Abstract
Age constitutes a salient feature of a face and signals group membership. There is evidence of greater attention to and better memory for own-age than other-age faces. However, little is known about the neural and behavioral mechanisms underlying processing differences for own-age vs. other-age faces. Even less is known about the impact of emotion expressed in faces on such own-age effects. Using fMRI, the present study examined brain activity while young and older adult participants identified expressions of neutral, happy, and angry young and older faces. Across facial expressions, medial prefrontal cortex, insula, and (for older participants) amygdala showed greater activity to own-age than other-age faces. These own-age effects in ventral medial prefrontal cortex and insula held for neutral and happy faces, but not for angry faces. This novel and intriguing finding suggests that processing of negative facial emotions under some conditions overrides age-of-face effects.
Keywords: prefrontal cortex, amygdala, faces, emotion expression, in-group/out-group
1. Processing Own-Age vs. Other-Age Faces: Neuro-Behavioral Correlates and Effects of Emotion
People have an extraordinary interest in human faces, likely due to the high emotional and social relevance of faces in everyday lives. Facial features such as age, race, and sex are important cues about biological and/or social group membership that have an impact on cognition and behavior. For example, there is growing evidence that faces of one’s own age group receive greater attention (Ebner et al., 2011a; Ebner and Johnson, 2010; He et al., 2011a) and are better remembered (see Rhodes and Anastasi (2012) for a review) than faces of another age group. There are similar effects for faces of one’s own race (Malpass and Kravitz, 1969; see Meissner and Brigham (2001) for a review) and gender (Lewin and Herlitz, 2002; Wright and Sladden, 2003).
Investigators have suggested various factors that may underlie differences in processing in-group vs. out-group faces, such as greater familiarity of, more elaborated schemas for, greater personal interest in, and/or social motivation for own-group than other-group individuals (Anastasi and Rhodes, 2005; Ebner and Johnson, 2009; Harrison and Hole, 2009; He et al., 2011a; Slone et al., 2000; see Hugenberg et al. (2010); Rhodes and Anastasi (2012) for reviews). A systematic examination of such factors is warranted in order to increase our understanding of processing differences between members of the in-group vs. the out-group.
One step in this direction comes from recent neuroimaging studies that compare neural processing of in-group vs. out-group faces. Most of these studies have focused on group membership based on race or experimental assignment to “minimal” groups. Yet, comparatively little is known about the neural processing of age-based in-group vs. out-group. To this date, only two functional magnetic resonance imaging (fMRI) studies have addressed processing differences between own-age and other-age faces (Ebner et al., 2011a; Wright et al., 2008).
Some factors, such as increased familiarity, greater preference and/or affective salience, or more available prototypes for the in-group than the out-group may operate in similar ways for group memberships based on age, race, or gender (see Ebner et al., 2011b, for a discussion). At the same time, there are some fundamental differences between different types of group memberships, which likely result in processing differences. For example, for age-based groups, individuals naturally transition out of one group (i.e., young adults) and into the other group (i.e., older adults) over the course of their lifetime. Also, having been young once, older adults have personal experience and autobiographical memory of membership in the now (young adult) out-group. These substantial differences argue for the importance and novelty of examining processes involved in age-based group memberships in particular, the first goal of the present study. Below, we review findings from neuroimaging studies that have investigated in-group vs. out-group effects.
1.1. Brain Regions Involved in Processing of In-Group vs. Out-Group Faces
Amygdala, fusiform gyrus, prefrontal cortex, and insula show differences in activity levels when processing in-group vs. out-group faces. However, evidence is mixed regarding the specific functions of these brain regions, suggesting at least partly different mechanisms at work when processing in-group vs. out-group faces based on age, race, or minimal group.
1.1.1. Amygdala
Amygdala activation has been associated with positive evaluation of faces and greater attention to and importance of in-group compared to out-group members (Van Bavel et al., 2008; see also Cunningham et al., 2005). Some studies using race or minimal group based definitions show greater amygdala activity for in-group than out-group faces (He et al., 2011b; Van Bavel et al., 2008; Wheeler and Fiske, 2005). Wright et al. (2008), as the only study to date that has examined amygdala involvement in processing age-based in-group vs. out-group faces, further supports this interpretation by showing greater activity in right amygdala (MNI: x = 27, y = −6, z = −15) to own-age than other-age faces in a passive viewing paradigm.
However, several studies that define group membership based on race found greater amygdala activity to out-group than in-group faces (Phelps et al., 2000; Wheeler and Fiske, 2005), with these effects at least partly attributed to less exposure to racial out-group faces (Hart et al., 2000; Lieberman et al., 2005). It has been suggested that these effects may also be driven by stereotypes and affective evaluations (Cunningham et al., 2004; Phelps et al., 2000), particularly negative ones toward the out-group (Lieberman et al., 2005).
1.1.2. Fusiform Gyrus
Fusiform gyrus is an area selectively activated by faces compared to other stimuli (Kanwisher et al., 1997; Puce et al., 1995) and plays a crucial role in face individuation (Rhodes et al., 2004), which may be particularly elaborated for members of the in-group compared to the out-group (Hugenberg et al., 2010). In support of this interpretation, studies using faces from different races or minimal groups have found greater fusiform gyrus activity to in-group than out-group faces (Golby et al., 2001; Kim et al., 2006; Van Bavel et al., 2008; but see He et al., 2011b).
1.1.3. Prefrontal Cortex
Medial prefrontal cortex is involved in self-referential processing (see Amodio and Frith (2006); Murray et al. (2012); Van Overwalle (2009), for reviews). Self-referential processing is likely more prevalent for own-age than other-age faces, due to increased similarity and identification with the own-age group. In line with this interpretation, Ebner et al. (2011a) found greater activity in a region of ventral medial prefrontal cortex (MNI: x = −6, y = 30, z = −6) when participants evaluated personality traits in own-age than other-age individuals. In addition, greater perceived similarity to the own-age vs. the other-age person was related to greater BOLD response to the own-age vs. other-age person in this region of medial prefrontal cortex, suggesting that perceived similarity contributes to processing differences for faces of different ages.
