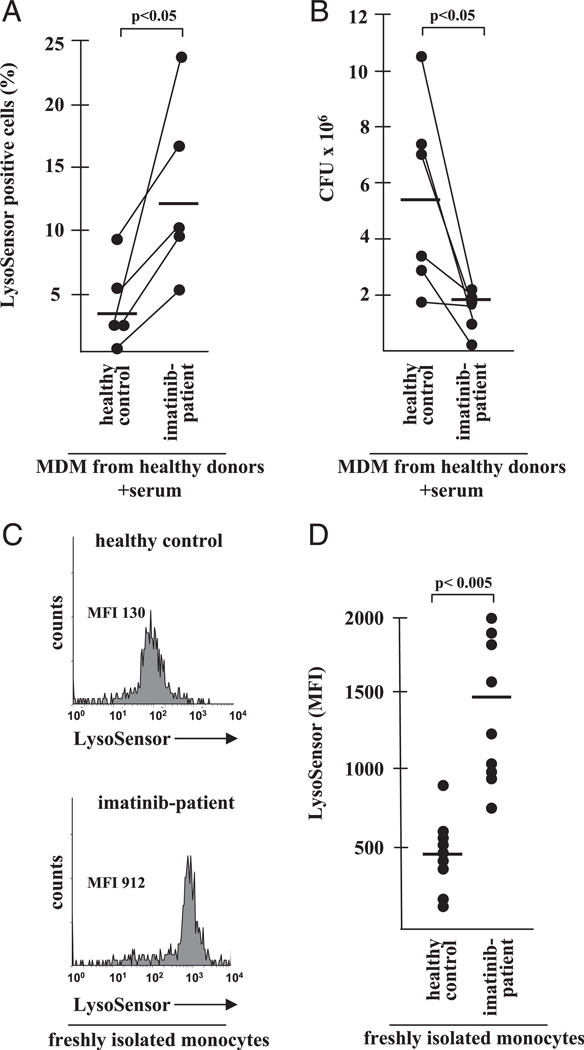
To determine whether the therapeutic inhibition of Abl tyrosine kinase would elicit biological effects in vivo in humans, we recruited patients receiving daily treatment with imatinib (400 mg) for CML. By this treatment, steady-state serum levels of 4.6 µM imatinib are achieved, which corresponds to the concentrations used for our in vitro experiments. First, we incubated MDM from healthy donors with serum (50%) from five patients and determined the percentage of LysoSensor-positive cells after 18 h of incubation by flow cytometry (Fig. 6A). The frequency of LysoSensor-positive cells was significantly higher in MDM cultured in the presence of serum from imatinib-treated patients as opposed to MDM cultured in control serum (12.2 versus 3.4%; p , 0.05). Importantly, the serum from patients reduced the survival of in-tracellular M. tuberculosis more efficiently than control serum (Fig. 6B). These experiments highlight the biological relevance of our initial findings because the imatinib concentrations achieved by oral therapy in vivo are as effective as the addition of purified imatinib (5 µM) in vitro.
FIGURE 6.
Effect of imatinib therapy on antimycobacterial activity and the pH of circulating monocytes. (A) MDM were incubated with serum (50%) of imatinib-treated CML patients or healthy controls for 18 h. MDM were then stained with LysoSensor (4 mM) and analyzed by fluorescence microscopy. The percentage of LysoSensor-positive cells was calculated from at least 100 cells/donor. The horizontal line shows the average percentage of LysoSensor-positive cells (n = 5). (B) MDM were infected with M. tuberculosis and incubated with serum (50%) of imatinib-treated or healthy control donors. The number of CFU was determined after 72 h of incubation. The figure gives the individual results of all six individual donors. The horizontal line shows the average number of CFU calculated from all six donors. (C) Whole blood from imatinib-treated patients or healthy controls was incubated with LysoSensor (4 mM) for 2 h, stained with anti–CD14-APC Abs, and analyzed by flow cytometry. At least 5 × 103 CD14+ monocytes were analyzed for the expression of LysoSensor. The histograms show a representative result from nine donors. (D) The graph shows the MFI from all healthy controls (n = 9) imatinib treated patients (n = 9) examined. The horizontal line shows the average MFI calculated from all donors.
