Abstract
Weight loss is the hallmark of any progressive acute or chronic disease state. In its extreme form of significant lean body mass (including skeletal muscle) and fat loss, it is referred to as cachexia. It has been known for millennia that muscle and fat wasting leads to poor outcomes including death. On one hand, conditions and risk factors that lead to cachexia and inadequate nutrition may independently lead to increased mortality. Additionaly, cachexia per se, withdrawal of nutritional support in progressive cachexia, and advanced age may lead to death via cachexia-specific pathways. Despite the strong and consistent association of cachexia with mortality, no unifying mechanism has yet been suggested as to why wasting conditions are associated with an exceptionally high mortality risk. Hence, the causality of the cachexia–death association, even though it is biologically plausible, is widely unknown. This century-long uncertainty may have played a role as to why the field of cachexia treatment development has not shown major advances over the past decades. We suggest that cachexia-associated relative thrombocytosis and platelet activation may play a causal role in cachexia-related death, while other mechanisms may also contribute including arrhythmia-associated sudden deaths, endocrine disorders such as hypothyroidism, and immune system compromise leading to infectious events and deaths. Multidimensional research including examining biologically plausible models is urgently needed to investigate the causality of the cachexia–death association.
Keywords: Cachexia, Cause of death, Platelet activation, Arrhythmias, Infection
Introduction
It has been known for millennia that muscle and fat wasting leads to poor outcomes including deaths in chronic disease states [1]. This strong and practically undeniable association is also observed in any acute condition that is associated with accelerated wasting such as acute illnesses that progress to unintentional weight loss including in critically ill patients. Circumstances that lead to inadequate nutrition are also associated with increased mortality including starvation due to natural disasters (war, famine, earthquakes, and other natural and man-made disasters), anorexia nervosa, intentional starvation such as in hunger strike, and inadequate nutritional support in patients on life support in critical care units or in terminal cancer or other terminal conditions where nutritional support is chosen to be withdrawn. In geriatrics, the aging-associated sarcopenia is another well-known predictor of death, even though the aging process per se is not an acute or chronic illness. Whereas the term “cachexia” is used to refer to a more severe form of weight loss with a low body mass index (BMI) (e.g., BMI <18.5 or <20.0 kg/m2), any wasting condition whereby muscle or fat is lost—sometimes referred to as “pre-cachexia”—can be a prelude to overt cachexia and may have a bearing on life-span [1].
Despite the age-old strong and consistent association of cachexia with mortality and despite the enormous public health burden of cachexia [2], it is surprising that to date no unifying mechanism has been suggested to explain why wasting conditions are associated with an exceptionally high mortality risk. Indeed, there are debates as to whether this association is causal. This century-long drawback may have played an important role in why the field of cachexia treatment research has not shown major advances, especially since it has frequently been argued that cachexia is a mere epiphenomenon and not a true cause of death. In this short review paper, we examine the causality of the cachexia–death association and review briefly potential pathophysiologic mechanisms whereby muscle and fat wasting results in premature death.