Another prefrontal cortex area, orbitofrontal cortex, is involved in representing subjective affective value (Kringelbach, 2005; Van Bavel et al., 2008). Greater orbitofrontal cortex activity to own-age than other-age faces could thus reflect greater liking of the own-age group. In support of this interpretation, Van Bavel et al. (2008) found greater activity in a lateral region of orbitofrontal cortex to experimentally assigned in-group compared to out-group faces, with this activity positively related to liking of in-group members.
1.1.4. Insula
Insula activity is associated with processing emotional and social information (Craig, 2009), and thus may play a crucial role in in-group vs. other-group face processing. In line with this suggestion, Lieberman et al. (2005) found greater insula activation for other-race than own-race faces.
Taken together, the existing literature converges on a set of brain regions involved in differentiating in-groups from out-groups. However, the specific function of each of these regions in processing own-age relative to other-age faces (as compared to own-race vs. other-race faces or group membership based on experimental assignment) and the underlying mechanisms are largely unknown. Thus, the first aim of the present study was to investigate neural processing differences between young and older faces in these selected regions of interest (ROIs) in samples of young and older adults.
1.2. Influence of Facial Expression on Processing Own-Age vs. Other-Age Faces
Our second aim was to investigate whether own-age effects varied as a function of emotional facial expressions. Previous studies of neural processing differences for own-age vs. other-age faces (Ebner et al., 2011a; Wright et al., 2008) used only neutral faces, and thus did not vary the emotional expression of the presented faces. However, both young and older adults find it easier to read positive than negative expressions (Ruffman et al., 2008; Svärd et al., 2012). Also, positive vs. negative facial expressions seem to be, at least to some extent, processed in different brain regions (Keightley et al., 2007; see also Ruffman et al., 2008), with some indication that this may be related to processing difficulty between these two types of facial expressions (Ebner et al., 2012). Thus, variations of age-of-face effects as a function of positive vs. negative emotional expression could arise from such differences in processing difficulty for both young and older adults. If the cognitive processes required for expression identification in angry compared to happy, and possibly also neutral, faces are more effortful, less attention may be directed at processing age information in angry than happy and neutral faces. This would then result in less pronounced own-age effects in angry than happy and neutral faces, in young and older adults alike.
1.3. The Present Study
In sum, the present study conducted the following analyses in the interest of two primary goals: (1) Age of Face by Participant Age Across Facial Expression: This analysis identified processing differences between young and older faces in selected brain areas (medial prefrontal cortex, orbitofrontal cortex, insula, fusiform gyrus, amygdala). We expected activity in these ROIs to be greater for own-age than other-age faces for both young and older adults (Hypothesis 1). (2) Age of Face By Participant Age As a Function of Facial Expression: We expected variations of age-of-face effects as a function of facial expression. In particular, we expected that in brain regions sensitive to differentiating between own-age and other-age faces, the own-age effect would be more pronounced for happy and neutral than angry faces in both young and older adults (Hypothesis 2). This prediction was based on the assumption that angry faces are more difficult to identify and thus less attention may be available for processing age-of-face information for angry faces.
2. Method
2.1. Participants
Participants were healthy young adults (n = 30 [16 females], M = 25.1 years [SD = 3.4; range = 20–31]) and healthy, active, independently living older adults (n = 32 [18 females], M = 68.2 years [SD = 2.5; range = 65–74]). All participants were in good health, with no known history of stroke, heart disease, or primary degenerative neurological disorder, had normal or corrected-to-normal vision, and none were known to take psychotropic medications. Grey and white matter lesions and/or abnormal extents of atrophy in older participants were ruled out by screening of both a T1-weighted and T2-weighted structural image through a radiologist. For a detailed description of the sample in terms of education and cognitive and affective measures see Ebner et al. (2012).
2.2. Stimuli
Face images were taken from the FACES database (Ebner et al., 2010). Forty-eight pictures of young (18–31 years) and 48 pictures of older (69–80 years) faces (all different face identities) were presented, with equal numbers of neutral, happy, or angry expressions displayed in young and older faces (i.e., 16 per Age of Face by Facial Expression). Stimulus presentation and response collection (accuracy and response time) were controlled using E-Prime (Schneider et al., 2002).
2.3. Procedure, Measures, and Design
The data reported here were from a larger project. Detailed study procedures are described in Ebner et al. (2012). The ethics committee at the Karolinska Institute approved the protocol, and informed consent was obtained from all participants.
During the fMRI session, participants worked on a Facial Expression Identification Task (Figure 1). This task had a mixed 2 Participant Age (Young, Older) X 2 Age of Face (Young, Older) X 3 Facial Expression (Neutral, Happy, Angry) factorial design, with Participant Age as a between-subjects factor and Age of Face and Facial Expression as within-subjects factors. As shown in Figure 1, participants saw faces, one at a time. Each face was presented for 3500 ms. Participants were asked to indicate whether the displayed face showed a happy, neutral, or angry expression by pressing one of three response buttons on a button box (index finger for “happy”, middle finger for “neutral”, and ring finger for “angry”). Response options appeared in black on a grey background below the faces and were always presented in the same order. In between faces, a black fixation cross appeared on a grey background on the screen. The interstimulus interval (ISI) pseudo-randomly varied between 3000–4000 ms in 250-ms increments (mean ISI = 3500 ms). In one-third of the trials (48 out of 144 total trials), “low-level baseline events” consisting of three black Xs on a grey background were presented. Participants pressed one of the three buttons that they also used for indicating the facial expressions to indicate appearance of a low-level baseline trial. Low-level baseline trials were not further considered in the present analyses.
Figure 1. Facial Expression Identification Task.

Trial event timing and sample faces used in the task.