Up to this point, all experiments were based on observations in macrophages derived from healthy donors. To evaluate whether the inhibition of Abl tyrosine kinase also affects the pH of monocytes in vivo, we obtained whole blood from imatinib-treated patients. Levels of monocyte acidification were estimated by double labeling of fresh blood with LysoSensor and the monocyte marker CD14 (Fig. 6C, 6D). The mean fluorescence intensity (MFI) of Lyso-Sensor staining was significantly higher in monocytes from imatinib-treated patients (average MFI in nine donors: 1462 ± 154) as compared with monocytes derived from nine healthy donors (average MFI: 434 ± 70). These results demonstrated that the neutralization of Abl tyrosine kinase in vivo increases the acidity of circulating monocytes and may therefore enhance the antimicrobial response to pathogens.
Discussion
The gradual acidification occurring during the maturation process of phagosomes to lysosomes is a fundamental cellular homeostatic mechanism that must protect against self-digestion but at the same time facilitate the elimination of intracellular pathogens. In this study, we demonstrate that Abl tyrosine kinase controls the acidity of these intracellular organelles by upregulation and redistribution of the proton pumping enzyme vATPase. Functionally, the vacuolar acidification triggered the activation of cathepsin D and an antimicrobial activity against the major human pathogen M. tuberculosis. Importantly, the anticancer drug imatinib, an established and well-tolerated treatment of leukemia, induced acidification of circulating monocytes in patients in vivo. It is tempting to speculate that imatinib could be used therapeutically to decrease the intracellular pH and enhance host antimicrobial responses in patients with severe forms of tuberculosis.
In monocytes and myeloid progenitor cells, the maturation to macrophages (24) and dendritic cells (25) is dependent on Abl tyrosine kinase, but information on the physiological role of Abl tyrosine kinase in resting macrophages is scarce. A key finding of this study is that the constitutive activity of Abl tyrosine kinase is essential for maintaining the physiological pH in CD63-positive lysosomes of primary human macrophages. Silencing of Abl tyrosine kinase induced spontaneous acidification in macrophages, underlining the specificity of our finding. This homeostatic regulation of lysosomal pH is not related to the CML-related Bcr Abl mutation because it was observed in macrophages derived from healthy donors and in imatinib-treated CML patients in complete genetic remission. Therefore, we describe a novel function for Abl tyrosine kinase in primary human macrophages.
Our findings provide insight into the mechanism by which Abl tyrosine kinase prevents acidification of intracellular compartments and how inhibition of the Abl tyrosine kinase permits acidification. The results support the concept that Abl tyrosine kinase controls lysosomal acidification by coordinating the activity of the vATPase, which regulates the phagosomal pH by its proton-pumping activity (17). Along these lines, inhibition of the host cell kinase AKT1 has been shown to prevent the growth of M. tuberculosis and Salmonella thyphimurium by interfering with phagosome–lysosome fusion (26). This does not rule out an additional effect on the dynamics of lysosomal fusion or maturation processes, even though we failed to detect imatinib-mediated changes in the frequency of lysosomes or endosomes (data not shown).
The molecular pathway affected by inhibition of Abl tyrosine kinase may involve activation of the transcription factor FoxO3a, which has been shown to regulate lysosomal functions in muscle cells (27). Stimulation of FoxO3a could stimulate the vATPase and the chloride channel CLC-3 because both have binding sites for FoxO3a in their promoter region and are required for acidification (28, 29). In addition, it is possible that Abl tyrosine kinase could affect the remodeling of the phagosome, which requires the fusion of secretory lysosomes, multivesicular bodies, and early phagosomes (6). Abl tyrosine kinase has an actin binding domain (30), acts on the cytoskeleton (31), and regulates autophagy, which requires directed movement of lysosomes (1, 3). The possibility that Abl tyrosine kinase affects lysosome physiology is further supported by a recent report that imatinib promotes the gene expression of lysosomal proteins such as lysosome-associated membrane proteins 1 and 2 and cathepsins in human phagocytes (32). In addition, inhibitors of host cell kinases have been shown to prevent the growth of M. tuberculosis and S. thyphimurium by interfering with phagolysosome fusion (26).
