Abstract
Background
In spite of some dissociation between muscle mass and strength, muscle strength is often used as a proxy to identify individuals with low muscle mass (sarcopenia). Thus, the aim of the present study was to investigate the relationship between muscle strength and the appendicular lean body mass index (app LBMI).
Methods
One hundred and five individuals were recruited. Knee extension and handgrip strength were measured. Body composition was assessed by DXA. App LBMI was calculated as appendicular lean body mass divided by height squared.
Results
At le level of the entire cohort, both handgrip (r = 0.73; p < 0.001) and knee extension strength (r = 0.57; p < 0.001) were associated with app LBMI. However, in women, knee extension strength (r = 0.32; p < 0.05) but not handgrip strength (r = 0.14; p = 0.35) was associated with app LBMI; while in men, handgrip strength (r = 0.43; p < 0.01) but not knee extension strength (r = 0.27; p = 0.09) was associated with app LBMI.
Conclusions
Muscle strength appears to be associated with lean body mass; however, handgrip strength may be preferentially used in men and knee extension strength in women to detect sarcopenic individuals. Future larger studies are now needed to confirm our findings and their clinical relevance.
Keywords: Sarcopenia, Muscle mass, Muscle strength, Older adults
Introduction
Sarcopenia, the age-related loss of muscle mass, is one of the most striking changes associated with advancing age. This loss of muscle mass is thought to result in decreased muscle strength, and consequently, negatively impact functional capacity/health. The interrelationship between these concepts led to the expansion of the definition of sarcopenia to include muscle strength, as illustrated by the European consensus proposed by the European Working Group on Sarcopenia in Older People (EWGSOP) [1]. In its broadest sense, sarcopenia has been associated with functional decline and disability [2–5], but also with increased mortality [6]. Furthermore, it appears that individuals with sarcopenia are twice likely to contract infection during a hospital stay than older patients with a normal skeletal muscle mass [7], suggesting a decreased immunity in these individuals. Ultimately, in 2000, the estimated direct healthcare cost attributable to sarcopenia in the USA was $18.5 billion, which represented about 1.5 % of total healthcare expenditures [8].
Diagnosis of sarcopenia is most of the time based on muscle mass or lean mass indexes. Depending on the context (clinical vs. research), a wide range of techniques can be used to assess muscle mass, from anthropometric measurements to MRI. One of the most commonly used indexes is the appendicular lean body mass index (App LBMI) which is calculated by dividing appendicular lean body mass (in kilogram) by height squared (in meter squared). Heymsfield et al. [9] first demonstrated that the assessment of appendicular lean body mass (by dual-energy X-ray absorptiometry (DXA)) was a highly accurate method of quantifying human skeletal muscle mass while being safe and accessible. Based on the calculation of body mass index (weight (kg)/height2 (m2)), Baumgartner et al. [2] then proposed to control appendicular lean body mass for height because of their strong relationship. Height squared appeared as the best denominator for minimizing the correlation of the index with height across all sex, ethnic, and age groups.
However, as suggested by the EWGSOP, muscle strength is a measurable variable of sarcopenia that may also be used to help identify sarcopenic individuals. Indeed, because of the role played by muscle strength in the relationship between muscle mass and functional disabilities, muscle strength is often used as a proxy to assess muscle mass. Among the measurements of muscle strength, measurements of handgrip and knee extension strength are particularly popular. The former, which is strongly related with lower extremity muscle function and a clinical marker of mobility [5], is particularly recognized for its convenience. On the other hand, knee extension strength is known to be more closely related to physical function, but its measurement in clinical practice is limited by the need for special equipment and training [1].
The belief that muscle strength is effective in identifying sarcopenic individuals is based on their common relationship with physical function rather than on a strong relationship between muscle mass indexes and strength. For instance, there is accumulating evidence that the relationship between muscle mass and strength is highly variable, which led to the recent distinction of these two parameters in the literature [10]. Moreover, few studies have considered possible sex differences in this relationship. This study attempts to answer this question by investigating the association between muscle strength and App LBMI in both men and women, separately. Furthermore, in order to better understand this association, we examined the relationship between muscle strength and total muscle mass, but also with different regional muscle mass measurements.