The presentation order of face identities was identical for each participant, with facial expressions counterbalanced across participants (each participant only saw each face with one expression). Lists were pseudo-randomized with the constraints that no more than three faces and no more than two null events were presented in a row, and no more than two faces of the same category (i.e., age, gender, facial expression) were repeated in a row. The task started with four practice trials. It was split into two runs, each lasting for 8.4 minutes.
At the end of the session, participants were debriefed and financially compensated for participation.
2.4. Imaging Details
Images were acquired using a 3T Siemens Magnetom Tim Trio scanner at Huddinge Hospital, Stockholm, Sweden. After localizer scans, two runs of 160 functional images each were acquired with a T2*-weighted echo-planar sequence (ep2d_bold; TR=2500 ms, TE=40 ms, flip angle=90 degrees, FOV=230mm, voxel size = 3 × 3 × 3 mm). Thirty-nine oblique axial slices were positioned parallel to the AC-PC line, and acquired interleaved. A 1 × 1 × 1 mm T1-weighted image was used for co-registration with functional images (MP-RAGE; TR = 1900 ms, TE = 2.52 ms, FoV = 256 mm).
2.5. fMRI Analyses
Data from this event-related fMRI study were analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Imaging Neuroscience). Pre-processing included slice timing correction, motion correction, co-registration of functional images to the participant’s anatomical scan, spatial normalization, and smoothing (9-mm full-width half maximum [FWHM] Gaussian kernel). Spatial normalization used a study-specific template brain composed of the average of the young and older participants’ T1 structural images (the detailed procedure for creating the template is available from the authors). After normalization to standard space, functional images had dimensions of 53×63×46 with 3mm isotropic voxels.
Standard general linear model (GLM) analyses were conducted on ROIs defined by the WFU PickAtlas v2.4 (Maldjian et al., 2004; Maldjian et al., 2003; http://www.nitrc.org/projects/wfu_pickatlas/; based on the Talairach Daemon). First-level, single-subject statistics were modeled by convolving each trial with the SPM canonical hemodynamic response function to create a regressor for each condition (Young Neutral, Young Happy, Young Angry, Older Neutral, Older Happy, Older Angry). Parameter estimates (beta images) of activity for each condition and each participant were then entered into a second-level random-effects analysis using a mixed 2 Participant Age (Young, Older) X 2 Age of Face (Young, Older) X 3 Facial Expression (Neutral, Happy, Angry) analysis of variance (ANOVA), with Participant Age as a between-subjects factor and Age of Face and Facial Expression as within-subjects factors. From within this model, an F-contrast was specified to examine the effects of Age of Face (Young, Older) X Participant Age (Young, Older), collapsed across all facial expressions. We refer to this F-contrast as Age of Face X Participant Age throughout the text.
For this F-contrast, two sets of ROI analyses were conducted: (1) We applied a mask to all cortical ROIs, comprising bilateral medial frontal gyrus, anterior cingulate gyrus, Brodmann areas 12 and 47 (orbitofrontal cortex; Chiavaras et al., 2001), insula, and fusiform gyrus as specified in the WFU PickAtlas. For this set of analyses we used a statistical threshold of p < .005 (uncorrected) and a cluster size of 22 contiguous voxels, which was determined by the AlphaSim tool from AFNI (Analysis for Functional NeuroImaging; Cox, 1996) to yield a cluster-level corrected threshold of p < .05 for the search region in our ROI mask. (2) We conducted a separate analysis on amygdala, as a subcortical, small, and well-circumscribed ROI, using the small volume correction implemented in SPM5. For this analysis we used a bilateral amygdala mask as specified in the WFU PickAtlas.
Within each region of activation identified by the F-contrast, beta values were extracted (in a 6-mm sphere around the peak voxel) to produce a single value for each condition of interest, for each participant. These values are depicted in the bar graphs of Figure 2. In the fashion of F- and t-tests in ANOVA, subsequent planned statistical comparisons of these values (p < .05) were conducted using IBM SPSS Statistics Version 20 to aid interpretation of the activations.
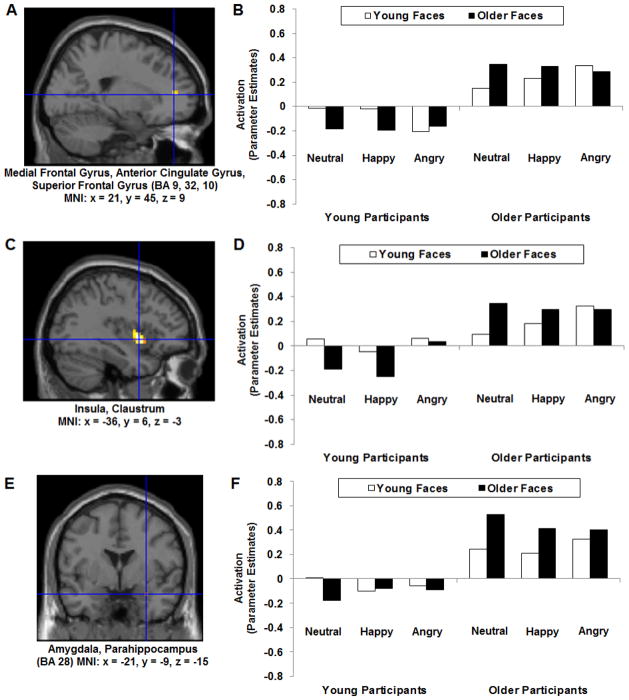
Figure 2. Age of Face X Participant Age.
(A) MNI: x = 21, y = 45, z = 9: Right medial prefrontal cortex, right anterior cingulate, right superior frontal gyrus (BA 9, 32, 10); (C) MNI: x = −36, y = 6, z = −3: Left insula, left claustrum, left putamen; and (E) MNI: x = −21, y = −9, z = −15: Left amygdala, left parahippocampus (BA 28); all showing own-age faces > other-age faces.
