Abstract
Background
Natural orifice transluminal endoscopic surgery (NOTES) mediastinoscopy (MED) through the esophagus has proved to be feasible in the animal model. However, injury of the adjacent pleura and pneumothorax has been reported as a frequent adverse event when using a blind access.
Objective
To assess the utility and safety of a CT-based image registration system (IRS) for navigation in the mediastinum.
Design
Prospective, randomized, controlled trial in 30 Yorkshire pigs. Thirty-minute MEDs were performed: 15 MEDs were performed with IRS guidance (MED-IRS), and 15 MEDs were performed with a blind access.
Setting
Animal research laboratory.
Interventions
In both groups, the mediastinum was accessed through a 10-cm submucosal tunnel in the esophageal wall. Timed exploration was performed with identification of 8 mediastinal structures.
Main Outcome Measurements
Technical feasibility, adverse events, and the number of mediastinal structures identified.
Results
Thirty animals weighing 31.5 ± 3.5 kg were included in this study. MED was not possible in 2 animals in the “MED with blind access” group but was possible in all MEDs performed with IRS. The mean number of identified organs was slightly higher in “with IRS-MED” (6.13 ± 1.3) than with MED with blind access (4.7 ± 2.3; P = .066). Moreover, the right atrium and vena cava were identified in more cases with IRS-MED than in MED with blind access (13 vs 3 and 15 vs 11, P = .000 and P = .03, respectively). There were 3 (23%) adverse events with IRS-MED and 4 (27%) with “MED with blind access” (P = not significant), with pneumothorax being the most frequent (2 and 3, respectively).
Limitations
Nonsurvival animal study.
Conclusions
This study demonstrates that the IRS system appears feasible in natural orifice transluminal endoscopic surgery MED and suggests that IRS guidance might be useful for selected procedures.
Natural orifice transluminal endoscopic surgery (NOTES) has been demonstrated to be a feasible approach to the mediastinum,1 and it has been suggested that the benefits of NOTES access to the mediastinum or thoracic cavity through the esophagus might be even greater than NOTES peritoneal access.2 Conventional video-assisted mediastinoscopy (MED) is associated with adverse events, some of them fatal (vessel injury and bleeding, bronchial rupture, recurrent laryngeal nerve lesions),3–5 and access to the posterior mediastinum and hilar regions is limited. Thoracic NOTES procedures could then be preferable to conventional video-assisted MED, especially for patients with cervical stiffness or a tracheotomy.
Previous reports on NOTES in the mediastinum focused on demonstrating the feasibility of certain procedures such as lymphadenectomy, vagotomy, esophagectomy, and pericardial window.6,7 However, the accuracy of systematic transesophageal endoscopic mediastinal exploration has never been evaluated. Injury of mediastinal organs has been reported in most of the transesophageal MED studies reported,8–10 and the use of EUS has been proposed to improve the safety of this technique. Our group recently showed that CT-based image registration system (IRS) navigation is feasible in NOTES peritoneoscopy and may be superior to conventional NOTES in efficiency and accuracy of probe positioning. Moreover, IRS-guided access might reduce the risk of adverse events.11 However, there are no reports on the feasibility of image-guided systems in transesophageal NOTES. The introduction of this technology in the mediastinum has the potential to improve procedure performance and to reduce the invasiveness of the procedure.
The aim of this study was to assess the utility and safety of a CT-based IRS navigation system to perform a systematic exploration of the mediastinum through transesophageal NOTES access.
METHODS
Animals
Experiments were performed in 30 female Yorkshire pigs weighing 27 to 35 kg. The studies were approved by the Animal Research Committee at the University of Barcelona. Animals underwent a 2-day quarantine and acclimation period during which they were individually caged and fed the same diet and had unlimited access to water. All procedures were performed with the pigs under general anesthesia by using desflurane and tracheal intubation.
Study protocol
A total of 30 NOTES MEDs were performed through the esophagus. The animals were randomized into 2 groups by using random numbers: NOTES with IRS guidance (IRS-MED, n = 15) and conventional NOTES without IRS guidance (MED, n = 15). Lymph nodes were retrieved for pathological study. After the procedure, necropsy was immediately performed.