Hill’s considerations for causality inferences in the observational studies of cachexia
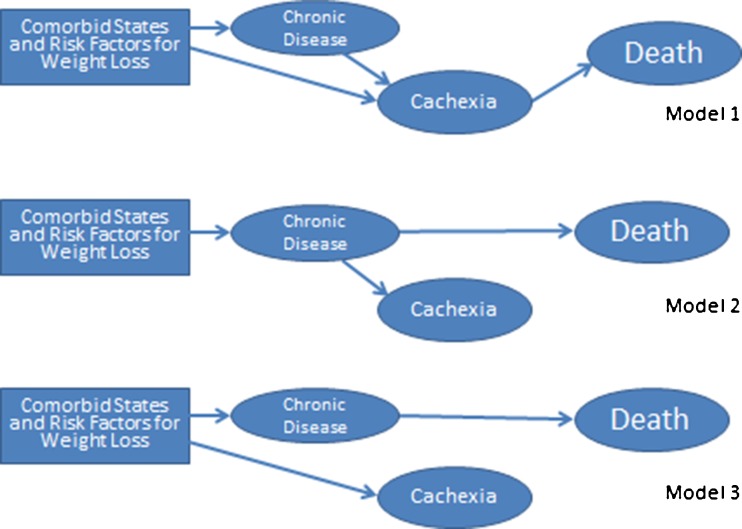
Given the inherent occurrence of cachexia as a universally unwanted terminal condition, it is highly unlikely that an interventional study to randomize human subjects to any cachectic versus non-cachectic state could be considered ethical. Hence, notwithstanding the potential role of animal models, the main source of evidence and inference pertaining to the cachexia–death association will remain observational or epidemiologic in nature. In epidemiological studies, the natural occurrences of the events are observed in a given population without an external “intervention” or “randomization” to an intervention. The ultimate goal of the epidemiological studies is to disclose the unbiased association between the candidate exposures—also called risk factor—and the outcomes of interest in the study population and then to extrapolate it to a larger population [3]. This association, however, is often confounded by third factors. Given the lack of randomization, the epidemiological associations—including those related to the cachexia–death association—are invariably subject to confounding bias. In other words, there is invariably a third factor or confounder, metastatic cancer for instance, that leads to both cachexia and death independent of each other, hence creating an apparent cachexia–death association that would not exist without the underlying cancer. Nevertheless, cachexia may also directly lead to death. We suggest that there are several biologically plausible scenarios pertaining to the cachexia–death association, as shown in Fig. 1.
Fig. 1.
Three hypothetical “causal” models of the cachexia–death association
Given the inherent limitations of epidemiologic studies, it can be argued that no matter how rigorous the confounders are adjusted for in sophisticated multivariate statistical models there may be a number of unknown residual confounders leading to the observed cachexia–death associations without true biologic plausibility [4]. Figure 1 shows a number of these associations. Whereas in model 1 cachexia is the true cause of death, in models 2 and 3, cachexia is a mere epiphenomenon and is secondary to the wasting disease or its underlying risk factors, respectively, which kill patient independent of cachexia (see Fig. 1). Hence, the inability of observational studies to prove causality is massively limited no matter what kind of statistical techniques are employed [5].
Despite the foregoing view about causal inferences in epidemiological studies of the cachexia–death association, there are some considerations to examine the leap from association to causation, the most well known of which was presented in the 1965 article of Sir Austin Bradford Hill, “The Environment and Disease: Association or Causation” [3], in which several benchmarks—subsequently refined and expanded to nine criteria—that “suggest” causality were listed (Table 1). The most important one is the “temporal relationship,” which indicates that the cause or “exposure,” e.g., weight loss to overt cachexia, should precede the effect or “outcome,” for instance death. An essential problem in studying the causes of death is the fact that death is inherently the final event preceded by presence of any risk factor or condition. Hence, temporality is universally present in this association and cannot help differentiate causality.
Table 1.
Hill’s considerations for causality inference in observational associations. Each causality benchmark is examined for cachexia–death association
| Benchmark | Definition/comments | Application to cachexia–death association |
|---|---|---|
| 1. Temporalitya | The cause (exposure) must precede the effect (outcome). | PRO: Wasting and weight loss occurs weeks, months, or years before death |
| CON: Death is inherently the final event. Death may also occur during weight gain, e.g., upon nutritional support. | ||
| 2. Strength of association | Stronger association may make causality more likely. | PRO: Most studies indicate strong and consistent associations between weight loss (pre-cachexia to cachexia) and imminent death. |
| CON: The reported strengths of the associations are not consistent. Disease severity, rather than cachexia, predicts death. | ||
| 3. Biological gradient (dose–response) | Greater exposure increases the incidence or magnitude of the effect. | PRO: Wasting severity or more rapid weight loss may be associated with higher likelihood of death. |