The region of activation represents the F-map of the contrast; it is displayed on the standard reference brain in SPM. The crosshair indicates the peak voxel (local maximum) within the region of activation. Bar graphs show the mean (B) right medial prefrontal cortex and (D) left insula, both showing own-age faces > other-age faces for young and older participants for neutral and happy but not angry faces; and (F) left amygdala, showing own-age faces > other-age faces for older but not young participants. Parameter estimates (beta values) separately for Age of Face, Participant Age, and Facial Expression; betas for this region of activation identified by the F-contrast Age of Face X Participant Age extracted for each individual from a 6-mm sphere around the local maximum within the region of activation and averaged to produce a single value for each condition of interest respectively.
Montreal Neurological Institute (MNI) coordinates are reported. Anatomical localization were verified using the Talairach Daemon (Lancaster et al., 1997; Lancaster et al., 2000) on coordinates transformed using icbm2tal (http://www.brainmap.org/icbm2tal/), and labels were confirmed visually using the Talairach and Tournoux (1988) atlas.
3. Results
3.1. Behavioral Data: Facial Expression Identification: Accuracy and Response Time
Due to technical problems with the response pad, behavioral data were lost for one older woman and one older man. For the remaining n = 30 young and n = 30 older participants, accuracy and response time (for accurate responses) data from the expression identification task, collapsed across facial expressions, were submitted to mixed 2 Participant Age (Young, Older) X 2 Age of Face (Young, Older) repeated-measures ANOVAs (see Table 1). For accuracy, the only significant effect was the main effect for Age of Face (F(1,58) = 23.10, p < .001, ηp2 = .29). Follow-up paired-sample t-tests showed that both young (t(29) = 4.31, p < .001) and older (t(29) = −2.90, p = .007) participants were more accurate in identifying expressions in young than older faces. There were comparable results for response time: The main effect for Age of Face (F(1,58) = 103.66, p < .001, ηp2 = .64) and the main effect for Participant Age (F(1,58) = 5.12, p = .027, ηp2 = .08) were significant, but there was no significant Participant Age X Age of Face interaction. As shown in Table 1, young participants responded faster than older participants and follow-up paired sample t-tests showed that both young (t(29) = −7.20, p < .001) and older (t(29) = 7.20, p < .001) participants were faster in identifying expressions in young than older faces (these accuracy and response time data were reported previously, see Ebner et al., 2012).
Table 1.
Means (M) and standard deviations (SD) for accuracy (%) and response time (ms) in facial expression identification for young and older participants
| Accuracy M(SD) | Response Time M(SD) | |||
|---|---|---|---|---|
|
| ||||
| Young Participants | Older Participants | Young Participants | Older Participants | |
| Across Facial Expressions | ||||
| Young Faces | 94.2 (9.6) | 96.4 (5.9) | 1236 (194) | 1360 (215) |
| Older Faces | 91.1 (9.8) | 93.3 (5.1) | 1359 (248) | 1485 (211) |
| Neutral Expressions | ||||
| Young Faces | 92.1 (11.9) | 96.0 (6.0) | 1292 (241) | 1318 (228) |
| Older Faces | 90.2 (10.7) | 91.3 (9.9) | 1469 (295) | 1567 (267) |
| Happy Expressions | ||||
| Young Faces | 95.8 (9.5) | 98.5 (3.6) | 1077 (145) | 1234 (241) |
| Older Faces | 94.6 (10.6) | 96.7 (5.9) | 1159 (216) | 1250 (212) |
| Angry Expressions | ||||
| Young Faces | 94.8 (9.6) | 94.6 (10.2) | 1340 (251) | 1527 (288) |
| Older Faces | 88.5 (12.9) | 91.9 (9.9) | 1451 (295) | 1638 (293) |
3.2. fMRI Data
3.2.1. Hypothesis 1: Age of Face by Participant Age Across Facial Expression
We expected greater activity to own-age than other-age faces for both young and older adults in medial prefrontal cortex, orbitofrontal cortex, insula, fusiform gyrus, and amygdala across facial expressions (Hypothesis 1). Such a result would be supported by a significant Age of Face X Participant Age interaction.
There was significant activity for Age of Face X Participant Age (F-contrast, across Facial Expression) in clusters of right ventral and dorsal regions of medial prefrontal cortex (see Table 2). In particular, Figure 2A shows that brain activity in an area of right ventral medial prefrontal cortex, right anterior cingulate, right superior frontal gyrus (BA 9, 32, 10; MNI: x = 21, y = 45, z = 9) was greater for own-age than other-age faces. To explore the significant interaction further, we extracted beta values at the peak voxel of activation of this region (F(1,60) = 13.47, p = .001, ηp2 = .18; see Figure 2B). Separate paired-sample t-tests in young and older participants on the extracted beta values showed that ventral medial prefrontal cortex activity for young (p = .001) and older (p = .05) participants was also significantly greater to own-age than other-age faces when considering each group individually, with this own-age effect more pronounced in young than older participants.
Table 2.
ROI Analysis: Areas showing an Age of Face X Participant Age interaction (F-contrast; all in the direction Own-Age Faces > Other-Age Faces)
| Activation Peak | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Hemi | BA | Anatomical Area | x | y | z | F-value | # vox |
| (1) ROI (mPFC, OFC, INS, FFG): p < .005, 22 contiguous voxels | |||||||
| R | 8 | Superior Frontal Gyrus, Medial Frontal Gyrus | 9 | 39 | 42 | 12.86 | 43 |
| R | 9, 32, 10 | Medial Frontal Gyrus, Anterior Cingulate Gyrus, Superior Frontal Gyrus | 21 | 45 | 9 | 15.45 | 44 |
| L | Insula, Claustrum | −36 | 6 | −3 | 17.27 | 33 | |
| (2) ROI (AMY): small volume correction | |||||||
| L | 28 | Amygdala, Parahippocampal Gyrus | −21 | −9 | −15 | 8.04 | 6 |
Notes. Areas sorted from dorsal to ventral. Bolded areas discussed in text and presented in Figures 3 to 5. MNI coordinates (x, y, z) and maximum F-value are given for the peak voxel (local maximum) within each region of activation. Hemi: hemisphere; L: left, R: right; BA: Brodmann Area; # vox: number of voxels in cluster. Full activation maps for all areas shown in the table are available from the authors; mPFC = medial prefrontal cortex, OFC = orbitofrontal cortex, INS = insula, FFG = fusiform gyrus, AMY = amygdala.
