Abstract
Signaling pathways for bone morphogenetic proteins (BMPs) are important in osteoblast differentiation. Although the precise function of type I BMP receptors in mediating BMP signaling for osteoblast differentiation and bone formation has been characterized previously, the role of type II BMP receptors in osteoblasts is to be well clarified. In this study, we investigated the role of type II BMP receptor (BMPR-II) and type IIB activin receptor (ActR-IIB) in BMP2-induced osteoblast differentiation. While osteoblastic 2T3 cells expressed BMPR-II and ActR-IIB, loss-of-function studies, using dominant negative receptors and siRNAs, showed that BMPR-II and ActR-IIB compensated each other functionally in mediating BMP2 signaling and BMP2-induced osteoblast differentiation. This was evidenced by two findings. First, unless there was loss of function of both type II receptors, isolated disruption of either BMPR-II or ActR-IIB did not remove BMP2 activity. Second, in cells with loss of function of both receptors, restoration of function of either BMPR-II or ActR-IIB by transfection of the wild-type forms, restored BMP2 activity. These findings suggest a functional redundancy between BMPR-II and ActR-IIB in osteoblast differentiation. Results from experiments to test the effects of transforming growth factor b (TGF-β), activin, and fibroblast growth factor (FGF) on osteoblast proliferation and differentiation suggest that inhibition of receptor signaling by double-blockage of BMPR-II and ActR-IIB is BMP-signaling specific. The observed functional redundancy of type II BMP receptors in osteoblasts is novel information about the BMP signaling pathway essential for initiating osteoblast differentiation.
Osteoblastic differentiation of mesenchymal cells is required for osteogenesis and postnatal bone formation. Bone morphogenetic proteins (BMPs), structurally related to the transforming growth factor β (TGF-β) superfamily, are important growth factors in controlling osteoblast differentiation. BMPs promote commitment of pluripotent mesenchymal cells into the osteoblast lineage by regulating signals that stimulate specific transcriptional programs required for bone formation during embryonic skeletal development and postnatal bone remodeling (Urist, 1965; Wozney et al., 1988; Chen et al., 2004; Zhao et al., 2008).
BMP signaling is mediated through type I and type II BMP receptors (Chen et al., 2004; Zhao et al., 2002, 2008). Like other receptor members of the TGF-β superfamily, both type I and type II BMP receptors have inducible intracellular serine/threonine kinase activity that transduces the external BMP signal to an intracellular phosphorylation cascade. Type II receptors, including the type II BMP receptor (BMPR-II), type II activin receptor (ActR-II), and type IIB activin receptor (ActR-IIB), serve as primary ligand-binding receptors. After binding to BMP ligands, homomeric dimers of the type II receptors form a tetrameric complex with homomeric dimers of the type I receptors, including the type IA BMP receptor (BMPR-IA), type IB BMP receptor (BMPR-IB), and type I activin receptor (ActR-I) (Koenig et al., 1994; ten Dijke et al., 1994; Kawabata et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Yamashita et al., 1995). In this heterotetrameric complex, type II receptors transphosphorylate the type I receptors through a GS domain, leading to activation of type I receptor kinase (Cárcamo et al., 1995; Wieser et al., 1995; Hoodless et al., 1996). The activated type I receptor acts as an effector in the signal transduction by recruiting and phosphorylating the pathway-restricted Smads (Smad1, Smad5, and Smad8) (Hoodless et al., 1996; Chen et al., 1997; Nishimura et al., 1998). After phosphorylation, Smads are released from the receptor, migrate into the nucleus with a chaperone Smad4, and activate transcription of specific target genes involved in osteoblastic differentiation and bone formation (Ducy et al., 1997; Nakashima et al., 2002; López-Rovira et al., 2002; Yagi et al., 2003; Ohyama et al., 2004).
As the primary binding receptors for BMPs, type II receptors have important roles in embryonic development. Gene manipulation or mutations of BMPR-II may result in developmental abnormalities of gastrulation and cardiogenesis (e.g., pulmonary hypertension) in mice and humans (Beppu et al., 2000, 2004, 2009; Newman et al., 2001; Song et al., 2005; Yu et al., 2005; Hong et al., 2008; Wang et al., 2009). Studies with genetically altered mouse lines have shown that ActR-II and ActR-IIB have distinct roles in embryonic patterning and pulmonary artery function (Matzuk et al., 1995a; Oh and Li, 1997, 2002; Oh et al., 2002; Ferguson et al., 2001). Moreover, disruption of these type II receptors in mutant mice induces defects in skeletal development, particularly in the teeth (Matzuk et al., 1995a; Ferguson et al., 2001). Studies on gene expression patterns in vivo and in vitro show that BMPR-II (Nishitoh et al., 1996; Yonemori et al., 1997; Onishi et al., 1998; Yeh et al., 1998, 2002; Ebisawa et al., 1999; Kloen et al., 2002; van der Horst et al., 2002; Nadiri et al., 2006; Garimella et al., 2007; Lehnerdt et al., 2007; Yu et al., 2008), ActR-II, and ActR-IIB (Nishitoh et al., 1996; Yonemori et al., 1997; Ebisawa et al., 1999; van der Horst et al., 2002) participate in osteoblast differentiation and may play a role in bone formation during fracture (Onishi et al., 1998; Kloen et al., 2002), otospongiosis (Lehnerdt et al., 2007), and osteosclerosis (Garimella et al., 2007). These findings suggest that type II BMP receptors are important mediators for skeletal development and bone formation.
BMP signaling initiates osteoblast differentiation in embryonic osteogenesis and postnatal bone formation. Although the importance of type I BMP receptors in controlling osteoblast differentiation has been confirmed previously (Chen et al., 1998; Zhao et al., 2002), the function of type II BMP receptors in osteoblast differentiation has not been well described. Some in vitro studies have identified potential roles of type II receptors in osteoblast cells (Nishitoh et al., 1996; Yeh et al., 1998, 2002; Ebisawa et al., 1999; van der Horst et al., 2002; Nadiri et al., 2006; Garimella et al., 2007; Yu et al., 2008); however, the mechanisms responsible for interaction of these receptors in mediating BMP signaling for osteoblast differentiation should be precisely elucidated.
In the present study, we investigated the role of BMPR-II and ActR-IIB in osteoblast differentiation. The results of loss-of-function experiments suggest that BMPR-II and ActR-IIB play redundant roles in the BMP signaling pathway in osteoblast differentiation.
Materials and Methods
Truncated receptor constructs
Intracellular kinase-truncated BMPR-II (dnBMPR-II) was generated by PCR using a template of human full length BMPR-II cDNA in the expression vector pCMV5. A 0.56 kb dnBMPR-II PCR product was amplified with the following primers: 5′-CGGAATTCTGGCCCAGGGATGACTTC (sense) and 5′-CGGAATTCAGCGTAGTCTGGGACGTCGTATGGGTATCTGTATCCAAAGCATAAG (antisense). The fragment encodes 173 amino acids of the N-terminus incorporating the extracellular domain, the transmembrane domain, and a sequence for the HA tag at the C-terminus. The PCR product was cloned into expression vector pcDNA3 (Invitrogen, San Diego, CA) at the EcoR I site. The positive clones were first selected by enzyme digestion for correct orientation and then sequenced to verify accuracy of the PCR amplification reaction. The same truncated form of ActR-IIB (0.52 kb) was amplified by PCR with the primers of 5′-CGGGGATCCGCGC CGCGGAACATGACGGCG (sense) and 5′-CTAGTCTAGATTACTTATCATCATCATCCTTATAATCATACATCCAGAAGGCCAGCAGG (antisense) using mouse full-length ActR-IIB cDNA in expression vector pCMV5-ActR-IIB. This deletion mutant ActR-IIB (dnActR-IIB) encodes 160 amino acids covering the ActR-IIB extracellular and transmembrane domains and also a Flag epitope tag at the C' terminus. The dnActR-IIB gene was cloned into a pcDNA3 vector at BamHI and XbaI sites and verified by sequencing.
siRNA knockdown
Synthesis of siRNA: Short complementary RNA oligonucleotides were synthesized and annealed to form siRNA duplexes. The targeting sequences (sense) of the oligonucleotides for mouse BMPR-II and ActR-IIB are 5′-GGAUGAGCGUCCAGUGCU and 5′-AACUUCUGCAACGAGCGC were previously validated (Rebbapragada et al., 2003; Perron and Dodd, 2009). The siRNAs and the irrelevant scrambled oligonucleotides (20 nM) were transfected into 2T3 and C2C12 cells using the transfection reagents (Dharmacon RNAi Technologies, Lafayette, CO) by following the protocol. The knockdown efficiency BMPR-II and ActR-IIB was verified by PCR.