All the experiments were performed by 1 endoscopist with the support of an on-site expert radiologist, both with previous experience with IRS guidance in mediastinum and abdomen.
The primary outcome of the study was the rate of adverse events; the secondary outcome measurement was the number of organs identified.
Image registration system
The IRS is a real-time guidance system with 1 synthetic display driven by the position and orientation of the endoscope tip. The display shows a 3-dimensional (3D) anatomic model of the anatomy derived from a volumetric CT dataset, and the tip of the tracked endoscope provides contextual information on its position with respect to the overall anatomy. The IRS uses established techniques for the visualization of the probe position and image registration but implements them in real time by using an electromagnetic tracker (3D Guidance trakSTAR; Ascension Technology Corp, Burlington, Vt) with a mid-range transmitter and a miniaturized sensor (2-mm outer diameter). The tracker system has been tested to meet International Electrotechnical Commission 60601-01 standards; it is easily sterilized and has been used in human subjects by our team. The electromagnetic sensor is securely attached to the tip of the endoscope by using Tegaderm and Steri-strips.
For these studies, the volumetric data were collected by using a Siemens Sensation 64-slice system (Siemens Medical Systems, Erlangen, Germany). Early arterial and venous scan phases were acquired by using 120 kV(p) and 200 mA. We used the bolus-tracking method at the descending aorta level and set 100 Hounsfield units as the triggering threshold for the arterial phase. The venous phase scan was taken 50 seconds after completing the arterial phase. Contrast agent (120 mL) (Ultravist; Bayer, St. Joan Despí, Spain) was injected by using a power injector at the rate of 3 mL/s. The images were reconstructed in a 512 × 512 matrix with a B30f reconstruction kernel and 1-mm slice thickness. The synthetic 3D models of reference anatomy were then created by using a semiautomatic approach12 by using the open-source image analysis software 3D slicer (www.slicer.org). Anatomic models were made for the rib cage, spine, lungs, trachea, heart, aorta, vena cava, pulmonary artery, pulmonary vein, brachiocephalic vein, and porcine bronchium.
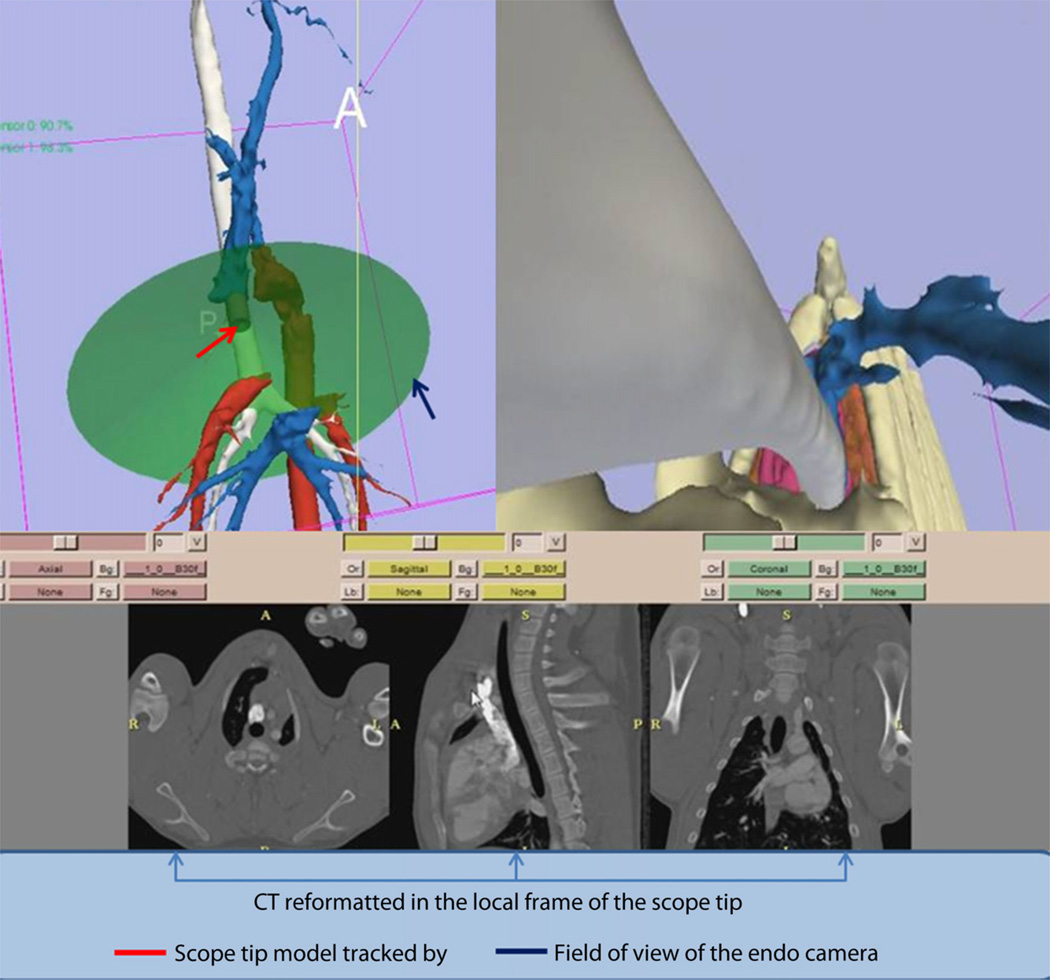
The IRS display was based on the display previously reported for peritoneal NOTES11 and was composed of 2 views: an external view of the anatomy and a virtual endoscopic view (Fig. 1). The external 3D view shows the tracked tip of the endoscope with respect to the displayed anatomy. The virtual endoscopic view shows the 3D anatomy from the viewpoint of the endoscope camera as a complement to the real endoscopic view.