A region of superior frontal gyrus, medial prefrontal cortex (BA 8; MNI: x = 9, y = 39, z = 42) showed similar results (F(1,60) = 9.25, p = .003, ηp2 = .13). Follow-up t-tests suggested that the main effect of greater dorsal medial prefrontal cortex activity to own-age than other-age faces in this region was driven by older (p = .001) but not young (p = .626) participants (see Ebner et al., 2012).
Also in line with our expectations, brain activity in left insula, left claustrum (MNI: x = −36, y = 6, z = −3) was greater for own-age than other-age faces (see Figure 2C). To explore the significant interaction further, we extracted beta values at the peak voxel of activation of this region (F(1,60) = 16.20, p < .001, ηp2 = .21; see Figure 2D). Paired-sample t-tests on the extracted beta values showed that insula activity for both young (p = .003) and older (p = .024) participants was greater to own-age than other-age faces.
Contrary to expectations, there was no significant difference in BOLD response to own-age than other-age faces in any area of orbitofrontal cortex or fusiform gyrus at our cluster-level corrected threshold. Post-hoc, we conducted an exploratory whole-brain analysis using a threshold of p < .001, uncorrected, with no minimum cluster threshold, on the F-contrast Age of Face X Participant Age (across Facial Expression). As shown in Table 3 (Supplementary Material 1), among those regions identified by this contrast were small clusters of orbitofrontal cortex and fusiform gyrus. Thus, although these areas do not meet strict criteria for significance under a multiple-comparisons-corrected threshold, they may still warrant further investigation in future studies with greater statistical power.
Using small volume correction, we observed significant clusters in bilateral amygdala (see Table 2). Figure 2E depicts brain activity in left amygdala, left parahippocampus (BA 28; MNI: x = −21, y = −9, z = −15) for the F-contrast Age of Face X Participant Age (across Facial Expression). To explore the significant interaction further, we extracted beta values at the peak voxel of activation of this region (F(1,60) = 5.23, p = .026, ηp2 = .08; see Figure 2F). Paired-sample t-tests on the extracted beta values showed that amygdala activity for older (p = .047), but not young (p = .290), participants was significantly greater to own-age than other-age faces.
3.2.2. Hypothesis 2: Age of Face by Participant Age As a Function of Facial Expression
In line with our hypotheses, we expected the own-age vs. other-age effect that was identified under Hypothesis 1 to be magnified for happy and neutral compared with angry faces (Hypothesis 2). First, the Participant Age (Young, Older) X Age of Face (Young, Older) X Facial Expression (Neutral, Happy, Angry) interaction was examined in F-tests on extracted beta values at the peak voxel of activation for all four areas separately. The 3-way interaction was significant for ventral medial prefrontal cortex (MNI: x = 21, y = 45, z = 9; F(2,59) = 4.08, p = .022, ηp2 = .12) and insula (MNI: x = −36, y = 6, z = −3; F(2,59) = 3.60, p = .034, ηp2 = .11) but not dorsal medial prefrontal cortex (MNI: x = 9, y = 39, z = 42; F(2,59) = 2.28, p = .111, ηp2 = .07) or amygdala (MNI: x = −21, y = −9, z = −15; F(2,59) = .96, p = .388, ηp2 = .03).
Follow-up Age of Face X Participant Age F-tests separately for each of the three facial expressions showed that in ventral medial prefrontal cortex, the Age of Face X Participant Age interaction was significant for neutral (F(1,60) = 10.66, p = .002, ηp2 = .15) and happy (F(1,60) = 9.05, p = .004, ηp2 = .13) but not angry (F(1,60) = 1.18, p = .281, ηp2 = .02; see Figure 2B) faces. Similarly, in insula, the Age of Face X Participant Age interaction was significant for neutral (F(1,60) = 11.05, p = .002, ηp2 = .16) and happy (F(1,60) = 7.85, p = .007, ηp2 = .12) but not angry (F(1,60) = .00, p = .998, ηp2 = .00; see Figure 2D) faces.
4. Discussion
The present study examined neural and behavioral correlates of differences in processing of young and older neutral, happy, and angry faces during facial expression identification in a relatively large sample of young (n = 30) and older (n = 32) adults. As discussed below, there were two primary and novel contributions of this study: Evidence of (1) own-age effects in medial prefrontal cortex and insula in both young and older adults, and amygdala in older adults; (2) variations of the own-age effect as a function of the facial expression in ventral medial prefrontal cortex and insula.
4.1. Greater Activity in Medial Prefrontal Cortex, Insula, and Amygdala to Own-Age than Other-Age Faces
We provide evidence for a role of medial prefrontal cortex, insula, and amygdala in processing own-age vs. other-age faces: Activity was greater for own-age than other-age faces in medial prefrontal cortex and insula for both young and older participants, and there was greater amygdala activity for own-age than other-age faces for older (but not young) participants.
In line with evidence from Ebner et al. (2011a), our finding of greater activity in medial prefrontal cortex for own-age than other-age faces may reflect greater similarity and identification with and/or greater preference and personal interest in own-age than other-age individuals. This interpretation is further supported by evidence that medial prefrontal cortex is associated with self-referential processing (Gutchess et al., 2007; Mitchell et al., 2009; see Murray et al. (2012); Van Overwalle (2009), for reviews), impression formation (Schiller et al., 2009), third-person perspective taking (Ames et al., 2008; D’Argembeau et al., 2007; Ruby and Decety, 2004), and evaluative processing (Bush et al., 2000; Cunningham et al., 2004; see also Amodio and Frith, 2006).