Cell culture and transfection
Osteoblast precursor 2T3 cells, derived from a transgenic mouse containing a BMP2 promoter driving the SV40 T-antigen transgene, were previously characterized (Ghosh-Choudhury et al., 1996). Cells were cultured with complete α-minimal essential medium (α-MEM) supplemented with 10% fetal calf serum (FCS), penicillin, streptomycin, and l-glutamine in air mixed with 5% CO2. After the seeded 2T3 cells were grown to 60–80% confluence, the cells were transfected with expression plasmids of wild-type or dominant-negative forms of type II receptors or their empty vectors using Lipofectamine Plus Reagent and the optimal medium (Gibco BRL, Rockville, MD) following the manufacturer's instruction. Cells transfected with dnBMPR-II and/or dnActR-II or pcDNA3 vector were treated with G418 (200–500 μg/ml). Cells were screened for expression of deletion mutant receptors by RT-PCR and Western blot. The selected stable colonies (five clones for each transfection group) that stably expressed high levels of mutant receptors were used in the following functional experiments.
In addition to 2T3 cells, osteoblastic C2C12 cells were also used in siRNA knockdown studies as described above. C2C12 cells were cultured in DMEM medium with the same supplements as the culture condition for 2T3 cells.
RT-PCR
Total RNA was extracted from untransfected 2T3 or transfected 2T3 cells using RNAzol B method (Tel-test Inc., Houston, TX). Purified RNA was reverse transcribed into cDNA (SuperScript III First-Strand Synthesis System, Invitrogen), and the synthesized cDNA was used as a template for PCR to amplify either wild-type and/or dn type II BMP receptors at 94°C (1 min), 56°C (1 min), and 72°C (1 min) for 35 cycles. The primers used were as follows: endogenous BMPR-II: 5′-GGGATGACTTCCTCGCTGCATC (sense), 5′-ACTGCTCCGTATCGACCCCG (antisense) (0.65 kb); endogenous ActR-II: TCGGGAAAATGGGAGCTGCTGC (sense), AGCTGCAATGGCTTCAA CCCTAG (antisense) (0.59 kb); endogenous ActR-IIB: 5′-GCGCCGCGGAACATGACGGCG (sense), 5′-GGTCCTGGAAAGGCAAGGGC (antisense) (0.61 kb); dnBMPR-II: 5′-TGGCCCAGGGATGACTTC (sense), 5′-AGCGTAGTCTGGGACGTCGTATGGGTA (antisense, HA tag) (0.56 kb); dnActR-IIB (Flag tag): 5′- GCGCCGCGGAACATGACGGCG (sense), 5′-CTTATCATCATCATCCTTATAATC (antisense, Flag tag) (0.52 kb).
Western blot
Cells were lysed in lysis buffer (50 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS) containing protease inhibitors and run on SDS-PAGE Ready Gels (Bio-Rad, Hercules, CA). Proteins were transblotted onto PVDF membrane (Bio-Rad) and blocked with 5% milk in TBST buffer (Tris-buffered saline with 0.1% Tween 20), at room temperature, for 2 h. Rabbit anti-HA polyclonal antibody (1:1,000) (Babco, Richmond, CA) and M2 mouse anti-Flag monoclonal antibody (1:2,000) (Sigma, St. Louis, MO) were incubated overnight with the membranes at 4°C to detect dnBMPR-II-HA and dnActR-IIB-Flag. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (1:5,000) (Amersham Life Science; GE Healthcare, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used as the second antibody. Signals on the blots were detected by an enhanced chemiluminescence system (ECL, Amersham Life Science) and X-ray film exposure.
Smad phosphorylation
Cells were seeded in six-well plates in α-MEM with 10% FCS. After overnight culture, when the cells reached a density of 80%, they were starved with α-MEM supplemented with 0.2% FCS for 24 h. Then, the cells were incubated with BMP2 (100 ng/ml) for 4 h. Cells were then lysed with the lysis buffer. Western blot of phosphorylated Smad1/5/8 was performed using an anti-phosphoserine antibody (Cell Signaling Technology, Inc., #9511, 1:1,000) to detect serine-phosphorylated Smad1/5/8. Non-phosphorylated Smad1 was used as a control detected by an antibody against Smad1 (Cell Signaling Technology, Inc., Boston, MA, #9743, 1:1,000). β-actin served as the protein control for normalization.
Immunofluorescence
Wild-type or mutant 2T3 cells, cultured in eight-well chamber slides at 1 × 104 cells per well, were transfected with Flag-tagged Smad1 expression vector for 24 h. Cells were starved with α-MEM supplemented with 0.2% FCS for 24 h and then treated with BMP2 (100 ng/ml) for 8 h. Cells were then fixed and incubated with FITC-conjugated mouse anti-Flag antibody (Sigma) diluted at 1:50 with PBS containing 1% BSA in room temperature for 2 h. After washing with PBS, fluorescence in respective wells was viewed with a fluorescent microscope.
BMP signaling reporter assay
A tandem consensus sequence (12 copies) of Smad binding element (SBE), GCCGCCGC, was synthesized. The annealed SBE DNA oligonucleotides were linked to mouse osteocalcin promoter (OCN, −155/+1), and the chimeric 12×SBE-OCN promoter cassette was inserted into the pGL3 luciferase reporter vector at the sites of Kpn I and Hind III. Osteoblastic 2T3 cells cultured in 24-well plates were transfected with p12SBE-OCN-Luc for 12 h. Subsequently, cells were starved in α-MEM with 1% FCS for 12 h, and then treated with BMP2 (100 ng/ml) for 24 h. The cells were lysed with the Reporter Lysis Buffer (Promega, Madison, WI). Luciferase activity was measured using a luminescence assay system (Promega). Data were normalized to the β-galactosidase activity of co-transfected pSV-β-Gal.
Mineralized matrix formation
Cells were plated into 12-well plates (4 × 104 cells per well) in α-MEM medium with 10% FCS. When the cells reached confluence, the medium was changed to α-MEM supplemented with 5% FCS, ascorbic acid (100 μg/ml), and β-glycerophosphate (5 mM), and the cells were cultured in the presence or absence of BMP2 (1–100 ng/ml) or activin A (0.5–50 ng/ml). The medium was refreshed every other day. After 2–3 weeks following the initial treatment, cells were fixed in phosphate buffered formalin, and the mineralized matrix was stained (von Kossa stain with van Gieson counterstain). Rehydrated cells reacted with silver nitrate solution (2%) and were exposed to sunlight for 20 min. Plates were rinsed with water, and sodium thiosulfate (5%) was added (3 min), followed by a water rinse. Yellow-red Van Gieson's Stain then was added to stain the collagen matrix. Plates were dehydrated and dried for image analysis. The area of dark brown or black von Kossa-stained matrix was imaged using the ImagePro Plus image analysis software (Media Cybernetics, Silver Spring, MD).