Figure 1.
Screen capture of the image registration system–guided mediastinoscopy that was displayed during the guided procedures. The upper left part of the screen shows a 3-dimensional display of the general anatomy and the location of the endoscope tip with respect to the rendered organs. The upper right part of the screen shows the virtual endoscopic image that is generated from the structures extracted from preoperative CT. The lower part of the screen shows the CT reformatted in the local frame with respect to the endoscope tip.
The preoperative images were registered to the coordinate system defined by the electromagnetic transmitter by using a continuous set of points acquired from the skin surface by using a separate electromagnetic sensor. Those points were aligned with the model of the skin by using the iterative closest point algorithm13 to yield a rigid body transformation. The accuracy and reproducibility of the endoscope tip measurements were previously reported.11
NOTES MED
A standard flexible gastroduodenoscope (GIF Q160; Olympus Europe, Hamburg, Germany) was used. Reusable endoscopic tools used throughout the procedure included conventional needle-knives, endoscopic forceps, hot biopsy forceps, and sclerosis needle.
The endoscope was inserted through the pig’s mouth, and the esophageal lumen was secured with an overtube. The technique of submucosal tunneling was used for accessing the mediastinum, as described elsewhere.14 In summary, at 25 cm from the incisors and by using the trachea as an anatomic reference in the blinded access or IRS guided, the site of interest in the esophagus was located and 5 mL of normal saline solution were injected to create a submucosal cushion. After the injection, a superficial 10-mm incision was created in the mucosal layer of the esophagus with a needle-knife. Then the endoscope was introduced into the submucosal space and was used for controlled blunt dissection to create a 10-cm long submucosal tunnel. At the end of the tunnel, a full-thickness incision was created through the muscularis propria with the needle-knife, and the endoscope was inserted in the posterior mediastinum. Once the endoscope was placed in the pretracheal space, dissection with endoscopic forceps was used to advance the endoscope until the carina was identified. Pneumomediastinum was maintained with CO2 insufflation through the endoscope. MED consisted of timed chest exploration (30 minutes) with identification of 8 predetermined structures in the following order: carina, right pulmonary artery, right atrium, vena cava, porcine bronchium, brachiocephalic vein, right vagus nerve, and lung. Systematic sampling of stations 2R, 2L, 4R, 4L, and 7 was carried out.