Even though there is compelling evidence that an acidic environment is unfavorable for M. tuberculosis (33), there are several lines of evidence that challenge this general assumption. First, mycobacteria express genes that confer resistance to acid, and this resistance is essential for virulence (34). Second, morphologically intact mycobacteria were detected in acidic vacuoles in vitro (35, 36) and in vivo (37). Finally, virulent mycobacteria, including Mycobacterium marinum, have evolved evasion mechanisms to escape the hazardous phagolysosomal compartment (38–41). These issues question whether phagolysosomal fusion and an acidic environment alone are sufficient to result in bacillary death. In conjunction with our findings that the low pH is required for growth inhibition (Fig. 5G), we propose a scenario in which the acidic microenvironment promotes antimicrobial macrophage functions such as the activation of lysosomal hydrolases (Fig. 4), the production of reactive oxygen, and nitrogen intermediates or increases the susceptibility of M. tuberculosis to toxic free fatty acids (42). Imatinib has also been shown to regulate autophagy 1,3, which is an alternative defense mechanism against M. tuberculosis (43). We also detected an increased frequency of autophagosomes in macrophages after imatinib treatment using LC3 labeling as a marker (data not shown). Nevertheless, the growth inhibition by the vATPase inhibitor concanamycin A (Fig. 5G) provides compelling evidence that acidification is required for imatinib-mediated growth reduction of M. tuberculosis. We favor the concept that acidification and autophagy are not two independent effector mechanisms but act in concert to support the antimicrobial activity of macrophages against intracellular pathogens. The antimicrobial effect of imatinib could hence be based on both enhanced delivery of mycobacteria to and subsequent acidification of lysosomal compartments.
In addition to affecting macrophage biology, Abl tyrosine kinase has profound effects on T cell function. Studies with Abl tyrosine-deficient mice demonstrated striking deficits in the development, proliferation, and function of T cells (44). In contrast, clinical and experimental observations in imatinib-treated CML patients show that pharmacological inhibition of Abl tyrosine kinase promotes the induction of Ag-specific T cell responses (45–47). An explanation for this paradox is that Abl tyrosine kinase may be required for the development of T lymphocytes but support activation of mature T cells in an adult organism. In the context of microbial infection, imatinib could therefore be beneficial to both arms of the immune system for innate immunity by acidifying lysosomes and activating digestive enzymes in M. tuberculosis-infected macrophages (Fig. 4, Fig. 5) and for acquired immunity by stimulating effector T cell subsets, including cytotoxic T cells (48), which have a unique role in immunity to intracellular pathogens (49).
Imatinib was developed in the 1990s and was approved by the U.S. Food and Drug Administration shortly after the turn of the millennium (50). The introduction of this molecular targeted therapy has revolutionized the treatment of CML, and the 5-y survival rate for newly diagnosed chronic phase patients has reached ~90% (50). Besides its enormous efficacy, imatinib has a very favorable tolerability. The majority of adverse effects such as edema, muscle cramps, diarrhea, nausea, skin rash, and myelosuppression are mild to moderate. Remarkably, the incidence of infections, which frequently complicate standard anticancer therapies, is low. Case reports describe sporadic infections with Varicella zoster (51), hepatitis B (52), candida (53), or tuberculosis (54–56). This is even more surprising because Abl tyrosine kinase has profound effects on the development, signaling, proliferation, and effector function of T cells (44), especially the CD8+ T cell compartment (44, 57, 58). Because CD8+ cytotoxic T cells play a special role in protection against human tuberculosis (59–62), it is puzzling why this infection is not observed with increased frequency during imatinib therapy. One potential explanation is provided by our finding that monocytes from imatinib-treated CML patients contain acidified intracellular vacuoles. Acidification of phagosomes provides a hostile environment for M. tuberculosis with the presence of acidic phagolysosomes, a hallmark of successful elimination of M. tuberculosis.