Similarly, insula has been shown to play a role in self-awareness (Allman et al., 2011; Modinos et al., 2009), and, in particular, left insula has been shown to be involved in positive and affiliative feelings (Craig, 2009), which may be the kind of feelings one typically experiences more for the own-age than the other-age group. It should be noted that, in contrast to the present study, Wright et al. (2008) did not find significant differences in insula activity to own-age compared to other-age faces in their exploratory whole-brain analysis. Also, Lieberman et al. (2005) observed a reversed effect, with greater insula activity for the other-race than the own-race group. A recent finding by Castle et al. (2012) furthermore showed greater insula activity to untrustworthy-looking compared to trustworthy-looking faces. These findings seem to suggest that increased insula activation may be associated with heightened negative visceral feelings, perhaps in the service of an “early warning system” (cf. Castle et al., 2012). However, in both Lieberman et al. (MNI: y = 14 and 21, respectively for left and right insula) and Castle et al. (MNI: y > 15), an anterior insula was activated, whereas in the present study more posterior insula was involved (MNI: y = 6). This pattern of findings across studies may suggest an interesting functional dissociation in insula (cf. Chang et al., 2013). In particular, anterior insula may be associated with heightened negative feelings and gut reactions (Castle et al.; Lieberman et al.). In contrast, posterior insula may be recruited during increased self-referential processing such as involved when processing own-age compared to other-age faces, when taking a first-person relative to a third-person perspective (David et al., 2006; Ruby and Decety, 2001), or during self-experienced pain relative to perceived pain in others (Singer et al., 2004; see also Lamm et al., 2007).
The greater amygdala activity we saw for own-age than other-age faces was driven by older participants and was not significant in young participants (see also Wright et al., 2008). It is possible that age represents a more salient and relevant feature for older than young adults. This may be due to age-associated declines in various domains of functioning (e.g., cognition, health); thus, older compared to young adults might be reminded of their age more frequently throughout their daily routines. This in-group effect in amygdala is consistent with several studies of face processing based on race and minimal groups (He et al., 2011b; Van Bavel et al., 2008). It may suggest an association of amygdala with more affective evaluation, and increased importance, of members of the own than the other age group. At the same time, our finding contrasts with results of some studies that used race as the definition for group membership and found greater amygdala activity for other-race than own-race faces (Hart et al., 2000; Phelps et al., 2000). Thus, it could well be that amygdala response to the in-group vs. the out-group varies as a function of personal motivation or situational/task-related demands (see Cunningham et al., 2005).
As detailed in the Supplementary Material 2, secondary analyses provided preliminary evidence of a positive correlation between self-reported frequency of contact, as a proxy for familiarity, and own-age relative to other-age individuals with amygdala activity to own-age relative to other-age faces in young but not older adults. Future studies will have to determine whether frequency of contact indeed constitutes one of the potential underlying factors for differences in processing in-group vs. out-group faces in amygdala (cf. Wright et al., 2008).
Evidence for reversed patterns in insula and amygdala response to age-based and race-based faces suggest that, at least in part, different processes underlie these types of group memberships (Ebner et al., 2011b). As suggested above, age-based in-group vs. out-group processing is unique compared to other types of group processing because individuals naturally transition from one age group to another. That is, young adults anticipate maturing into older adults, and older adults retain memories of being a young adult. This makes age-based in-groups vs. out-groups less exclusive categories and may imply relatively higher self-relevance of both the age-based, compared to race-based, in-group and out-group. However, in addition to age- or race-specific processes, there may be general mechanisms deployed for differentiating between salient stimulus categories (e.g., in-group vs. out-group) which may operate in similar ways for different types of group memberships and may rely on factors such as familiarity, greater preference and/or affective salience, or more available prototypes for the in-group than the out-group. For example, Ebner et al. (2011b) showed striking similarity between the electrophysiological correlates of processing young and older faces and those that differentiated between Black and White faces in the context of a gender categorization task. It will be particularly important to determine in future research the specific lower-level perceptual mechanisms (e.g., compositional detail) and/or higher-level cognitive mechanisms (e.g., differential representations based on prototypicality, familiarity, or expertise, and/or differences related to attention, arousal, or social motivation) involved in different types of in-group vs. out-group processing.
There were no significant effects for orbitofrontal cortex or fusiform gyrus at our cluster-level corrected threshold. This is in line with findings by Wright et al. (2008; see also He et al., 2011b) but differs from our expectation and other studies on in-group vs. out-group processing based on race and/or minimal groups (Golby et al., 2001; Kim et al., 2006; Van Bavel et al., 2008).
Finally, even though we found neural processing differences for own-age vs. other-age faces in several ROIs, there was no evidence of an own-age effect in facial expression identification. Rather, in line with previous work (Ebner et al., 2011c; Riediger et al., 2011), expressions in young faces were more correctly and quickly identified than expressions in older faces, for both young and older adults. Low-level perceptual differences between young and older faces (see Ebner et al., 2011b, for a discussion) may play a role here as well as increased affect involved in processing young faces and more cognitive control in processing the more complex older faces (see Ebner et al., 2012, for a discussion). In contrast, own-age effects are typically seen in face recognition (Rhodes and Anastasi, 2012). Also, recent eye-tracking studies suggest increased attention to own-age than other-age faces (Ebner et al., 2011c; He et al., 2011a). It will be interesting in future research to follow up on the differential impact of the age of the face on attention and memory processes related to face identity, facial emotion, facial age, and facial attractiveness, respectively.
4.2. Own-Age Effects Pronounced in Neutral and Happy but not Angry Faces
Importantly, this project is the first to systematically vary the emotional expression of the presented young and older faces, with the goal to examine the impact of facial expression on the own-age effect and thus clearly goes beyond earlier research (Ebner et al., 2011a; Wright et al., 2008). As expected, there was greater activity in ventral medial prefrontal cortex and insula for own-age than other-age faces for neutral and happy but not angry faces in young and older adults. This finding is novel and intriguing in that it suggests that processing of negative facial emotions may override age-of-face effects under certain conditions.