The animals were killed immediately after the procedure by using intravenous intracardiac pentobarbital and potassium chloride.
Statistical analysis
The sample size was calculated to achieve 90% power to detect a difference in the incidence of adverse events of 20% and an α value of .05. The calculation resulted in a required sample size of 15 swine in each group (a total of 30 swine).
All continuous variables were expressed as mean ± 1 standard deviation or as range and median where appropriate. The times of the procedure were expressed as range (mean), and quantitative variables were compared with a nonparametric Mann-Whitney U test or χ2 test as appropriate.
P < .05 was considered statistically significant. All analyses were performed with SPSS for Windows, version 17.0 (SPSS Inc, Chicago, Ill).
RESULTS
Thirty animals weighing 31.5 ± 3.5 kg were included in this study. Twenty-eight procedures were completed (15 IRS-MED, 13 MED with blind access). MED was not possible in 2 cases: in 1 animal because the mediastinum could not be reached because of technical difficulties and in the second, a bilateral pneumothorax was created with hemodynamic and respiratory repercussion and the animal was euthanized.
Characteristics of the procedures are described in detail in Table 1. The mean time for accessing the mediastinum was no different between groups (MED with blind access: 8.4 ± 4 minutes; IRS-MED: 7.13 ± 1.8 minutes; P = .46), and submucosal tunnels were created without any adverse event. The mean number of identified structures was slightly higher in IRS-MED (6.13 ± 1.3) than in MED with blind access (4.67 ± 2.3) (P = .06). Moreover, the right atrium and vena cava were identified in more cases with IRS-MED than with MED with blind access (13 vs 3 and 15 vs 11, P = .000 and P = .03, respectively) (Table 2, Fig. 2). The number of lymph nodes identified and confirmed by histology was higher with MED with blind access (9 lymph nodes) than with IRS-MED (1 lymph node) (Table 1).
TABLE 1.
Characteristics of the procedures performed with IRS-MED and blind MED
| IRS-MED (n = 15) |
Blind MED (n =15) |
P value |
|
|---|---|---|---|
| Weight, kg, mean ± SD | 32.04 ± 4.4 | 30.9 ± 2.3 | .77 |
| Incision time, mean (SD), minutes | 7.13 ± 1.8 | 8.4 ± 4.0 | .46 |
| Total no. of MEDs | 15 | 13 | .14 |
| Visualized structures, mean ± SD | 6.13 ± 1.3 | 4.67 ± 2.32 | .06 |
| No. of lymph nodes retrieved | 1 | 9 | .003 |
| Procedural adverse events, no. (%) | 3 (20) | 4 (27) | .52 |
IRS-MED, Image registration system–guided mediastinoscopy; MED, mediastinoscopy.
TABLE 2.
Identification of mediastinal structures during natural orifice transluminal endoscopic surgery
| IRS-MED (n = 15) |
MED (n = 15) |
P value |
|
|---|---|---|---|
| Carina | 15 (100) | 13 (87) | .14 |
| Right pulmonary artery | 15 (100) | 13(87) | .14 |
| Vena cava | 15 (100) | 11 (73) | .03 |
| Right atrium | 13 (87) | 3 (20) | .001 |
| Porcine bronchium | 12 (80) | 11(73) | .66 |
| Right vagus nerve | 9 (60) | 7 (47) | .46 |
| Lung | 8 (53) | 6 (40) | .46 |
| Brachiocephalic vein | 5 (33) | 7 (47) | .46 |
Values shown are number (%).
IRS-MED, Image registration system–guided mediastinoscopy; MED, mediastinoscopy.