Methods
Participants
One hundred and five Caucasian individuals (53 women and 52 men) aged between 50 and 75 years were recruited among registered members of the YMCAs of Montreal. To be included in the study, the participants had to meet the following criteria: no history of CVD and diabetes and no medication that could influence metabolism (except hormonal therapy). For the present paper, participants with other comorbidities (such as CKD, cancer, or depression) were also excluded from the analyses, so that the 105 participants were all healthy. All procedures were approved by the ethics committee of the Department of Kinanthropology of the University of Quebec at Montreal. All participants were fully informed about the nature, goal, procedures, and risks of the study and gave their informed consent.
Study procedure
After screening, for the aforementioned inclusion criteria, participants were invited to visit their YMCA center for a muscle strength (handgrip and knee extension strength) assessment. They were then invited for a visit to the Department of Kinanthropology at the University of Quebec at Montreal where their body composition was assessed by DXA.
Body composition
Body weight (BW) was determined using an electronic scale (Omron HBF-500CAN, USA). Lean body mass (LBM) and fat mass were evaluated by DXA (General Electric Lunar Corporation version 6.10.019, Madison, USA) (Fig. 1). Forearm lean body mass was estimated by manually defining an area from the wrist to the elbow joint. Arm lean body mass was estimated by manually defining an area from the shoulder to the elbow joint. Thigh lean body mass was estimated by manually defining an area from the basin to the knee joint. Measures were considered usable only if the parts were perfectly distinguishable from each other. Height was measured using a stadiometer (Seca, Hanover, MD) affixed to the wall. Waist circumference was measured to the nearest 0.5 cm using a nonelastic plastic tape, with the participant standing upright. Body mass index [BMI = Body weight/Height (m2)] and appendicular lean body mass index [AppLBMI = AppLBM/Height (m2)] were also calculated.
Fig. 1.
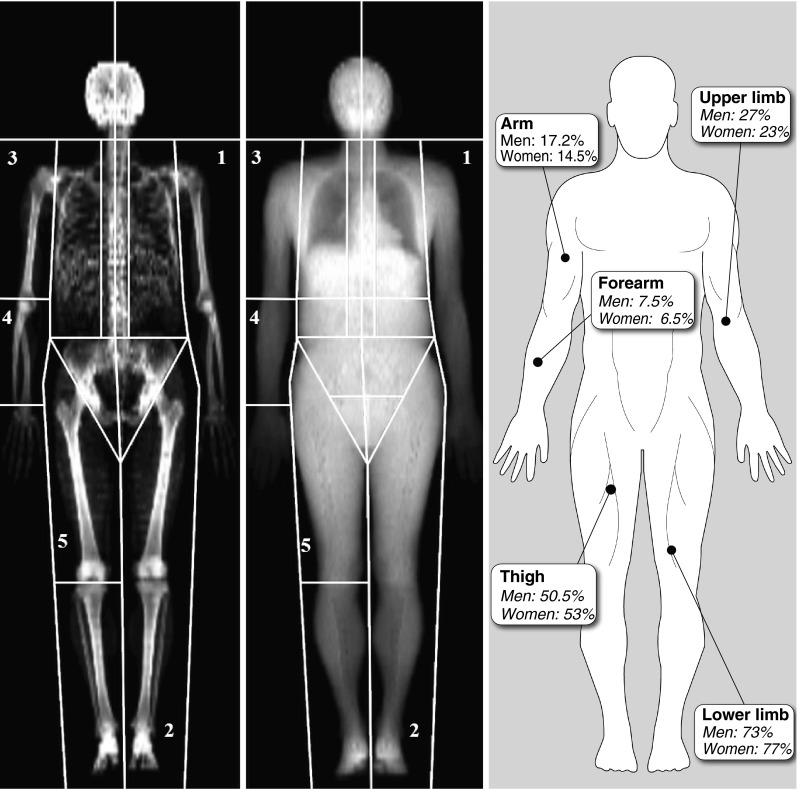
Body segmentation by DXA and distribution of lean body mass. 1, upper limb area; 2, lower limb area; 3, arm area; 4, forearm area; 5, thigh area right panel; values represent the contribution (percent) of lean body mass for each region in appendicular lean body mass
Men and women were then characterized by dividing their respective groups into tertiles based on appendicular lean body mass index.