One explanation for this effect could be that facial expression identification in the present study was better for happy than angry faces (see Table 1; see also Ebner et al., 2012; Ruffman et al., 2008). The greater difficulty of identifying angry faces may have resulted in less attention to processing age-of-face information, thus decreasing the own-age effect for angry faces. At the same time, facial emotion of anger constitutes a salient and immediate situational cue of danger and threat that may receive preferential attention (over age-of-face information), as it is crucial for well-being and survival (LeDoux, 1998; Öhman and Mineka, 2001; cf. Ebner and Johnson, 2010). Age cues in faces, even though also an important social signal, in contrast, may just have less likely immediate practical consequences and thus may be processed with lower priority. This interpretation is supported by evidence of a “threat advantage” (Hansen and Hansen, 1988; Öhman et al., 2001; see Palermo and Rhodes (2007) for a review), seen in both young and older adults (Hahn et al., 2006; Mather and Knight, 2006). Also, a functional intergroup argument could be made in that happy expressions of in-group faces as well as angry expressions of out-group faces may form a congruent category, whereas happy expressions of out-group faces or angry expressions of in-group faces may form an incongruent category, with the latter inducing negative or at least ambivalent emotions (see also Weisbuch and Ambady, 2008).
Interestingly, the present study’s evidence of an “overriding effect” of emotion over age of face differs from results by Ebner and Johnson (2010) that suggested a “potentiating effect” of emotion and age of face. In particular, in young but not older adults, task-unrelated angry own-age faces were more distracting than angry other-age faces, with no comparable effect for happy faces. For older adults, task-unrelated happy compared to angry faces were more distracting, and this effect did not differ for own-age compared to other-age faces. This finding is in line with evidence that older compared to young adults prioritize processing of positive over negative emotions (Carstensen and Mikels, 2005; Mather and Carstensen, 2005), an effect that is further supported by evidence of a shift from subcortical to cortical processing of negative information in older compared to young adults (Fischer et al., 2010; St. Jacques et al., 2009; Samanez-Larkin and Carstensen, 2011).
Ebner and Johnson’s (2010) participants processed number trials while task-unrelated faces were shown in the background and behavioral interference effects (slowing of response time) for different types of faces (young vs. older, and neutral vs. happy vs. angry) were measured. In contrast, participants in the present study were asked to explicitly process the faces, with a particular focus on the emotion expressed in the faces, while their brain activity and their ability to identify the facial expressions was assessed. The difference in findings across these two studies is interesting and seems to suggest that under some conditions emotion-of-face effects are potentiated by age-of-face effects, whereas under other conditions emotion-of-face effects are primary and override age-of-face effects. Examination of these kinds of interactions of the age of the face and the emotion of the face under varying conditions in future studies would help identify conditions under which either the emotion or the age of a face “trumps” the other.
4.3. Conclusion
The present study extends the literature on processing in-group vs. out-group faces in several ways: By targeting a wider range of regions of interest based on the literature, it provides evidence of insula in addition to the previously shown medial prefrontal cortex (Ebner et al., 2011a) and amygdala (Wright et al., 2008) involvement in differentiating between age-based in-group vs. out-group faces in young and older adults. It furthermore supports evidence of neural processing differences for own-age faces compared to other-age faces in the context of an emotion identification task that drew attention away from the age-of-face information as compared to a passive viewing task (Wright et al.) or a personality rating task (Ebner et al.). Most importantly, however, the present study systematically varied the emotional expression of the presented young and older faces, to determine the impact of facial expression on the own-age effect. Thus, it clearly goes beyond earlier research (e.g., Ebner et al.; Wright et al.) and provides novel evidence that under certain conditions emotional facial expression, particularly anger, can override the own-age effect in specific brain areas sensitive to own-age effects.
The present study does not allow for a direct comparison of effects of race-based vs. age-based group membership. However, it further qualifies the literature on in-group vs. out-group processing and underscores the importance of considering varying definitions of group membership. In addition, methodological and design-related differences among the present study and previous research suggest that orienting tasks (e.g., passive viewing, emotion identification, implicit vs. explicit processing), details of face presentation (i.e., single vs. repeated), and features of face stimuli (neutral vs. emotional) are important sources of variance that need to be sorted out in future research in order to clarify the mechanisms driving differences in processing members of the in-group vs. the out-group.
Supplementary Material
Acknowledgments
This research was conducted at the Karolinska Institute MR-Center, Huddinge Hospital, Stockholm, Sweden, and supported by the Swedish Research Council (2008–2356) and Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse awarded to HF, National Institute on Aging grant R37AG009253 to MKJ, and German Research Foundation Research Grant DFG EB 436/1-1 to NCE. The authors wish to thank Sebastian Gluth for assistance in programming the tasks and Joakim Svärd and Lisa Lidberg for assistance in data collection and data entry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Natalie C. Ebner, Email: natalie.ebner@ufl.edu.
Matthew R. Johnson, Email: Matthew-r.Johnson@nottingham.edu.my.
Anna Rieckmann, Email: rieckmann@nmr.mgh.harvard.edu.
Kelly A. Durbin, Email: kelly.durbin@yale.edu.
Marcia K. Johnson, Email: marcia.johnson@yale.edu.
Håkan Fischer, Email: hakan.fischer@psychology.su.se.