Alkaline phosphatase (ALP) activity
Cells were grown to 50–70% confluence in 48-well plates with α-MEM supplemented with 10% FCS, and then incubated with BMP2 (0–200 ng/ml) or activin A (0–200 ng/ml) in α-MEM supplemented with 1% FCS for 48 h. Cells were washed twice with PBS, and lysed with 0.05% Triton X-100. The cell lysate was frozen and thawed twice. ALP activity in the cell lysate was determined using ALP detection reagents (Sigma) by reading absorbance of the reaction product p-nitrophenol at 405 nm; p-nitrophenol was used to construct a standard curve of ALP activity. ALP activity was calculated by normalizing the amount of protein in cell lysates harvested from each well.
Northern blot
Cells cultured in six-well plates were treated with BMP2 (100 ng/ml) for 4–8 days. Then, total RNA was isolated from the cells using the RNAzol B method (Tel-test Inc., Houston, TX). Enriched polyadenylated RNA was obtained using oligo(dT) cellulose columns (Stratagene, San Diego, CA). Denatured poly-(A+) RNA (5 μg) was run on a 1% agarose gel containing formaldehyde (2.2 M). RNA was transferred to a filter (Nytran; Schleicher and Schuell, Keene, NH) and then cross-linked to the filter by ultraviolet irradiation. Prehybridization and hybridization were performed as described previously (Chen et al., 1998). The 32P-labeled cDNA probes of Runx2, osteocalcin (OCN) and GAPDH were used in the blot. Hybridized signals of Runx2 and OCN were normalized by GAPDH.
Cell proliferation assay
Cells were cultured in 96-well plates with α-MEM medium and 10% FCS. When cells had grown to approximately 50% confluence, they were starved with α-MEM and 0.2% FCS for 12 h, and then incubated with TGF-β1 (0–0.06 ng/ml) or acidic FGF (0–0.4 ng/ml). After 40 h of incubation, cell proliferation was determined with an assay kit (CellTiter 96® Non-Radioactive Cell Proliferation Assay Kit; Promega) by adding dye from the assay kit to the culture for 4 h and then determining the absorbance (at 570 nm) of formazan product, which is directly proportional to the number of viable cells.
Results
Expression of type II receptors for BMP and their dominant negative forms in osteoblasts
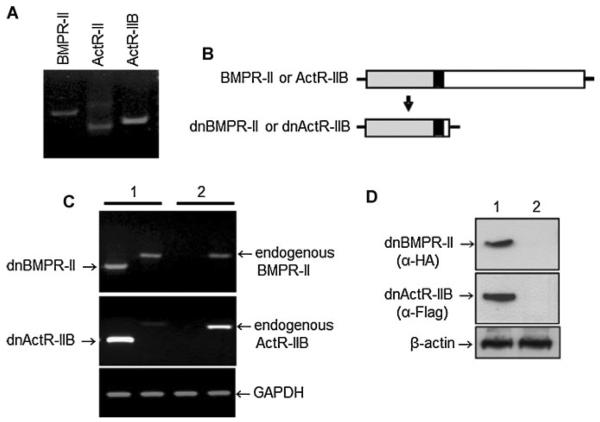
We used immortalized mouse osteoblast precursor 2T3 cells, previously shown to express type I receptors for BMP (BMPRIA and BMPR-IB) and to respond to stimulation by BMP2 (Ghosh-Choudhury et al., 1996; Chen et al., 1998; Zhao et al., 2002). We examined expression of type II receptors for BMP in 2T3 cells and found that these cells expressed all three type II BMP receptors (BMPR-II, ActR-IIB, and ActR-II) (Fig. 1A). With a high sequence homology, the two type II activin receptors ActR-II and ActR-IIB functionally overlap in many of their biological activities. We found that the expression level of ActR-IIB was higher than other type II receptors in 2T3 cells, suggesting that ActR-IIB might be a predominant form of the type II activin receptors in osteoblastic cells.
Fig. 1.
Expression of type II BMP receptors in osteoblastic 2T3 cells. A: Expression of endogenous BMPR-II, ActR-II, and ActR-IIB detected by RT-PCR. B: Molecular structures of BMPR-II and ActRIIB. Top: Wild-type consists of the extracellular ligand-binding domain (gray), transmembrane domain (black), and intracellular kinase domain (blank). Bottom: Dominantnegative C' terminal, intracellular domain, truncated forms, dnBMPR-II, and dnActR-IIB. C, D: Expression of dnBMPR-II and dnActR-IIB. Expression plasmids of (1) the dominantnegative mutantsor (2) their empty vectors were stably transfected into 2T3 cells. Expression of these mutant receptors and endogenous receptors was determined by RT-PCR (C) and Western blot (D) with anti-HA and anti-Flag antibodies for dnBMPR-II and dnActR-IIB.
Both BMPR-II and ActR-IIB function as major signaling mediators in the same BMP pathway, and ActR-IIB also distinctly transduces signaling upon activin binding. Therefore, we investigated the function and relation of BMPR-II and ActRIIB in BMP-induced osteoblast differentiation. For this purpose, we generated dominant negative (dn) mutants of these type II receptors, dnBMPR-II, and dnActR-IIB. The structure of type II receptors is composed of an extracellular N-terminal domain capable of binding BMP ligands, a transmembrane domain, and an intracellular C-terminal domain responsible for transactivating the type I receptor to transduce BMP signal. In the mutant dnBMPR-II and dnActR-IIB forms, the intracellular C' terminal regions were deleted from the site of Arg173 (dnBMPR-II) and Tyr160 (dnActR-IIB) just behind the transmembrane domains and replaced by hemagglutinin (HA) (dnBMPR-II) and a Flag (dnActR-IIB) epitopes. As a result, the kinase activity of the receptors was removed (Fig. 1B). Expression plasmids of the truncated receptors, pcDNA3-dnBMPR-II and/or pcDNA3-dnActR-IIB, were stably transfected into 2T3 cells. Expression of truncated dnBMPR-II and dnActR-IIB in individual colonies was determined by RT-PCR and Western blot using anti-HA or anti-Flag antibody (Fig. 1C, D). Five confirmed colonies for each transfection groups that express truncated receptors at high levels with minor variations over endogenous wild type receptors (Fig. 1C, lane 1, 2) were selected for the following functional measurements.
Effects of dnBMPR-II and dnActR-IIB on BMP signaling
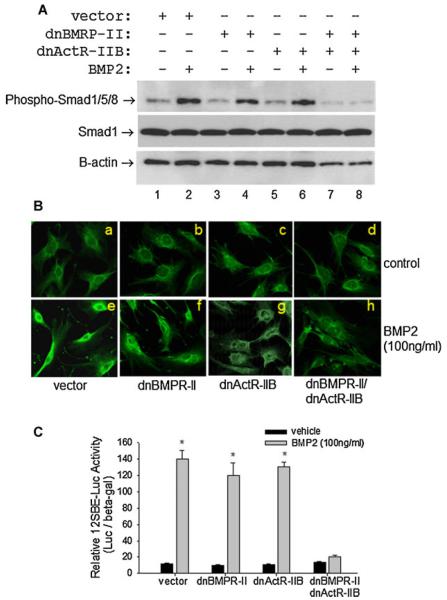
Type II receptor-initiated BMP signaling is mediated through type I receptors that phosphorylate BMP-specific Smad1, Samd5, and Smad8 (Smad1/5/8). The phosphorylated active Smads then migrate into the nuclei and transactivate target genes. We determined the effects of the dominant negative receptors on BMP-induced Smad1/5/8 phosphorylation. Western blot results showed that treatment of control 2T3 cells with BMP2 for 6 h substantially increased levels of the phosphorylated BMP-specific Smads (Fig. 2A, lanes 1 and 2). Unexpectedly, we found that overexpression of either dnBMPR-II or dnActR-IIB in 2T3 cells did not decrease BMP2-induced phosphorylation of Smad1/5/8 (Fig. 2A, lanes 3–6). However, when 2T3 cells were forced to simultaneously overexpress both dnBMPR-II and dnActR-IIB, the BMP2-enhanced Smad phosphorylation was completely abolished (Fig. 2A, lanes 7 and 8). In all these cells with single or double dominant negative receptors, protein levels of non phosphorylated Smad1 were not notably changed (Fig. 2A), suggesting that these mutant receptors do not affect the stability of the endogenous Smad proteins.