Muscle strength measurements
Knee extension strength
Participants’ maximum knee extension muscle strength (KES) was determined by one repetition maximum (1RM). After a five-repetition warm-up, a resistance was chosen that was estimated to be slightly below the subject’s 1RM value. If the subject was able to complete the repetition, the resistance was increased by 5 kg and another trial performed after a 60-s rest. Each participant was given six lifting attempts in order to achieve their 1RM. A repetition was valid if the participant used correct form and was able to complete the entire lift in a controlled manner without assistance. The last resistance that was successfully completed was recorded as the 1RM.
Handgrip strength
Maximum voluntary handgrip strength (HGS) was measured using a hand dynamometer with an adjustable grip (Hand Dynamometer; Lafayette Instrument, Lafayette, IN). This method has been shown to be reliable [11]. Participants were standing upright and were instructed to apply as much handgrip pressure as possible for at least 4 s, performing the test with the right and left hands in turn. They performed three trials for each hand. The maximal score was recorded.
Muscle quality
Arm and leg muscle quality (MQ) were calculated by dividing HGS or KES by arm LBM or leg LBM, respectively.
Statistical analysis
Results are presented as means ± SD. Normality was verified using the kurtosis test. Independent t tests were used to compare differences between men and women as well as between tertiles of AppLBMI. Pearson’s correlation and partial correlation between muscle strength (handgrip and knee extension strength) and LBM measurements (LBM and App LBMI) were performed, as well as between age and LBM measurement, App LBMI, muscle strength, and quality indexes. Multiple stepwise linear regression analyses were performed to identify predictors of AppLBMI among muscle strength measurements. p ≤ 0.05 was considered statistically significant. Analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL).
Results
Participant’s characteristics
Participant’s characteristics are presented in Table 1. Briefly, for a similar age and BMI, men were significantly taller, heavier, and stronger than women (p < 0.001). Men also had more regional and overall LBM, as well as an app LBMI higher than that of women (p < 0.001). There were differences in the distribution of lean body mass between men and women (Fig. 1). Arm, forearm, and upper limb lean body mass accounted for a significantly greater proportion of appendicular lean body mass in men than in women (p < 0.001), while thigh and lower limb lean body mass accounted for a significantly greater proportion of appendicular lean body mass in women than in men (p < 0.001).