References
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann N Y Acad Sci. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP. Taking another person’s perspective increases self-referential neural processing. Psychol Sci. 2008;19:642–644. doi: 10.1111/j.1467-9280.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anastasi JS, Rhodes MG. An own-age bias in face recognition for children and older adults. Psychon Bull Rev. 2005;12:1043–1047. doi: 10.3758/bf03206441. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Curr Dir Psychol Sci. 2005;14:117–121. [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, Taylor SE. Neural and behavioral bases of age differences in perceptions of trust. Proc Natl Acad Sci U S A. 2012;109:20848–20852. doi: 10.1073/pnas.1218518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavaras MM, LeGoualher G, Evans A, Petrides M. Three-dimensional probabilistic atlas of the human orbitofrontal sulci in standardized stereotaxic space. NeuroImage. 2001;13:479–496. doi: 10.1006/nimg.2000.0641. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of Black and White faces. Psychol Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn Affect Behav Neurosci. 2005;5:202–211. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- David N, Bewernick BH, Cohen MX, Newen A, Lux S, Fink GR, Shah NJ, Vogeley K. Neural representations of self versus other: Visual-spatial perspective taking and agency in a virtual ball-tossing game. J Cogn Neurosci. 2006;18:898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Gluth S, Johnson MR, Raye CL, Mitchell KJ, Johnson MK. Medial prefrontal cortex activity when thinking about others depends on their age. Neurocase. 2011a;17:260–269. doi: 10.1080/13554794.2010.536953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, He Y, Fichtenholtz HM, McCarthy G, Johnson MK. Electrophysiological correlates of processing faces of younger and older individuals. Soc Cogn Affect Neurosci. 2011b;6:526–535. doi: 10.1093/scan/nsq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, He Y, Johnson MK. Age and emotion affect how we look at a face: Visual scan patterns differ for own-age versus other-age emotional faces. Cogn Emot. 2011c;25:983–997. doi: 10.1080/02699931.2010.540817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK. Young and older emotional faces: Are there age group differences in expression identification and memory? Emotion. 2009;9:329–339. doi: 10.1037/a0015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK. Age-group differences in interference from young and older emotional faces. Cogn Emot. 2010;24:1095–1116. doi: 10.1080/02699930903128395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, Fischer H. Neural mechanisms of reading facial emotions in young and older adults. Front Psychol. 2012;3:1–19. doi: 10.3389/fpsyg.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. FACES- A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav Res Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Fischer H, Nyberg L, Bäckman L. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex. 2010;46:490–497. doi: 10.1016/j.cortex.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nat Neurosci. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychol (Amst) 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Hansen RD. Finding the face in the crowd: An anger superiority effect. J Pers Soc Psychol. 1988;54:917–924. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Harrison V, Hole GJ. Evidence for a contact-based explanation of the own-age bias in face recognition. Psychon Bull Rev. 2009;16:264–269. doi: 10.3758/PBR.16.2.264. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner NC, Johnson MK. What predicts the own-age bias in face recognition memory? Soc Cogn. 2011a;29:97–109. doi: 10.1521/soco.2011.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Johnson MK, McCarthy G. Fast neural detection of race and group membership: Evidence from ERP and fMRI studies. 2011b Manuscript under review. [Google Scholar]
- Hugenberg K, Young SG, Bernstein MJ, Sacco DF. The categorization-individuation model: An integrative account of the other-race recognition deficit. Psychol Rev. 2010;117:1168–1187. doi: 10.1037/a0020463. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL. Age-related differences in brain activity underlying identification of emotional expressions in faces. Soc Cogn Affect Neurosci. 2007;2:292–302. doi: 10.1093/scan/nsm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Yoon HW, Kim BS, Jeun SS, Jung SL, Choe BY. Racial distinction of the unknown facial identity recognition mechanism by event-related fMRI. Neurosci Lett. 2006;397:279–284. doi: 10.1016/j.neulet.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuefner D, Cassia VM, Picozzi M, Bricolo E. Do all kids look alike? Evidence for an other-age effect in adults. J Exp Psychol Hum Percept Perform. 2008;34:811–817. doi: 10.1037/0096-1523.34.4.811. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lewin C, Herlitz A. Sex differences in face recognition-Women’s faces make the difference. Brain Cogn. 2002;50:121–128. doi: 10.1016/s0278-2626(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malpass RS, Kravitz J. Recognition for faces of own and other race. J Pers Soc Psychol. 1969;13:330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends Cogn Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61:P54–P57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Brigham JC. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychol Public Policy Law. 2001;7:3–35. [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychol Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS One. 2009;4:e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. J Pers Soc Psychol. 2001;80:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O‘Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji MR. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Byatt G, Michie PT, Puce A. Is the fusiform area specialized for faces, individuation, or expert individuation? J Cogn Neurosci. 2004;16:189–203. doi: 10.1162/089892904322984508. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, Anastasi JS. The own-age bias in face recognition: A meta-analytic and theoretical review. Psychol Bull. 2012;138:146–174. doi: 10.1037/a0025750. [DOI] [PubMed] [Google Scholar]
- Riediger M, Voelkle MC, Ebner NC, Lindenberger U. Beyond “happy, angry, or sad?: Age-of-poser and age-of-rater effects on multi-dimensional emotion perception. Cogn Emot. 2011;25:968–982. doi: 10.1080/02699931.2010.540812. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J Cogn Neurosci. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neurosci Biobehav Rev. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Carstensen LL. Socioemotional functioning and the aging brain. In: Decety J, Cacioppo JT, editors. The Oxford Handbook of Social Neuroscience. Oxford University Press; New York: 2011. pp. 507–521. [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime Reference Guide. Pittsburgh, PA: Psychology Software Tools Inc; 2002. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slone AE, Brigham JC, Meissner CA. Social and cognitive factors affecting the own-race bias in Whites. Basic Appl Soc Psych. 2000;22:71–84. [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: Fronto-amygdalar differences during emotional perception and episodic memory. J Int Neuropsychol Soc. 2009;15:819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svärd J, Wiens S, Fischer H. Superior recognition performance for happy masked and unmasked faces in both younger and older adults. Front Psychol. 2012;3:520. doi: 10.3389/fpsyg.2012.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3- dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: A functional magnetic resonance imaging investigation. Psychol Sci. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbuch M, Ambady N. Affective divergence: Automatic responses to others’ emotions depend on group membership. J Pers Soc Psychol. 2008;95:1063–1079. doi: 10.1037/a0011993. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice: Social-cognitive goals affect amygdala and stereotype activation. Psychol Sci. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. NeuroImage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DB, Sladden B. An own gender bias and the importance of hair in face recognition. Acta Psychol (Amst) 2003;114:101–114. doi: 10.1016/s0001-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.