| CON: The wasting severity or cachexia progression rapidity has inconsistent and in some studies even weak association with death risk. | ||
| 4. Consistency | The association can be replicated in studies in different settings using different methods. | PRO: Different weight loss patterns, e.g., anorexia nervosa and cancer cachexia, and different types of wasting, i.e., both fat loss and muscle loss, lead to death |
| CON: Some types of weight loss such intentional weight loss or fat loss may be fully reversed without any risk of death (e.g., yoyo dieting). | ||
| 5. Biologic plausibility | The association is consistent with known biological or pathological processes.b | PRO: Cachexia may lead to thromboembolic events, arrhythmia, sudden cardiac death, immune system disarrays and higher rates of cardiovascular and infectious disease events and death. |
| CON: There is essentially no confirmed pathophysiologic pathway between cachexia and death. | ||
| 6. Experimentation | The putative effect can be altered (prevented or mitigated) by an experimental regimen. | PRO: In some animal models, starvation and weight loss can lead to death. Improving cachexia in human subjects improves survival. |
| CON: The current cachexia animal models are scarce and not convincing. Trials of nutritional support or anti-cachectic interventions in human subjects have often not improved survival. | ||
| 7. Specificity | A single cause produces the effect without other pathways. | PRO: Preceding wasting and weight loss can fully explain death events. |
| CON: Cachexia is only one of the correlates of chronic progressive disease states and likely an epiphenomenon (see Fig. 1, models 2 and 3). Death often happens in various acute and chronic diseases independent of cachexia. | ||
| 8. Biologic coherence | The association is consistent with the natural history of the disease or laboratory findings. | PRO: A lower risk of death should result from preventing weight loss or by nutritional support. |
| CON: Natural history of death in chronic disease states has little to do with wasting and weight loss if any. | ||
| 9. Analogy | The effect of similar factors may be considered in other populations or under different settings. | PRO: Wasting, fat, and muscle mass loss precede death as seen in any risk factors that precede mortal events. |
| CON: There is no biologically plausible analogy in death due to other conditions, such as cardiovascular (atherosclerosis) or cancer death. |
aNote that temporality is the only necessary (but not sufficient) condition of causality
bHowever, studies that disagree with established understanding of biological processes may force a reevaluation of accepted beliefs
It is important to note that even though Hill’s consideration can advance steps in the direction of causal inferences, epidemiology can virtually never prove causality. Table 1 shows pros and cons pertaining to each of the nine Hill’s considerations. Given the inconsistency of these benchmarks in the cachexia–death association, the current state of knowledge makes it virtually impossible to allow naming weight loss or even full-blown cachexia as the main cause of death in chronic and progressive disease states. Nevertheless, the consistency of the associative data, the remarkable strength of the cachexia–death association, the early occurrence of death following progressive weight loss, and emerging evidence from basic science and animal models suggest that the causality element may be present and can soon be discovered.
Putative pathophysiology of the cachexia–death link
Several hypotheses have been advanced to explain a biologically plausible association between cachexia and death, as shown in Table 2. We favor the platelet pathway hypothesis, according to which relative thrombocytosis (increased platelet count), potentially further enhanced by platelet function activation or other platelet aspect augmentation such as increase in platelet volume, leads to a predisposition to thromboembolic events including cardiovascular or cerebrovascular events and sudden death. In a recent cohort study of 40,000 people with end-stage kidney disease undergoing chronic hemodialysis treatment, Molnar et al. [6] have shown that relative thrombocytosis, i.e., platelet count >300,000 × 103/μL, was incrementally associated with higher death risk. Interestingly, this association was mitigated after adjusting for surrogates of malnutrition–inflammation complex including protein–energy wasting (PEW), which suggests that the platelet link may be in the causal pathway between PEW and mortality.
Table 2.