Table 1.
Participant’s characteristics
| Variables | All (n = 105) | Women (n = 53) | Men (n = 52) | p |
|---|---|---|---|---|
| Age (years) | 60.7 ± 7.7 | 60.5 ± 7.2 | 60.9 ± 8.2 | 0.81 |
| Height (cm) | 166.5 ± 9.4 | 160.7 ± 7.1 | 172.7 ± 7.4 | <0.001 |
| BW (kg) | 71.4 ± 16.3 | 65.8 ± 17.8 | 77.2 ± 12.3 | <0.001 |
| BMI (km/m2) | 25.6 ± 4.8 | 25.4 ± 5.9 | 25.9 ± 3.4 | 0.64 |
| HGS (kg) | 35.6 ± 9.9 | 27.2 ± 0.7 | 42.3 ± 9.3 | <0.001 |
| KES (kg) | 27.6 ± 14.2 | 20.5 ± 10.1 | 35.7 ± 13.9 | <0.001 |
| Forearm LBM (kg) | 0.81 ± 0.25 | 0.60 ± 0.08 | 1.02 ± 0.17 | <0.001 |
| Arm LBM (kg) | 1.86 ± 0.61 | 1.35 ± 0.21 | 2.38 ± 0.40 | <0.001 |
| Upper limb LBM (kg) | 2.93 ± 0.93 | 2.09 ± 0.32 | 3.73 ± 0.59 | <0.001 |
| Thigh LBM (kg) | 5.99 ± 1.45 | 4.92 ± 0.65 | 7.04 ± 1.23 | <0.001 |
| Lower limb LBM (kg) | 8.60 ± 1.92 | 7.25 ± 0.94 | 10.08 ± 1.47 | <0.001 |
| App LBM (kg) | 23.13 ± 5.45 | 18.72 ± 2.33 | 27.64 ± 3.78 | <0.001 |
| Whole-body LBM (kg) | 47.29 ± 10.07 | 38.99 ± 4.87 | 55.58 ± 6.39 | <0.001 |
| App LBMI (kg/m2) | 8.23 ± 1.31 | 7.22 ± 0.66 | 9.27 ± 0.94 | <0.001 |
BW body weight, BMI body mass index, HGS handgrip strength, KES knee extension strength, LBM lean body mass, App LBMI appendicular lean body mass index
Age and muscle characteristics
In women, there was a significant inverse correlation between age and KES (r = −0.47, p = 0.001), leg MQ (r = −0.46, p = 0.001), forearm (r = −0.36, p = 0.009), arm (r = −0.30, p = 0.032), upper limb (r = −0.43, p = 0.002) and appendicular (r = −0.37, p = 0.007) LBM, as well as with app LBMI (r = −0.31, p = 0.027), but neither with HGS, nor arm MQ, thigh, lower limb, and whole-body LBM. Furthermore, BMI was significantly correlated with forearm (r = 0.32, p = 0.021), arm (r = 0.29, p = 0.037), thigh (r = 0.29, p = 0.037), lower limb (r = 0.30, p = 0.033), and whole body (r = 0.32, p = 0.025) LBM, as well as with app LBMI (r = 0.31, p = 0.027), but not with HGS, KES, arm MQ, leg MQ, upper limb, and app LBM. Correlations between age and lean body mass, muscle strength, and muscle quality indexes in women are presented in Fig. 2a. Correlations between local and overall lean body mass measurements with age in women are presented in Fig. 3a.
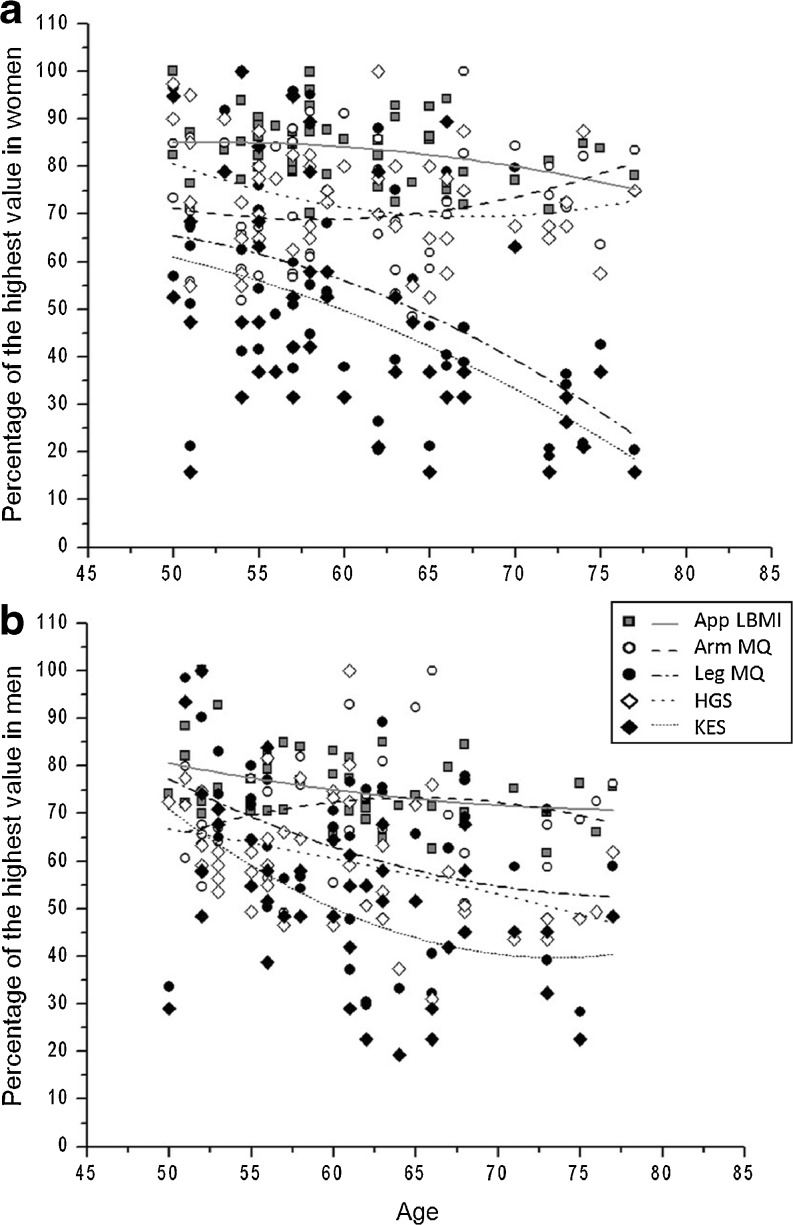
Fig. 2.