Fig. 2.
Effects of dnBMPR-II and dnActR-IIB on BMP signaling. A: Smad phosphorylation. 2T3 cells, transfected with empty vector, dnBMPR-II, ActR-IIB, or dnBMPR-II/dnActR-IIB expression vector, were treated with BMP2 (100 ng/ml) for 6 h. Levels of phosphorylated BMP-specific Smads were determined by Western blot using an anti-phospho-Smad1/5/8 antibody, with normalization to non-phosphorylated Smad1 and β-actin. B: Smad1 nuclear translocation. The mutant receptor-carrying cells were co-transfected with Flag-Smad1 plasmid and treated with BMP2 for 6 h. After fixation, cells were probed with FITC-conjugated anti-Flag antibody. Immunofluorescence of Smad1 was recorded. C: BMP/Smad signaling reporter assay. Cells were transfected with 12×SBELuc reporter construct and treated with BMP2 for 24 h. Relative reporter luciferase activity was determined and normalized by β-galactosidase activity. *P < 0.01 versus vehicle controls.
We evaluated the effects of these mutant receptors on Smad1 nuclear migration by immunofluorescence in 2T3 cells that had been treated with BMP2 for 6 h. In the wells for the empty vector, dnBMPR-II, and dnActR-IIB, the intensity of Smad1 fluorescence was much higher in the nuclei than in the cytoplasm, indicating that stimulation with BMP2 induced Smad1 nuclear translocation in these cells (Fig. 2B, e, f, g vs. a, b, c). However, the BMP2-induced Smad1 nuclear migration was markedly inhibited by dnBMPR-II + dnActR-IIB double blockage (Fig. 2B, h vs. d).
Smads mediate BMP signaling to the target genes by interacting with a specific Smad binding element (SBE). We made a BMP-responding reporter gene, p12SBE-OCN-Luc, using a chimeric cassette of 12 copies of the SBE DNA sequence and a mouse osteocalcin promoter that drives a luciferase gene. This BMP signaling reporter gene was transfected into 2T3 cells, and the BMP2 responsiveness was determined by luminescence measurement. This showed that overexpression of only dnBMPR-II or dnActR-IIB did not affect the BMP2-mediated increase in BMP/Smad-specific luciferase activity, but dnBMPR-II/dnActR-IIB co-action completely removed the luciferase responsiveness of the cells to the BMP2 treatment (Fig. 2C). These results suggest that the functions of BMPR-II and ActR-IIB in mediating BMP action in osteoblast cells are redundant and mutually compensated, because inhibition of only one receptor is not enough to block BMP signaling.
Effects of dnBMPR-II and dnActR-IIB on osteoblast differentiation
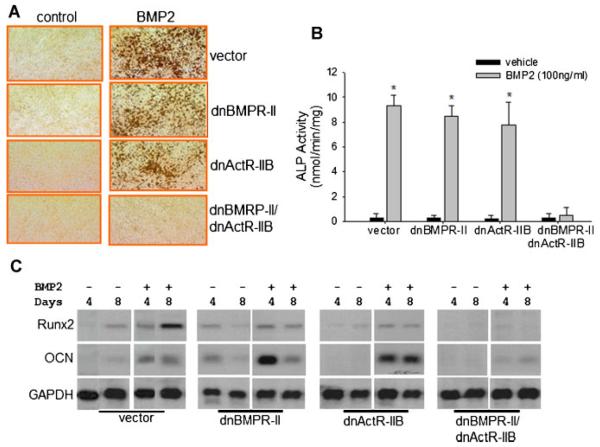
BMP signaling is an important activator of osteoblast differentiation. BMPR-II and ActR-IIB have been implicated in osteoblast differentiation (Nishitoh et al., 1996; Yonemori et al., 1997; Yeh et al., 1998, 2002; Ebisawa et al., 1999; van der Horst et al., 2002; Nadiri et al., 2006; Garimella et al., 2007; Yu et al., 2008), but they have not been confirmed yet by loss-of-function studies. Therefore, we determined whether these dominant negative receptors influence the osteoblastic differentiation of 2T3 cells. These osteoblast precursor cells are capable of mineralization in culture (Ghosh-Choudhury et al., 1996; Chen et al., 1998; Zhao et al., 2002, 2003, 2006, 2009). We performed an in vitro assay of mineralized matrix formation. Cells stably transfected with dominant negative receptors were cultured in osteogenic culture media in the presence or absence of BMP2. Two weeks after the initial BMP2 treatment, von Kossa staining showed that BMP2 substantially increased bone nodule formation in the culture of the 2T3 cells transfected with vector (Fig. 3A, upper). When we overexpressed either dnBMPR-II or dnActR-IIB, the BMP2-induced formation of mineralized bone matrix by 2T3 was not markedly affected (Fig. 3A, middle). However, cells that overexpressed both dnBMPR-II and dnActR-IIB did not form bone nodules in response to BMP2 (Fig. 3A, bottom).
Fig. 3.
Effects of dnBMPR-II and dnActR-IIB on osteoblast differentiation. A: Mineralized matrix formation. Osteoblastic 2T3 cells expressing dominantnegative receptors were treated with BMP2 (100 ng/ml) in an osteogenic culture medium for 2 weeks. Mineralized matrix formation was determined by combined von Kossa and van Gieson staining. B: ALP activity. Cells were treated with BMP2 for 2 days. ALP activity of cell lysates was measured with cell protein normalization. *P < 0.01 versus vehicle controls. C: Expression of osteoblast marker genes. At 4 or 8 days after BMP2 treatment, total RNA was extracted from these cells. mRNA levels of Runx2 and OCN were determined by Northern blotting with GAPDH normalization.
Furthermore, BMP2 treatment for 2 days markedly increased alkaline phosphatase (ALP) activity in 2T3 cells. Single blockage with dnBMPR-II or dnActR-II did not attenuate the BMP2-enhanced ALP activity; however, BMP2-mediated stimulation of ALP activity was completely inhibited by dnBMPR-II/dnActR-IIB double mutants (Fig. 3B).
We also examined gene expression of osteoblast maturation markers in these cells. Northern blot electrophoresis showed that in the vector-control 2T3 cells, treatment with BMP2 for 4 and 8 days increased expression of Runx2 and osteocalcin. Although there were some variations in basal levels of these genes and their responsiveness to BMP2 treatment between the cell clones, overexpression of either dnBMPR-II or ActR-IIB did not eliminate BMP2 stimulation of these osteoblast-specific marker genes. However, the cells in which signaling via both BMPR-II and ActR-IIB were blocked by overexpression of dnBMPR-II and dnActR-IIB had a substantial reduction in Runx2 and osteocalcin expression levels with or without BMP2 treatment (Fig. 3C). These functional observations suggest that BMPR-II and ActR-IIB play a redundant role in mediating BMP2 stimulation of osteoblast differentiation.