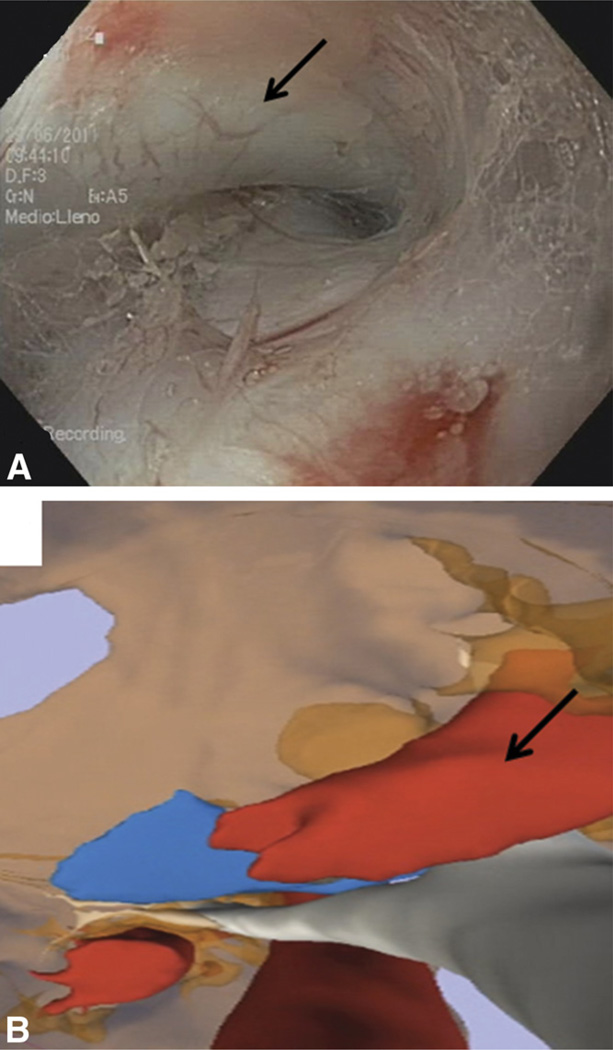
Figure 2.
Endoscopic (A) and virtual endoscopic (B) views of the carina and right pulmonary artery (arrows).
There were 3 adverse events (20%) with IRS-MED (2 pneumothorax and 1 chylothorax) and 4 (27%) with MED with blind access (3 pneumothorax and 1 hypotension) (P = .52). Four of 5 cases of tension pneumothorax were relieved by insertion of a percutaneous catheter. The chylothorax was produced by thoracic lymphatic duct lesion with fluid leakage. Hypotension was resolved with usual measures.
DISCUSSION
One of the major challenges in transesophageal NOTES procedures is to access the mediastinum without adverse events. Because the posterior mediastinum is located near the esophagus, some vital structures such as the heart and pulmonary trunk can be easily damaged. In initial reports published, hemorrhage and pneumothorax were fre- quently described.6,15 Therefore, the use of EUS (which can visualize structures beyond the esophageal wall) has been proposed to minimize the risk of adverse events, but no other alternative methods have been evaluated.16
Image navigation systems are usually used in otorhinolaryngology, neurosurgery, and interventional cardiology, and its usefulness has also been demonstrated when performing guided therapies in the abdomen and mediastinum. 17,18 This is the first study that uses an IRS not only to select the best point of access but also to guide the navigation of the endoscope inside the mediastinum. The use of the IRS increased the complexity of the surgical procedure but was feasible and reduced the number of adverse events (not significantly) compared with the unguided MED; 2 planned surgeries were not possible because of iatrogenic events.
Another potential expected advantage of a guidance system is to improve the number of structures identified. Our study included only a timed MED, and this lack of complexity could explain why the mean number of identified organs was similar between groups. However, the use of the IRS improved the detection of the right atrium and superior vena cava, suggesting that this system may be helpful when targeting these structures. The logical next step would be to assess the performance of the IRS during more complex surgeries or when targeting difficult structures. Because of the greater availability of EUS, a comparative study between IRS and EUS (transesophageal and/or transbronchial) would also be warranted.
The issue of endoscope orientation outside the GI lumen was previously addressed by our group in a study in the peritoneal cavity,11 and we concluded that guidance may be useful for supporting navigation with an increased smoothness of motion. Although the benefit of a guidance system might be considered less relevant in the narrow mediastinal space, the use of the IRS had a positive effect on the endoscopists who felt more confident when working in the mediastinum. Moreover, the introduction of sophisticated technology in the laboratory did not have a negative impact on efficiency.