Correlations between lean body mass, muscle strength, and muscle quality indexes in women (a) and men (b). App LBMI, appendicular lean body mass index; KES, knee extension strength; HGS, handgrip strength; MQ, muscle quality. For each variable, the obtained measurement for an individual was expressed as a percentage of the highest value obtained in the group. a In women, App LBMI, KES, and leg MQ were significantly correlated with age (p ≤ 0.05). Furthermore, r for App LBMI, KES, HGS, Arm MQ, and leg MQ were −0.31, –0.47, –0.21, 0.17, and −0.47, respectively. b In men, App LBMI, KES, and HGS were significantly correlated with age (p ≤ 0.05). r for App LBMI, KES, HGS, arm MQ, and leg MQ were −0.37, −0.35, –0.40, 0.10, and −0.23, respectively
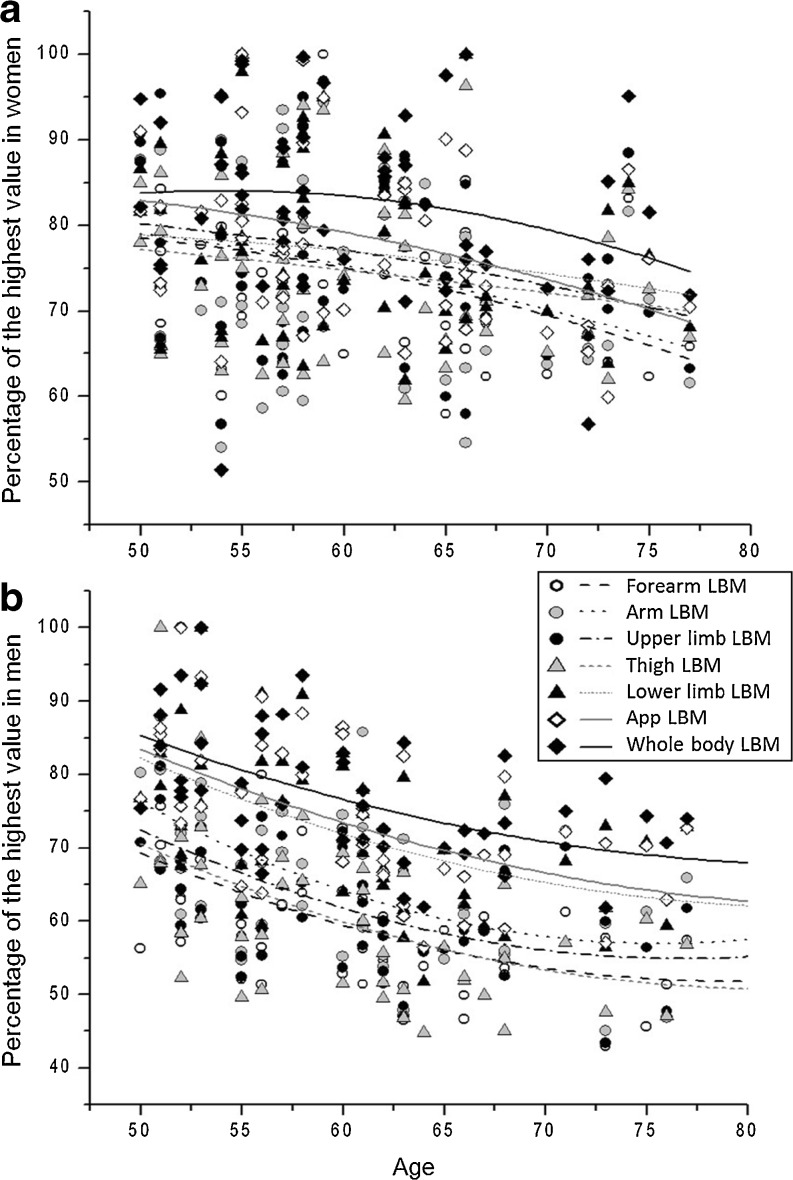
Fig. 3.