Effects of dnBMPR-II/dnActR-IIB double blockage on osteoblast proliferation
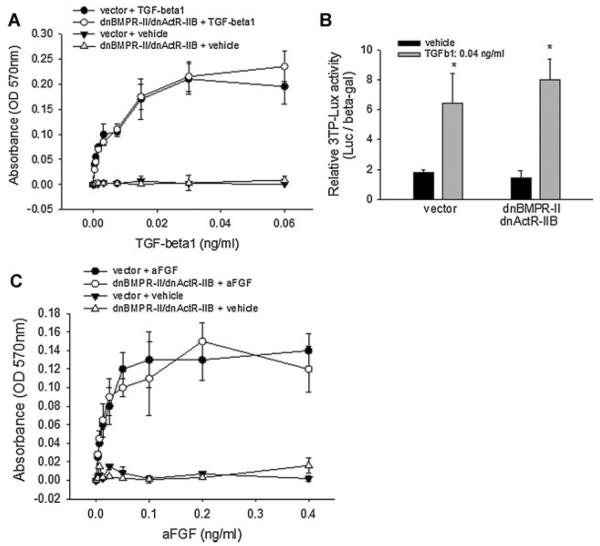
Both BMPR-II and ActR-IIB are serine-threonine kinase receptors of the TGF-β superfamily in which type I receptors including BMPR-IA, BMPR-IB, ActR-IA, and ActR-IB share a common activin receptor like kinase (ALK) activity with the TGF-β type I receptor (TβR-I). To address the signaling specificity of dnBMPR-II/dnActR-IIB-induced inhibition of the type II receptor, we performed a cell proliferation assay to determine whether the double blockage affects TGF-β signaling, because TGF-β is known to stimulate osteoblast growth (Gosain et al., 2000; van der Zande et al., 2008). The dnBMPR-II/dnActR-IIB double-blocked and vector-control 2T3 cells described above were incubated with TGF-β1 for 48 h and cell proliferation was determined. Results showed that treatment with TGF-β1 (0–0.06 ng/ml) caused a dose-dependent increase in the proliferation rate of 2T3 cells, and there was no significant difference in this stimulation between control cells and dnBMPR-II/dnActR-IIB cells (Fig. 4A), suggesting that dnBMPR-II/dnActR-IIB double blockage did not affect TGF-β signaling.
Fig. 4.
Effects of dnBMPR-II/dnActR-IIB double blockage on osteoblast proliferation. A, C: Cell proliferation assay. 2T3 cells transfected with vector or dnBMPR-II/dnActR-IIB double mutants were treated with (A) TGF-β1 (0–0.06 ng/ml), (C) aFGF (0–0.4 ng/ml), or their vehicle for 40 h. Cell proliferation was determined by adding an assay reagent (CellTiter, Promega, Madison, WI) and reading absorbance at 570 nm. B: TGF-β signaling reporter assay. Both control and mutant cells were transfected with TGF-β signaling reporter 3TP-Lux and incubated with TGF-β1 (0.04 ng/ml) for 36 h. The reporter luciferase activity was determined by luminescence with β-gal normalization. *P < 0.05 versus vehicle control.
A TGF-β-signaling specific luciferase reporter, 3TP-Lux, was used to confirm this finding. Cells that carried control empty vector or dnBMPR-II/dnActR-IIB mutants were transfected with the 3TP-Lux reporter. We found that both types of cells responded to stimulation of TGF-β1 to the same level of luciferase activity (Fig. 4B).
We also examined the effects of fibroblast growth factor (FGF), a positive regulator of osteoblast proliferation (Rodan et al., 1987; Globus et al., 1988). Similar to the result with TGF-β1, treatment with acidic FGF (aFGF) stimulated cell proliferation in both control and double-dominant negative mutant 2T3 cells, evidenced by the parallel dose-dependent stimulation curves (Fig. 4C). Although BMP2 is a powerful stimulator of osteoblast differentiation, it is not an important regulator of osteoblast proliferation. Therefore, we did not compare the effects of BMP2 on cell growth in these cells. These cell proliferation studies, with the other observations above, suggest that blocking type II receptors by dnBMPR-II/ dnActR-IIB is specific for BMP signaling.
Restoration of dnBMPR-II/dnActR-IIB-impaired BMP signaling and osteoblast differentiation
Next, studies to restore function were performed to confirm the redundancy of BMPR-II and ActR-IIB in BMP signaling in osteoblasts. Osteoblastic 2T3 cells, in which the BMP signaling was inhibited by double blockage of type II receptors with dnBMPR-II and dnActR-IIB, were co-transfected with p12SBE-OCN-Luc and expression plasmids of either wild-type BMPR-II, or wild-type ActR-IIB, or an empty vector. Cells were treated with BMP2 (100 ng/ml) for 24 h, and the luciferase activity was determined by fluorescence and normalized to β-galactosidase activity. In cells with dnBMPR-II/dnActR-IIB double blockage, forced overexpression of either wild-type BMPR-II or ActR-IIB resulted in marked recovery of the inhibited responsiveness of 12SBE-OCN-Luc activity to BMP2 treatment, compared with the vector control (Fig. 5A). Furthermore, BMP2-induced ALP activity was markedly restored by the overexpression of either wild-type BMPR-II or ActR-IIB in the impaired 2T3 cells (Fig. 5B). These data show that isolated restoration of either BMPR-II or ActR-IIB restored the impaired BMP signaling and osteoblast differentiation that results from co-inhibition of dnBMPR-II and dnActR-IIB, and strongly suggest that the functions of BMPR-II and ActR-IIB in mediating the BMP2 osteogenic signal are redundant.
Fig. 5.
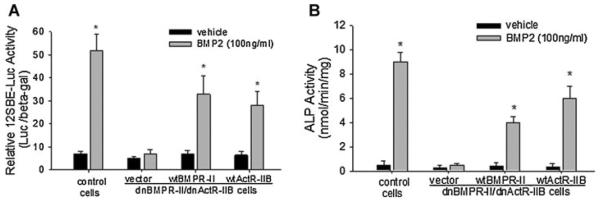
Restoration of dnBMPR-II/dnActR-IIB-impaired BMP signaling and osteoblast differentiation. Osteoblastic 2T3 cells, in which BMP signaling was blocked by expression of dnBMPR-II/dnActR-IIB double mutants, were transfected with wild-type BMPR-II, wild-type ActR-IIB, or an empty vector. After BMP2 treatment, (A) the BMP signaling reporter (12×SBE-Luc) activity and (B) ALP activity in the cell lysates were determined as described in Figures 1 and 2. *P < 0.01 versus vehicle control.
Effects of knockdown of BMPR-II and ActR-IIB genes on BMP signaling and osteoblast differentiation
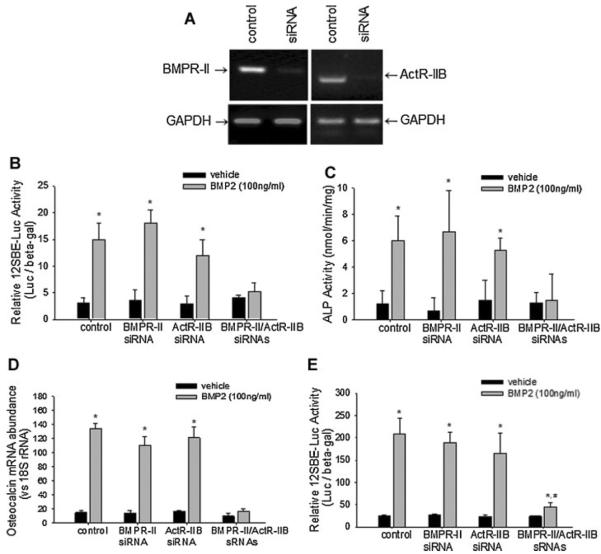
In addition to dominant negative receptors, we also used small interfering RNA (siRNA) knockdown to determine the effects of loss of function of endogenous BMPR-II and ActR-IIB on osteoblast differentiation. RT-PCR results showed that transfection of siRNAs specific for BMPR-II or ActR-IIB in 2T3 cells substantially decrease the mRNA levels of these endogenous receptors, compared with control/scrambled RNA oligonucleotides (Fig. 6A). A luciferase reporter assay using the 12SBE-OCN-Luc vector showed that, similar to control siRNA treatment, knockdown of only BMPR-II or ActR-IIB did not abolish the stimulatory effects of BMP2 on Smad-induced activation of the reporter gene; however, the cells that interfered with expression of both BMPR-II and ActR-IIB had no responsiveness of the BMP signaling reporter to BMP2 treatment (Fig. 6B). Similarly, BMP2-induced ALP activity was completely abolished only in these double knockdown cells, but single knockdown of either BMPR-II or ActR-IIB did not affect BMP2-enhancement of ALP activity (Fig. 6C). Real time PCR has shown that BMP2-induced osteocalcin expression was completely removed in the cells with both mutant receptors, compared to other control cells (Fig. 6D).