Very recently, it was reported that inhibition of imatinib-sensitive kinases limit the growth of M. tuberculosis in a mouse model of tuberculosis (8). This important study raised several key questions, including which of several kinases is critical for host immunity, the mechanism by which imatinib induced an antimicrobial activity, and the potential efficacy of imatinib in vivo in humans. In this study, genetic knockdown experiments definitively identify Abl tyrosine kinase as a master switch for controlling the acidity in human macrophages by regulating the expression of the proton pump vATPase. Importantly, sera obtained from the blood of imatinib-treated leukemia patients induced acidification and anti-mycobacterial activity in human macrophages, demonstrating that these effects occur in vivo at therapeutic imatinib levels. Taken together the in vivo results in the mouse model, and our mechanistic and translational human studies warrant clinical trials evaluating imatinib as a complementary treatment for tuberculosis, in particular for disease caused by multidrug-resistant and extremely drug-resistant disease.
Acknowledgments
We acknowledge the support and advice of Frank Kirchoff, Bernd Eikmanns, Barry Bloom, Stefan Ehlers, Kerstin Walter, and the Institute of Transfusion Medicine in Ulm.
This work was supported by the German Ministry for Education and Science (Comprehensive Infectious Disease Center Ulm and “TB or not TB”) and the European Union (Framework Program 7, NewTBVAC).
Abbreviations used in this article
- Abl
Abelson
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- BPF
bodipy-pepstatin-FL
- CM
complete medium
- CML
chronic myeloid leukemia
- EEA1
early endosome Ag 1
- MDM
monocyte-derived macrophage
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- qPCR
quantitative PCR
- siRNA
small interfering RNA
- vATPase
vacuolar-type H+-adenosine triphosphatase
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Yogalingam G, Pendergast AM. Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J. Biol. Chem. 2008;283:35941–35953. doi: 10.1074/jbc.M804543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu JJ, Ryu JR, Pendergast AM. Abl tyrosine kinases in T-cell signaling. Immunol. Rev. 2009;228:170–183. doi: 10.1111/j.1600-065X.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ertmer A, Huber V, Gilch S, Yoshimori T, Erfle V, Duyster J, Elsässer HP, Schätzl HM. The anticancer drug imatinib induces cellular auto-phagy. Leukemia. 2007;21:936–942. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 4.Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315:758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 6.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.Jayaswal S, Kamal MA, Dua R, Gupta S, Majumdar T, Das G, Kumar D, Rao KV. Identification of host-dependent survival factors for in-tracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. 2010;6:e1000839. doi: 10.1371/journal.ppat.1000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10:475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Skinner MA, Shi W, Yu QC, Wildeman AG, Chan YM. The 16 kDa subunit of vacuolar H+-ATPase is a novel sarcoglycan-interacting protein. Biochim. Biophys. Acta. 2007;1772:570–579. doi: 10.1016/j.bbadis.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 11.Pielage JF, Powell KR, Kalman D, Engel JN. RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization. PLoS Pathog. 2008;4:e1000031. doi: 10.1371/journal.ppat.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beatty WL, Russell DG. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 2000;68:6997–7002. doi: 10.1128/iai.68.12.6997-7002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 14.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 15.Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved a-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulinbinding IQ motif. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 16.Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen: a novel lysosomal membrane glyco-protein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J. Biol. Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- 17.Stevens TH, Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. 1997;13:779–808. doi: 10.1146/annurev.cellbio.13.1.779. [DOI] [PubMed] [Google Scholar]