Correlations between local and overall lean body mass measurements with age in women (a) and men (b). LBM, lean body mass. For each variable, the obtained measurement for an individual was expressed as a percentage of the highest value obtained in the group. a In women, forearm, arm, and appendicular LBM were significantly correlated with age (p ≤ 0.05). Furthermore, r for forearm, arm, upper limb, thigh, lower limb, appendicular, and whole-body LBM were −0.36, –0.30, –0.25, –0.19, –0.19, –0.37, and −0.26, respectively. b In men, forearm, arm, upper limb, thigh, lower limb, appendicular, and whole-body LBM were significantly correlated with age (p ≤ 0.05). r for forearm, arm, upper limb, thigh, lower limb, appendicular, and whole-body LBM were −0.48, –0.45, –0.47, –0.50, –0.52, –0.56, and −0.54, respectively
In men, there was a significant inverse correlation between age and HGS (r = −0.40, p = 0.004), KES, (r = −0.35, p = 0.017), forearm (r = −0.48, p ≤ 0.001), arm (r = −0.45, p = 0.001), upper limb (r = −0.47, p = 0.001), thigh (r = −0.50, p ≤ 0.001), lower limb (r = −0.52, p ≤ 0.001), appendicular (r = −0.56, p ≤ 0.001), and whole-body (r = −0.49, p ≤ 0.001) LBM, as well as with app LBMI (r = −0.37, p =0.008), but not with arm and leg MQ. Furthermore, BMI was significantly correlated with forearm (r = 0.41, p = 0.003), upper limb (r = 0.30, p = 0.033), thigh (r = 0.43, p = 0.002), appendicular (r = 0.33, p = 0.022), and whole body (r = 0.47, p ≤ 0.001), as well as with app LBMI (r = 0.49, p ≤ 0.001), but not with HGS, KES, arm LBM, arm and leg MQ. Correlations between age and lean body mass, muscle strength, and muscle quality indexes in men are presented in Fig. 2b. Correlations between local and overall lean body mass measurements with age in men are presented in Fig. 3b.
Muscle strength and lean mass
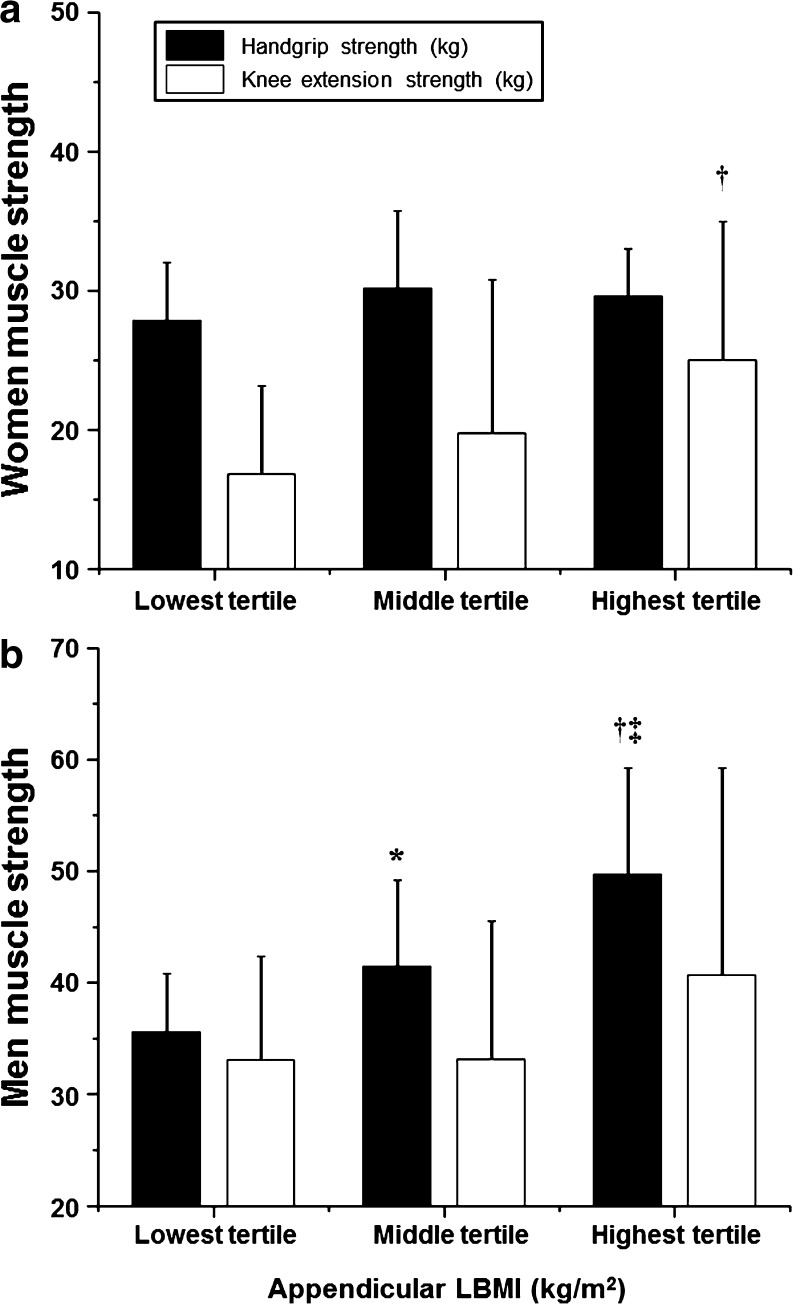
In the entire cohort, after controlling for age and BMI, both HGS and KES were significantly correlated with regional (forearm, arm, upper limb, thigh, lower limb, and appendicular) and overall lean body mass measurements, as well as with the appendicular LBMI (p < 0.001). In women, after controlling for age and BMI, HGS was significantly correlated with regional (forearm, arm, upper limb, thigh, lower limb, and appendicular) and overall LBM measurements (p ≤ 0.05), but not with appendicular LBMI while KES strength was significantly correlated with regional (forearm, arm, upper limb, thigh, lower limb, and appendicular) and overall LBM measurements as well as with the appendicular LBMI (p < 0.05). Differences in handgrip and knee extension strength in women classified into tertiles based on appendicular lean body mass index are presented in Fig. 4a. In men, after controlling for age and BMI, HGS