Fig. 6.
Effects of knockdown of BMPR-II and ActR-IIB on BMP signaling and osteoblast differentiation. A: siRNA knockdown. Wild-type 2T3 cells were transfected with targeting siRNAs for BMPR-II and/or ActR-IIB, or control scrambled RNA, at 20 nM for 24 h and mRNA levels of endogenous BMPR-II and ActR-IIB were examined by PCR. B, E: BMP signaling reporter assay. Osteoblastic 2T3 (B) and C2C12 (E) cells were co-transfected with siRNAs and 12SBE-Lucreporter, and then treated with BMP2 for 48 h. The reporter luciferase activity was measured with β-gal normalization. *P < 0.05 versus vehicle control. #P < 0.05 versus BMP2 treatment in other groups. C: ALP activity assay. Osteoblastic 2T3 cells transfected with siRNAs were incubated with BMP2 for 48 h, followed by measurement of ALP activity as described in Figure 3. *P < 0.05 versus vehicle control. D: Osteocalcin expression. 2T3 cells transfected with siRNAs were treated with BMP2 for 24 h. Osteocalcin mRNA levels were determined by real time PCR with 18S rRNA normalization. *P < 0.05 versus vehicle control.
To verify the redundancy between BMPR-II and ActR-IIB that we identified in osteoblast 2T3 cells as described above, we also performed siRNA studies in osteoblastic C2C12 cells and examined the effects of deficiency of endogenous receptors on BMP2 signaling. Consistently, we found that only in BMPR-II and ActR-IIB double knockdown cells, BMP2 activation of Samd-responsive reporter activity was significantly reduced, compared the BMP2 stimulation in other groups (Fig. 6E). These results confirm the findings obtained from the above studies using dominant negative receptors that BMPR-II and ActR-IIB are functionally redundant in BMP2-induced osteoblast differentiation.
Effects of activin on osteoblast differentiation
The receptor ActR-IIB also binds to activin ligands (in addition to BMP ligands) and mediates their signals. We determined whether activin signaling is involved in osteoblast differentiation in this cell system. Wild-type 2T3 cells were cultured in the presence of BMP2 or activin A for 2 days to measure ALP activity, or for 21 days to examine mineralized matrix formation. Unlike BMP2 (0–200 ng/ml), which markedly increased ALP activity in a dose-dependent manner, we found that activin A (0–200 ng/ml) did not show any increasing effect on ALP activity (Fig. 7A). Similarly, in the assay of mineralized matrix formation, BMP2 increased mineralized nodule formation in the cell culture in a dose-dependent manner, 21 days after initial treatment, but activin A did not cause mineralized nodule formation (Fig. 7B). Together with above results, these data suggest that normal function of BMPR-II and ActR-IIB is required for BMP signaling, but not activin signaling, for osteoblast differentiation.
Fig. 7.
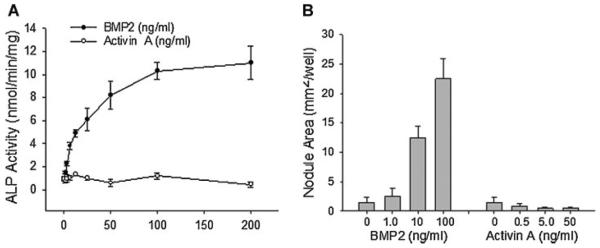
Effects of activin on osteoblast differentiation. A: ALP activity. Wild-type 2T3 cells were treated with BMP2 or activin A for 48 h. ALP activity of cell lysates was determined with normalization to cell protein. B: Mineralized matrix formation. Wild-type 2T3 cells were cultured in osteogenic media in the presence of BMP2 (0.5–50 ng/ml) or activin A (1–100 ng/ml) for 2 weeks. Areas of mineralized bone nodules in the culture were quantitated with von Kossa stain.
Discussion
Osteoblast precursor 2T3 cells that were used in this study were originally isolated and cloned from the primary calvarial osteoblastic cells of a transgenic mouse containing the BMP2 promoter driving the SV40 T-antigen transgene. These cells respond to BMP2 and undergo bone matrix formation in vitro (Ghosh-Choudhury et al., 1996; Chen et al., 1998; Zhao et al., 2002, 2003, 2006, 2009). We previously characterized expression and function of type I BMP receptors (BMPR-IA and BMPR-IB) in this cell line and demonstrated that BMPR-IB is a predominant type I receptor for BMP2 signaling in initiating osteoblastic differentiation and bone formation in vitro and in vivo (Chen et al., 1998; Zhao et al., 2002). In this study, we investigated the role of type II receptors for BMP2 signaling in these osteoblast cells. Expression of type II receptors for BMP (BMPR-II, ActR-II, and ActR-IIB) is essential for mediating BMP signaling in osteoblast differentiation (Nishitoh et al., 1996; Yeh et al., 1998, 2002; Ebisawa et al., 1999; van der Horst et al., 2002; Nadiri et al., 2006; Garimella et al., 2007; Yu et al., 2008), bone formation, and fracture healing (Yonemori et al., 1997; Onishi et al., 1998; Kloen et al., 2002; Lehnerdt et al., 2007). We found that 2T3 cells express all three types of II BMP receptors. Therefore, these receptors may play an important role in BMP2-induced osteoblast differentiation. Measurement of mRNA levels has shown that 2T3 cells have higher expression levels of ActR-IIB than the other two types of II receptors (Fig. 1A).
Although other studies have shown that the two type II activin receptors ActR-II and ActR-IIB which share 75% homology in their sequence exhibit some diverse and overlapping biological functions (Ferguson et al., 2001; Oh et al., 2002), they have been reported to possess similar functions in mediating BMP signaling in osteoblasts (Nishitoh et al., 1996; Ebisawa et al., 1999; van der Horst et al., 2002). Our expression studies suggested that ActR-IIB might serve as a major type II activin receptor in osteoblast 2T3 cells. Thus, we investigated the function of ActR-IIB, rather than ActR-II, in osteoblasts. We had hypothesized that BMPR-II and ActR-IIB are both necessary for BMP2 function in osteoblasts because the sequences of ActR-II and ActR-IIB are 75% homologous and both receptors possess overlapping function in mediating both BMP and activin signaling, distinguishing them from BMPR-II. However, the results of the loss-of-function studies, using either dominant negative receptors or siRNA, showed that BMPR-II and ActR-IIB are functionally redundant in osteoblasts and functionally compensate for each other in mediating BMP2 signaling and osteoblast differentiation. This was confirmed by these findings: (a) Blocking either BMPR-II or ActR-IIB did not affect BMP2 signaling and osteoblast differentiation; (b) BMP2-initiated events were completely abolished only when both BMPR-II and ActR-IIB receptors were blocked (Figs. 2, 3, 6); and (c) restoring the function of either BMPR-II or ActR-IIB with their wild-type forms restored the impaired phenotype in the double-blocked cells (Fig. 5). Nevertheless, our results do not exclude the involvement of ActR-II in the functional redundancy between type II BMP receptor and type II activin receptors, as ActR-II, like ActR-IIB, also has been demonstrated to participate in osteoblast-related functions (Nishitoh et al., 1996; Ebisawa et al., 1999; van der Horst et al., 2002). This warrants further investigation.