The IRS approach is more robust and stable in MED for explorations compared with retroperitoneal NOTES. The registration step was more reliable and easy to perform because of the stability of the thoracic cavity, which is used as a reference for the registration. The correspondence between the structures seen with the virtual endoscopic view and the endoscopic camera was good for all the cases in which IRS-MED was performed.
This study confirms the applicability of the transesophageal submucosal tunnel technique and produces a thorough examination of the mediastinal compartment. In this animal model, the tracheal tube did not disturb the cre- ation of the tunnels, but this point should be taken into consideration when performing this technique in humans. We found that blunt forceps dissection was sufficient to get an excellent view of the mediastinum. However, the stiffness of the forceps could explain the high frequency of pneumothorax, an adverse event that could be avoided with the use of dedicated instruments such as a flexible dissector.
Although EUS has been used in other studies to perform a lymphadenectomy, this is not a required condition, as demonstrated in our study. Mediastinal lymphadenectomy was easily accomplished by using standard endoscopic forceps, and lymph nodes were retrieved for pathological examination. The number of lymph nodes identified with the IRS was significantly lower than in blind MEDs. The fact that in guided MEDs we only tried to retrieve the lymph nodes visible on previous CT imaging could explain this finding. It is well-known that malignant lymph nodes can be underdiagnosed by CT because of the criteria used that relies on the size of the node.19
Several limitations must be addressed. These experiments were conducted in an animal model, with the animals breathing. Moreover, porcine anatomy differs quite substantially from that of humans, making the pig a suboptimal model for determining immediate clinical applicability. Another limitation of the study is the small number of animals included.
In conclusion, the IRS appears feasible in mediastinal NOTES and could be superior to conventional NOTES in selected procedures. Pneumothorax is the most common adverse event when the exploration is performed with flexible instruments not designed specifically for this purpose.
Take-home Message.
The image registration system appears feasible in mediastinal natural orifice transluminal endoscopic surgery (NOTES) and could be superior to conventional NOTES in selected procedures.
Pneumothorax is the most common adverse event when exploration is performed with flexible instruments not designed specifically for this purpose.
Acknowledgments
This research project was supported by a grant from the Ministerio de Ciencia e Innovación (SAF2010-15635). Dr Córdova was supported by the Instituto Nacional de Salud Carlos III. Dr San José Estepar was supported by NIH grant K25 HL104085. Dr Vosburgh was supported by the Center for Integration of Medicine and Innovative Technology and the National Center for Image Guided Therapy, NIH grant P41 RR019703.
Abbreviations
- 3D
3-dimensional
- IRS
image registration system
- MED
mediastinoscopy
- NOTES
natural orifice transluminal endoscopic surgery
Footnotes
DISCLOSURE: All authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Turner BG, Gee DW, Cizginer S, et al. Endoscopic transesophageal mediastinal lymph node dissection and en bloc resection by using mediastinal and thoracic approaches (with video) Gastrointest Endosc. 2010;72:831–835. doi: 10.1016/j.gie.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritscher-Ravens A, Patel K, Ghanbari A, et al. Natural orifice transluminal endoscopic surgery (NOTES) in the mediastinum: long-term survival animal experiments in transesophageal access, including minor surgical procedures. Endoscopy. 2007;39:870–875. doi: 10.1055/s-2007-966907. [DOI] [PubMed] [Google Scholar]