was significantly correlated with thigh, lower limb, and appendicular LBM (p < 0.05), as well as with appendicular LBMI (p < 0.01), but not with forearm, arm, upper limb, and whole-body LBM. Furthermore, KES was significantly correlated with regional and overall LBM measurements (p < 0.05), but not with appendicular LBMI. Differences in handgrip and knee extension strength in men classified into tertiles based on appendicular lean body mass index are presented in Fig. 4b.
Fig. 4.
Differences in handgrip and knee extension strength in women (a) and men (b) classified into tertiles based on appendicular lean body mass index. †: significant difference between highest and lowest tertile (women, p = 0.022; men, p < 0.001). ‡: significant difference between highest and middle tertile (men, p = 0.009). *: significant difference between lowest and middle tertile (men, p = 0.020)
Discussion
Muscle strength is often used as a proxy to identify sarcopenic individuals [1]. However, accumulating evidence suggests that this relationship is weaker than previously thought. Thus, the aim of the present study was to investigate the association between measures of muscle strength and appendicular LBMI, an index that is commonly used to identify sarcopenic individuals. Two measures of muscle strength were performed; handgrip and knee extension strength. The major finding of our analyses is the sex-specific association that seems to exist between muscle strength and app LBMI. For instance, in women, knee extension but not handgrip strength was associated with app LBMI. Inversely, in men, handgrip but not knee extension strength was associated with app LBMI. According to these results, only knee extension strength measurements may be used to screen for sarcopenic women, while only handgrip strength measurements may be used to screen for sarcopenic men aged 50 years and more.
To our knowledge, this is the first study to report such a sex-specific relationship between app LBM, app LBMI, and muscle strength. The joint or independent effect of muscle mass and strength on functional capacity or mortality has been extensively studied [6, 12–15] and the belief that muscle mass and strength are strongly related is mostly based on their common relationship with physical function rather than on the study of their relationship. Nevertheless, few recent studies have examined this relationship, and findings usually result from secondary analyses [13]. Our results highlight the need to separately consider men and women. Indeed, at the level of the entire cohort, both handgrip and knee extension strength were strongly correlated with regional and overall LBM measurements, as well as with appendicular LBMI, while differences exist when each sex is considered separately.