Through the Northern blot assay, although we conclude that single dnBMPR-II or dnActR-IIB did not block BMP2-induced Runx2 and osteocalcin expression, we noticed that basal levels of Runx2 and osteocalcin and their responsiveness to BMP2 stimulation varies between the cell clones that overexpress dnBMPR-II or dnActR-IIB. In establishing the stable cell clones with these truncated receptors, we found that most cell clones revealed different basal levels of these genes, some higher and some lower between the clones, but they all responded well to BMP2 stimulation, compared to control cells and double mutant cells. The cell variation also was seen between the cell clones overexpressing both dnBMPR-II and ActR-IIB. Four of five clones substantially reduced expression of these genes and their response to BMP2 at various levels. In immunofluorescence studies, Samd1 intensity in cytoplasm appeared to be increased when responding to BMP2 treatment in the cells transfected with control vector or single mutant receptor. We noticed that the increased Smad1 fluorescence intensity concentrated locally at the surrounding nuclei, which disappeared in the double-blocking cells. This suggests that BMP2 induces Smad1 migration from cytoplasm towards nuclei. However, BMP2 may increase Smad1 levels by antagonizing Smurf1-mediated Samd1 degradation (Zhu et al., 1999; Ying et al., 2003), thus, we did not completely exclude the possibility of a slight increase in Smad1 level, although our Western blot analysis did not provide a support for this notion.
This observed redundancy might be a result of a heteromeric receptor complex. A type I receptor dimer forms a tetrameric complex with a type II receptor dimer. Similar to a type I receptor dimer, a type II dimer can be either a homomeric or heteromeric dimer, such as BMPR-II/BMPR-II or BMPR-II/ActR-IIB. In a BMPR-II/ActR-IIB heteromeric dimer within the tetrameric receptor complex, BMPR-II still can transduce the BMP signal in the absence of ActR-IIB function. In the cells that express both dnBMPR-II and ActR-IIB, these dominant negative receptors occupy the positions of native BMPR-II and ActR-IIB in the tetramers, and this dysfunctional receptor complex compete with native receptor complex for BMP ligands leading to blockage of receptor signaling. However, truncated forms of receptors may block broader signaling than the one through corresponding receptors. Considering this potential limitations in specificity and efficiency in blocking receptor signaling using these dominant negative receptors, we also performed siRNA knockdown studies and verified that the redundancy between endogenous BMPR-II and ActR-IIB. This phenomenon was observed in BMP signaling studies in other cell systems (Yeh et al., 1998; Upton et al., 2009). In endothelial cells, normal responsiveness of the cells to BMP9 was retained despite siRNA-mediated knockdown of BMPR-II or ActR-II, but double siRNAs of these receptors significantly removed BMP signaling activity, such as Smad1/5 phosphorylation and Id1 expression (Upton et al., 2009). Similarly, in fetal rat calvarial cells, loss-of-function of only BMPR-II by siRNA was not sufficient to reduce the baseline level of ALP activity and had a very mild inhibitory effect of BMP7-induced ALP activity (Yeh et al., 1998). These previous findings are consistent with functional redundancy between these type II receptors discovered in this study. Another possible mechanism for the signaling blockage in the dnBMPR-II/dnActR-IIB cells may be a functional disruption of type I receptors by these dysfunctional type II receptors, either prohibiting their gene expression or interfering their assembly within the receptor complex. Because of the research scope, we did not examine this potential mechanism in this study.
Although these previous and current findings support the concept that type II BMP receptors are redundant for BMP signaling in osteoblast cells, it has been observed in vivo with animal models that conventionally (Oh and Li, 1997, 2002; Beppu et al., 2000, 2004; Oh et al., 2002; Song et al., 2005) or conditionally (Yu et al., 2005; Hong et al., 2008; Beppu et al., 2009) targeted disruption of a single version of any of these type II receptors introduces specific phenotypic abnormalities, particularly during embryonic development. Deficiency of BMPR-II causes defects in cardiogenesis, such as pulmonary arterial hypertension (Beppu et al., 2000, 2004, 2009; Song et al., 2005; Yu et al., 2005; Hong et al., 2008) and deletion of ActR-II and/or ActR-IIB impairs embryonic asymmetrical patterning (Oh and Li, 1997, 2002; Matzuk et al., 1995a; Ferguson et al., 2001; Oh et al., 2002). However, these animal models are either early embryonically lethal or not specific to osteoblasts, and this may limit investigators from evaluating the precise role of these receptors in osteoblasts. Therefore, these in vivo observations do not necessarily exclude the possibility of redundancy between these type II receptors, especially in osteoblast cells. Recently, a BMPR-II transgenic mouse model was described in which a dominant negative BMPR-II (BMPR-DN) was driven by osteoblast-specific Col1a1-2.3 promoter, and the BMPR-DN transgenic mice had low bone mass (Yang et al., 2010). However, that study lacked histomorphometric characterization of the osteoblastic bone phenotype. It is unknown whether disruption of BMPR-II interferes with expression and function of ActR-II and ActR-IIB in these mice. Addressing these issues may help clarify the redundancy of BMPR-II with other type II receptors in vivo.
The BMP and TGF-β signaling pathways share a common ALK activity in the type I receptors, and there is cross-talk between type I and II receptors responsible for each pathway (Persson et al., 1997). Unlike the BMP signaling pathway that has 3 type II receptors, the TGF-β signaling pathway has only one type II receptor (TβR-II). Recent studies of TβR-II knockout mice have shown that deletion of TβR-II in osteoblasts leads to a high bone mass phenotype by regulating PTH activity (Qiu et al., 2010), suggesting that type II TGF-β receptor and type II BMP receptors have distinguishing functions in bone homeostasis. We examined the effects of dnBMPR-II/dnActR-IIB on TGF-β action in osteoblasts to test the specificity of the redundancy of the type II BMP receptors. The data have shown that the double blockage of type II receptors does not affect TGF-β signaling activity and TGF-β-mediated osteoblast proliferation (Fig. 4A, B), evidencing that the functional redundancy between BMPR-II and ActR-IIB is BMP pathway specific in osteoblast cells. This concept also was verified by the examination of effects of aFGF on osteoblast proliferation (Fig. 4C).
Type II activin receptors (ActR-II and ActR-IIB) bind not only with BMP ligands, but also bind with activin ligands to mediate receptor signaling. The lack of overlap between phenotypes of ActR-II-deficient, ActR-IIB-deficient (Matzuk et al., 1995a; Oh and Li, 1997, 2002; Ferguson et al., 2001; Oh et al., 2002), and activin-deficient (Vassalli et al., 1994; Matzuk et al., 1995b) mice is evidence that the BMP and activin signaling pathways may be involved in diverse activities through type II activin receptors during embryonic development. Although BMP2 has been characterized to have anabolic effects in inducing osteoblast differentiation, it is unknown whether activin growth factors are essential for osteoblasts.
In this study, we found that treatment with activin A did not have any stimulatory effects on osteoblast differentiation (Fig. 7). These data provide further evidence for the activity of BMP signaling through the BMPR-II/ActR-IIB pathway in promoting osteoblast differentiation.
Acknowledgments
The authors would like to acknowledge Dr. Joan Massagué and Dr. Jeffrey Wrana for kindly providing BMPR-II and ActR-IIB cDNAs, and Smad1 expression vectors, respectively. They also thank Mr. Carl Richmond for editing the article.