- 3.Leschber G, Sperling D, Klemm W, et al. Does video-mediastinoscopy improve the results of conventional mediastinoscopy? Eur J Cardiothorac Surg. 2008;33:289–293. doi: 10.1016/j.ejcts.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;16:2152–2165. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 5.Krasna MJ, Deshmukh S, McLaughlin JS. Complications of thoracoscopy. Ann Thorac Surg. 1996;61:1066–1069. doi: 10.1016/0003-4975(96)00021-5. [DOI] [PubMed] [Google Scholar]
- 6.Fritscher-Ravens A, Cuming T, Olagbaiye F, et al. Endoscopic transesophageal vs. thoracoscopic removal of mediastinal lymph nodes: a prospective randomized trial in a long term animal survival model. Endoscopy. 2011;43:1090–1096. doi: 10.1055/s-0030-1256768. [DOI] [PubMed] [Google Scholar]
- 7.Woodward T, McCluskey D, Wallace MB, et al. Pilot study of transesophageal endoscopic surgery: NOTES esophagomyotomy, vagotomy, lymphadenectomy. J Laparoendosc Adv Surg Tech A. 2008;18:743–745. doi: 10.1089/lap.2008.0226. [DOI] [PubMed] [Google Scholar]
- 8.Fritscher-Ravens A, Cuming T, Eisenberger CF, et al. Randomized comparative long-term survival study of endoscopic and thoracoscopic esophageal wall repair after NOTES mediastinoscopy in healthy and compromised animals. Endoscopy. 2010;42:468–474. doi: 10.1055/s-0029-1244019. [DOI] [PubMed] [Google Scholar]
- 9.von Delius S, Wilhelm D, Feussner H, et al. Natural orifice transluminal endoscopic surgery: cardiopulmonary safety of transesophageal mediastinoscopy. Endoscopy. 2010;42:405–412. doi: 10.1055/s-0029-1243948. [DOI] [PubMed] [Google Scholar]
- 10.Gee DW, Willingham FF, Lauwers GY, et al. Natural orifice transesophageal mediastinoscopy and thoracoscopy: a survival series in swine. Surg Endosc. 2008;22:2117–2122. doi: 10.1007/s00464-008-0073-z. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Esparrach G, San José Estépar R, Guarner-Argente C, et al. The role of a computed tomography-based image registered navigation system for natural orifice transluminal endoscopic surgery: a comparative study in a porcine model. Endoscopy. 2010;42:1096–1103. doi: 10.1055/s-0030-1255824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.San José Estépar R, Stylopoulos N, Ellis RE, et al. Towards scarless surgery: an endoscopic-ultrasound navigation system for transgastric access procedures. Comput Aided Surg. 2007;12:311–324. doi: 10.3109/10929080701746892. [DOI] [PubMed] [Google Scholar]
- 13.Besl PJ, McKay HD. A method for registration of 3-D shapes. IEEE Transactions on pattern analysis and machine intelligence. 1992;12:239–256. [Google Scholar]
- 14.Yoshizumi F, Yasuda K, Kawaguchi K, et al. Submucosal tunneling using endoscopic submucosal dissection for peritoneal access and closure in natural orifice transluminal endoscopic surgery: a porcine survival study. Endoscopy. 2009;41:707–711. doi: 10.1055/s-0029-1214959. [DOI] [PubMed] [Google Scholar]
- 15.Fritscher-Ravens A, Cuming T, Jacobsen B, et al. Feasibility and safety of endoscopic full-thickness esophageal wall resection and defect closure: a prospective long-term survival animal study. Gastrointest Endosc. 2009;69:1314–1320. doi: 10.1016/j.gie.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Fritscher-Ravens A, Ghanbari A, Cuming T, et al. Comparative study of NOTES alone vs. EUS-guided NOTES procedures. Endoscopy. 2008;40:925–930. doi: 10.1055/s-2008-1077732. [DOI] [PubMed] [Google Scholar]
- 17.Abi-Jaoudeh N, Gossop N, Dake M, et al. Electromagnetic navigation for thoracic aortic stent graft deployment: a pilot study in swine. J Vasc Interv Radiol. 2012;21:888–895. doi: 10.1016/j.jvir.2009.12.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller SA, Maier-Hein L, Tekbas A, et al. Navigated liver biopsy using a novel soft tissue navigation system versus CT-guided liver biopsy in a porcine model: a prospective randomized trial. Acad Radiol. 2010;17:1282–1287. doi: 10.1016/j.acra.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Billé A, Pelosi E, Skanjeti A, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440–445. doi: 10.1016/j.ejcts.2009.04.003. [DOI] [PubMed] [Google Scholar]