- 18.Sun-Wada GH, Tabata H, Kawamura N, Aoyama M, Wada Y. Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J. Cell Sci. 2009;122:2504–2513. doi: 10.1242/jcs.050443. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi N, Maurer A, Nieke S, Kalbacher H. Cathepsin D: a cellular roadmap. Biochem. Biophys. Res. Commun. 2008;376:5–9. doi: 10.1016/j.bbrc.2008.08.099. [DOI] [PubMed] [Google Scholar]
- 20.Chen CS, Chen WN, Zhou M, Arttamangkul S, Haugland RP. Probing the cathepsin D using a BODIPY FL-pepstatin A: applications in fluorescence polarization and microscopy. J. Biochem. Biophys. Methods. 2000;42:137–151. doi: 10.1016/s0165-022x(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 21.Dro¨se S, Bindseil KU, Bowman EJ, Siebers A, Zeeck A, Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry. 1993;32:3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- 22.Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 2000;3:35–42. doi: 10.1016/s1369-5274(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 23.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, Lyons AB. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 25.Appel S, Boehmler AM, Grünebach F, Müller MR, Rupf A, Weck MM, Hartmann U, Reichardt VL, Kanz L, Bru¨mmendorf TH, Brossart P. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood. 2004;103:538–544. doi: 10.1182/blood-2003-03-0975. [DOI] [PubMed] [Google Scholar]
- 26.Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–730. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Reggio H, Bainton D, Harms E, Coudrier E, Louvard D. Antibodies against lysosomal membranes reveal a 100,000-mol-wt protein that crossreacts with purified H+,K+ ATPase from gastric mucosa. J. Cell Biol. 1984;99:1511–1526. doi: 10.1083/jcb.99.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Wang T, Zhao Z, Weinman SA. The ClC-3 chloride channel promotes acidification of lysosomes in CHO-K1 and Huh-7 cells. Am. J. Physiol. Cell Physiol. 2002;282:C1483–C1491. doi: 10.1152/ajpcell.00504.2001. [DOI] [PubMed] [Google Scholar]
- 30.Van Etten RA, Jackson PK, Baltimore D, Sanders MC, Matsudaira PT, Janmey PA. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J. Cell Biol. 1994;124:325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodring PJ, Litwack ED, O’Leary DD, Lucero GR, Wang JY, Hunter T. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 2002;156:879–892. doi: 10.1083/jcb.200110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appel S, Rupf A, Weck MM, Schoor O, Brümmendorf TH, Weinschenk T, Grünebach F, Brossart P. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-κB and Akt signaling pathways. Clin. Cancer Res. 2005;11:1928–1940. doi: 10.1158/1078-0432.CCR-04-1713. [DOI] [PubMed] [Google Scholar]
- 33.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 34.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli: reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borelli V, Vita F, Soranzo MR, Banfi E, Zabucchi G. Ultrastructure of the interaction between mycobacterium tuberculosis- H37Rv-containing phagosomes and the lysosomal compartment in human alveolar macrophages. Exp. Mol. Pathol. 2002;73:128–134. doi: 10.1006/exmp.2002.2452. [DOI] [PubMed] [Google Scholar]
- 37.Bouley DM, Ghori N, Mercer KL, Falkow S, Ramakrishnan L. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infect. Immun. 2001;69:7820–7831. doi: 10.1128/IAI.69.12.7820-7831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myrvik QN, Leake ES, Wright MJ. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis: a correlate of virulence. Am. Rev. Respir. Dis. 1984;129:322–328. [PubMed] [Google Scholar]