We also investigated the relationship between aging, muscle strength, muscle quality, and regional and overall LBM. As observed in previous epidemiological studies, muscle mass or LBM and strength were well associated with aging [16]. However, some sex-associated differences persist. For instance, age was not associated with arm or leg muscle quality in men, suggesting that LBM and muscle strength declined at a similar rate while in women, leg muscle strength declined at a higher rate than lower limb lean body mass, resulting in a decreased leg muscle quality. These results are somewhat contradictory with previous results reporting a decreased muscle quality in both men and women for arm and leg muscle quality [16, 17]. These findings may be explained by the relatively small sample size of our group compared with cohorts of epidemiological studies.
There is no obvious reason to explain this sex-specific relationship. The slight differences in the distribution of lean body mass between men and women might be partly involved in this phenomenon. For instance, the fact that lower limb lean body mass accounted for a greater proportion of app LBM in women than in men may be put in parallel to the fact that knee extension strength, but not handgrip strength, was associated with app LBMI in women. Similarly, the greater proportion of upper limb to app LBM in men than in women may be related to the fact that handgrip strength, but not knee extension strength, was associated with app LBMI in men. Nevertheless, this hypothesis may be questioned since in both men and women, thigh lean mass, which is responsible for the strength generated during a knee extension, accounted for a large part (50.5 and 53 %, respectively) of appendicular lean body mass.
Coactivation of agonist and antagonist muscles is another potential mechanism that may be involved [18]. Although conflicting, evidence suggests that coactivation is higher in elderly than in young adults, which may disrupt the relationship between muscle mass and muscle strength, as observed in the present study. The question remains as to whether there are sex- or region-associated differences in muscle coactivation.
The study of the relationship between muscle strength and regional LBM measurements also raises some questions. One might expect that arm and thigh LBMs are the best correlates of handgrip and knee extensors strength, respectively. Surprisingly, our results suggest that this relationship is not that obvious. Indeed, according to our results, there would be no relationship between arm or upper limb LBM and handgrip strength in men, while lower limb LBM or app LBM would be the best correlates of handgrip strength. Similarly, Forearm LBM appears to be the measurement that best correlates with knee extension strength in men. Rather than a seemingly inconsistent relationship between regional LBM measurements and muscle strength, our findings may be explained by the used of clinical methods to assess muscle strength. Our results do not question the validity and relevance of these measures, but they might suggest that in small samples, more accurate devices may be used to avoid misleading results.
This study is not without its limitations. First, our cohort is composed of healthy Caucasian older adults aged 50 years and over. Thus, our results are limited to this population and cannot be generalized to frail or otherwise active individuals for example. Second, our sample is relatively small and our results need to be confirmed in larger cohorts and more heterogeneous populations. However, our results are strengthened by the use of DXA to assess body composition, a device recognized as being extremely accurate. Finally, handgrip and knee extension strength are two measurements very commonly used and that have been shown to be both relevant and accessible. Nonetheless, they are less accurate than research tools (e.g., dynamometer).
Conclusion
Appendicular lean body mass index is presently used to identify sarcopenic individuals and muscle strength measurements (handgrip or knee extension strength) are often used as clinical proxies for app LBMI in both men and women. Our results showed that indeed, muscle strength in associated with LBM; however, they also suggest that handgrip strength may be preferentially used in men, and knee extension strength in women. Future larger studies are now needed to confirm our findings.
Acknowledgments
We would like to thank the YMCAs of Quebec for supporting us, as well as Frédéric Bernard for his artistic input. SBA is supported by the Canadian Institute of Health Research. MAL is supported by the Fonds de la Recherche en Santé du Québec (FRSQ). The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;1:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Melton L, Jr, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–30. [PubMed] [Google Scholar]
- 5.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96:895–901. doi: 10.1017/BJN20061943. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 9.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–8. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 10.Clark BC, Manini TM. Sarcopenia ≠ Dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 11.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–6. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 12.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 13.Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56:B443–8. doi: 10.1093/gerona/56.10.B443. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard DR, Heroux M, Janssen I. Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J Aging Health. 2010;23:313–28. doi: 10.1177/0898264310388562. [DOI] [PubMed] [Google Scholar]
- 15.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 17.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 18.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–51. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]