Literature Cited
- Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Beppu H, Malhotra R, Beppu Y, Lepore JJ, Parmacek MS, Bloch KD. BMP type II receptor regulates positioning of outflow tract and remodeling of atrioventricular cushion during cardiogenesis. Dev Biol. 2009;331:167–175. doi: 10.1016/j.ydbio.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo J, Zentella A, Massagué J. Disruption of transforming growth factor beta signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol Cell Biol. 1995;15:1573–1581. doi: 10.1128/mcb.15.3.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bhushan A, Vale W. Smad8 mediates the signaling of the ALK-2 [corrected] receptor serine kinase. Proc Natl Acad Sci USA. 1997;94:12938–12943. doi: 10.1073/pnas.94.24.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Heikinheimo K, Nomura M, Oh P, Li E, Sharpe PT. The role of effectors of the activin signalling pathway, activin receptors IIA and IIB, and Smad2, in patterning of tooth development. Development. 2001;128:4605–4613. doi: 10.1242/dev.128.22.4605. [DOI] [PubMed] [Google Scholar]
- Garimella R, Kacena MA, Tague SE, Wang J, Horowitz MC, Anderson HC. Expression of bone morphogenetic proteins and their receptors in the bone marrow megakaryocytes of GATA-1(low) mice: A possible role in osteosclerosis. J Histochem Cytochem. 2007;55:745–752. doi: 10.1369/jhc.6A7164.2007. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Windle JJ, Koop BA, Harris MA, Guerrero DL, Wozney JM, Mundy GR, Harris SE. Immortalized murine osteoblasts derived from BMP 2-T-antigen expressing transgenic mice. Endocrinology. 1996;137:331–339. doi: 10.1210/endo.137.1.8536632. [DOI] [PubMed] [Google Scholar]
- Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988;123:98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- Gosain AK, Song LS, Santoro T, Weihrauch D, Bosi BO, Corrao MA. Effects of transforming growth factor-beta and mechanical strain on osteoblast cell counts: An in vitro model for distraction osteogenesis. Plast Reconstr Surg. 2000;105:130–136. doi: 10.1097/00006534-200001000-00022. [DOI] [PubMed] [Google Scholar]
- Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Chytil A, Moses HL. Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J Biol Chem. 1995;270:5625–5630. doi: 10.1074/jbc.270.10.5625. [DOI] [PubMed] [Google Scholar]
- Kloen P, Doty SB, Gordon E, Rubel IF, Goumans MJ, Helfet DL. Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am. 2002;84:1909–1918. doi: 10.2106/00004623-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3cells. Mol Cell Biol. 1994;14:5961–5674. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnerdt G, Metz KA, Trellakis S, Jahnke K, Neumann A. Signaling by way of type IB and II bone morphogenetic protein receptors regulates bone formation in otospongiosis. Laryngoscope. 2007;117:812–816. doi: 10.1097/MLG.0b013e31803300a2. [DOI] [PubMed] [Google Scholar]
- López-Rovira T, Chalaux E, Massagué J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995a;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995b;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Lesot H. Expression patterns of BMPRs in the developing mouse molar. Cell Tissue Res. 2006;324:33–40. doi: 10.1007/s00441-005-0120-1. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345:319–324. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. Gene-dosage-sensitive genetic interactions between inversus viscerum (iv), nodal, and activin type IIB receptor (ActRIIB) genes in asymmetrical patterning of the visceral organs along the left-right axis. Dev Dyn. 2002;224:279–290. doi: 10.1002/dvdy.10103. [DOI] [PubMed] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749–2754. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Nifuji A, Maeda Y, Amagasa T, Noda M. Spaciotemporal association and bone morphogenetic protein regulation of sclerostin and osterix expression during embryonic osteogenesis. Endocrinology. 2004;145:4685–4692. doi: 10.1210/en.2003-1492. [DOI] [PubMed] [Google Scholar]
- Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, Sampath TK, ten Dijke P, Sakou T. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–612. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- Perron JC, Dodd J. ActRIIA and BMPRII Type II BMP receptor subunits selectively required for Smad4-independent BMP7-evoked chemotaxis. PLoS One. 2009;4:e8198–e8198. doi: 10.1371/journal.pone.0008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U, Souchelnytskyi S, Franzén P, Miyazono K, ten Dijke P, Heldin CH. Transforming growth factor (TGF-beta)-specific signaling by chimeric TGF-beta type II receptor with intracellular domain of activin type IIB receptor. J Biol Chem. 1997;272:21187–21194. doi: 10.1074/jbc.272.34.21187. [DOI] [PubMed] [Google Scholar]
- Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol. 2010;12:224–234. doi: 10.1038/ncb2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan SB, Wesolowski G, Thomas K, Rodan GA. Growth stimulation of rat calvaria osteoblastic cells by acidic fibroblast growth factor. Endocrinology. 1987;121:1917–1923. doi: 10.1210/endo-121-6-1917. [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J Biol Chem. 2009;284:15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- van der Horst G, van Bezooijen RL, Deckers MM, Hoogendam J, Visser A, Löwik CW. Differentiation of murine preosteoblastic KS483cells depends on autocrine bone morphogenetic protein signaling during all phases of osteoblast formation. Bone. 2002;31:661–669. doi: 10.1016/s8756-3282(02)00903-1. [DOI] [PubMed] [Google Scholar]
- van der Zande M, Walboomers XF, Briest A, Springer M, Alava JI, Jansen JA. The effect of combined application of TGFbeta-1, BMP-2, and COLLOSS E on the development of bone marrow derived osteoblast-like cells in vitro. J Biomed Mater Res. 2008;86:788–795. doi: 10.1002/jbm.a.31645. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Wang H, Li W, Zhang W, Sun K, Song X, Gao S, Zhang C, Hui R, Hu H. Novel promoter and exon mutations of the BMPR2 gene in Chinese patients with pulmonary arterial hypertension. Eur J Hum Genet. 2009;17:1063–1069. doi: 10.1038/ejhg.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yagi K, Tsuji K, Nifuji A, Shinomiya K, Nakashima K, DeCrombrugghe B, Noda M. Bone morphogenetic protein-2 enhances osterix gene expression in chondrocytes. J Cell Biochem. 2003;88:1077–1083. doi: 10.1002/jcb.10467. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang L, Wan M, Cao X. Generation of a mouse model with expression of bone morphogenetic protein type II receptor lacking the cytoplasmic domain in osteoblasts. Ann NY Acad Sci. 2010;1192:86–91. doi: 10.1111/j.1749-6632.2009.05248.x. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Betchel KP, Lee JC. Inhibition of BMP receptor synthesis by antisense oligonucleotides attenuates OP-1 action in primary cultures of fetal rat calvaria cells. J Bone Miner Res. 1998;13:1870–1879. doi: 10.1359/jbmr.1998.13.12.1870. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Zavala MC, Lee JC. Osteogenic protein-1 and interleukin-6 with its soluble receptor synergistically stimulate rat osteoblastic cell differentiation. J Cell Physiol. 2002;190:322–331. doi: 10.1002/jcp.10064. [DOI] [PubMed] [Google Scholar]
- Ying SX, Hussain ZJ, Zhang YE. Smurf1 facilitates myogenic differentiation and antagonizes the bone morphogenetic protein-2-induced osteoblast conversion by targeting Smad5 for degradation. J Biol Chem. 2003;278:39029–39036. doi: 10.1074/jbc.M301193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori K, Imamura T, Ishidou Y, Okano T, Matsunaga S, Yoshida H, Kato M, Sampath TK, Miyazono K, ten Dijke P, Sakou T. Bone morphogenetic protein receptors and activin receptors are highly expressed in ossified ligament tissues of patients with ossification of the posterior longitudinal ligament. Am J Pathol. 1997;150:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem. 2008;283:3877–3888. doi: 10.1074/jbc.M706797200. [DOI] [PubMed] [Google Scholar]
- Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Ko SY, Liu JH, Chen D, Zhang J, Wang B, Harris SE, Oyajobi OB, Mundy GR. Inhibition of microtubule assembly in osteoblasts stimulates bone morphogenetic protein 2 expression and bone formation through transcription factor Gli2. Mol Cell Biol. 2009;29:1291–1305. doi: 10.1128/MCB.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Mundy GR, Oyajobi BO, Padalecki SS, Sterling JA, Elefterious F. Bone morphogenetic proteins - cytokines and bone remodeling. In: Marcus B, Kelsey J, Feldman D, editors. Osteoporosis. 3rd edition Academic Press; New York: 2008. pp. 503–509. [Google Scholar]
- Zhao M, Qiao M, Harris SE, Chen D, Oyajobi BO, Mundy GR. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 expression in osteoblasts in response to hedgehog signaling. Mol Cell Biol. 2006;26:6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Qiao M, Oyajobi BO, Mundy GR, Chen D. E3 ubiquitin ligase Smurf1 mediates core-binding factor alpha1/Runx2 degradation and plays a specific role in osteoblast differentiation. J Biol Chem. 2003;278:27939–27944. doi: 10.1074/jbc.M304132200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]