- 39.McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [Published erratum appears in 1993 Infect. Immun. 61:4021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 2003;198:1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ, van der M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 42.Vandal OH, Nathan CF, Ehrt S. Acid resistance in Mycobacte-rium tuberculosis. J. Bacteriol. 2009;191:4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 44.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J. Immunol. 2007;179:7334–7343. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 45.Sato N, Narita M, Takahashi M, Yagisawa K, Liu A, Abe T, Nikkuni K, Furukawa T, Toba K, Aizawa Y. The effects of STI571 on antigen presentation of dendritic cells generated from patients with chronic myelogenous leukemia. Hematol. Oncol. 2003;21:67–75. doi: 10.1002/hon.705. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Cheng F, Cuenca A, Horna P, Zheng Z, Bhalla K, Sotomayor EM. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood. 2005;105:1135–1143. doi: 10.1182/blood-2004-01-0027. [DOI] [PubMed] [Google Scholar]
- 47.Chen CI, Maecker HT, Lee PP. Development and dynamics of robust T-cell responses to CML under imatinib treatment. Blood. 2008;111:5342–5349. doi: 10.1182/blood-2007-12-128397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riva G, Luppi M, Barozzi P, Quadrelli C, Basso S, Vallerini D, Zanetti E, Morselli M, Forghieri F, Maccaferri M, et al. Emergence of BCR-ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood. 2010;115:1512–1518. doi: 10.1182/blood-2009-06-230391. [DOI] [PubMed] [Google Scholar]
- 49.Stenger S, Modlin RL. Cytotoxic T cell responses to intracellular pathogens. Curr. Opin. Immunol. 1998;10:471–477. doi: 10.1016/s0952-7915(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 50.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 51.Mattiuzzi GN, Cortes JE, Talpaz M, Reuben J, Rios MB, Shan J, Kontoyiannis D, Giles FJ, Raad I, Verstovsek S, et al. Development of Varicella-Zoster virus infection in patients with chronic myelogenous leukemia treated with imatinib mesylate. Clin. Cancer Res. 2003;9:976–980. [PubMed] [Google Scholar]
- 52.Lakhani S, Davidson L, Priebat DA, Sherker AH. Reactivation of chronic hepatitis B infection related to imatinib mesylate therapy. Hepatol. Int. 2008;2:498–499. doi: 10.1007/s12072-008-9099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speletas M, Vyzantiadis TA, Kalala F, Plastiras D, Kokoviadou K, Antoniadis A, Korantzis I. Pneumonia caused by Candida krusei and Candida glabrata in a patient with chronic myeloid leukemia receiving imatinib mesylate treatment. Med. Mycol. 2008;46:259–263. doi: 10.1080/13693780701558969. [DOI] [PubMed] [Google Scholar]
- 54.Takashima M, Igaki N, Matsuda T, Ohyama M, Kanda S, Tamada F, Goto T. Malignant gastrointestinal stromal tumor of the small intestine complicated with pulmonary tuberculosis during treatment with imatinib mesylate. Intern. Med. 2005;44:114–119. doi: 10.2169/internalmedicine.44.114. [DOI] [PubMed] [Google Scholar]
- 55.Senn L, Kovacsovics T, Tarr PE, Meylan P. Peritoneal tuberculosis after imatinib therapy. Arch. Intern. Med. 2009;169:312–313. doi: 10.1001/archinternmed.2008.581. [DOI] [PubMed] [Google Scholar]
- 56.Daniels JM, Vonk-Noordegraaf A, Janssen JJ, Postmus PE, van Altena R. Tuberculosis complicating imatinib treatment for chronic myeloid leukaemia. Eur. Respir. J. 2009;33:670–672. doi: 10.1183/09031936.00025408. [DOI] [PubMed] [Google Scholar]
- 57.Mumprecht S, Matter M, Pavelic V, Ochsenbein AF. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 2006;108:3406–3413. doi: 10.1182/blood-2006-04-018705. [DOI] [PubMed] [Google Scholar]
- 58.Sinai P, Berg RE, Haynie JM, Egorin MJ, Ilaria RL, Jr, Forman J. Imatinib mesylate inhibits antigen-specific memory CD8 T cell responses in vivo. J. Immunol. 2007;178:2028–2037. doi: 10.4049/jimmunol.178.4.2028. [DOI] [PubMed] [Google Scholar]
- 59.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, Modlin RL. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 60.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melia´n A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 61.Stegelmann F, Bastian M, Swoboda K, Bhat R, Kiessler V, Krensky AM, Roellinghoff M, Modlin RL, Stenger S. Coordinate expression of CC chemokine ligand 5, granulysin, and perforin in CD8+ T cells provides a host defense mechanism against Mycobacterium tuberculosis. J. Immunol. 2005;175:7474–7483. doi: 10.4049/jimmunol.175.11.7474. [DOI] [PubMed] [Google Scholar]
- 62.Bruns H, Meinken C, Schauenberg P, Ha¨rter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cellmediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]