Putative mechanisms of action underlying the higher mortality associated with protein–energy wasting in chronic kidney disease
| System affected | Effect of PEW | Mechanism of action | Chronic disease populations studied |
|---|---|---|---|
| 1. Platelets | ↑ Platelet count (due to inflammation or iron deficiency); | Thrombosis leading to expansion of unstable atherosclerotic plaques leading to acute coronary syndrome and/or sudden cardiac death | CKD, almost all cachectic conditions |
| ↑ Platelet activation; | |||
| ↑ Platelet volume | |||
| 2. Anemia | ↓ Hemoglobin due to anemia of inflammation and/or chronic disease; | ↓ ESA responsiveness; | CKD, CHF, cancer cachexia |
| Functional iron deficiency mediated by hepcidin | ↑ ESA dose requirements; | ||
| ↑ IV iron requirements predisposing to infections, iron overload, and oxidative stress; | |||
| Likely not a main cause of death in cachexia | |||
| 3. Arrhythmia | Probably the most common cause of sudden cardiac death in cachectic patients | Widely unknown | CHF, CKD, cancer cachexia, virtually all severe forms of cachexia |
| 4. Endothelial dysfunction | Cardiovascular events and death | May be related to endotoxin–lipoprotein hypothesis (see below); | All forms of cachexia |
| Direct consequence of fat loss associated ↓ in anti-inflammatory cytokines adiponectin and IL-10 | |||
| 5. Endotoxin-lipoprotein | Likely via inducing inflammation and endothelial dysfunction | ↓ Circulating cholesterol levels leads to unbound circulating endotoxins; | CHF |
| Pro-inflammatory conversion of HDL | |||
| 6. Inflammatory cytokines | ↑ CRP and IL-6; | Pro-inflammatory cytokines lead to endothelial dysfunction and ↑ atherosclerotic plaque formation | |
| ↓ IL-10; | |||
| ↑ Myeloperoxidase | |||
| 7. Immune system | Immune deficiency predisposing to acute and chronic infectious events and infection related death | ↑ Susceptibility to bacterial or viral infections; | All forms of cachexia |
| Poor wound healing | |||
| 8. Adipose tissue | ↓ Fat tissue; | ↓ Uremic toxin sequestration; | CKD, all forms of cachexia |
| ↓ Adiponectin levels | ↓ Production of anti-inflammatory cytokines and adiponectin; | ||
| ↑ Levels of advanced glycation end products | |||
| 9. Skeletal muscle | Sarcopenia; | ↓ Skeletal, respiratory, and cardiac muscle function; | CKD |
| ↑ Circulating actin | ↓ Oxidative metabolism with ↓ antioxidant defense; | ||
| ↓ Bioavailability of activated vitamin D and gelsolin | |||
| 10. Thyroid hormone deficiency | Inflammation resulting in ↓ triiodothyronine levels | ↓ Cardiac contractility, | CKD and CHF |
| Endothelial dysfunction; | |||
| Atherosclerosis | |||
| 11. Testosterone deficiency | Low testosterone may be a consequence of cachexia, but some nutritional deficiencies (i.e., zinc) can cause testosterone deficiency | CAD; | CKD, chronic disease populations |
| Endothelial dysfunction; | |||
| Arterial calcification | |||
| 12. Psychosocial | Depression; | Progressive depression aggravates starvation | Cancer cachexia, all forms of cachexia |
| Pain; | |||
| ↓ Quality of life | |||
| 13. Gastrointestinal | Gut lining atrophy; | ↓ Absorption of nutrients and | CHF, CKD |
| ↓ Intestinal secretions; | ↑ absorption of endotoxins | ||
| Altered gut flora | |||
| 14. Nutritional | Anorexia and dietary restrictions leading to ↓ intake of fresh fruits and vegetables, legumes, dairy product and high-value proteins | Atherogenic effects of (self-) imposed diet; | |
| ↓ Levels of anti-oxidative vitamins and trace elements; | ↓ Protein intake leads to further PEW and mortality; | ||
| ↓ Levels of both nutritional and activated vitamin D | ↑ Oxidative stress, with consequent inflammation, endothelial dysfunction, and atherosclerosis; | ||
| ↑ Vascular calcification |
PEW protein–energy wasting, CKD chronic kidney disease, CHF congestive heart failure, ESA erythropoiesis-stimulating agent, IV intravenous, HDL high-density lipoprotein, CRP C-reactive protein, CAD coronary artery disease
There are several other reports about the causes and consequences of relative thrombocytosis and its relationship with thromboembolic events and death including in iron deficiency [7, 8]. Similarly, hypothyroidism, which can occur under wasting conditions, may also lead to increased platelet numbers as well as platelet activation [9]. Of note, a recent study in dialysis patients found that plasma level of gelsolin, a marker of healthy muscle mass, is decreased in dialysis patients, while circulating actin, a marker of myocyte damage, is increased [10]. Low gelsolin and high actin levels may activate platelet pathways, hence explaining why sarcopenia and muscle mass loss over time are strongly associated with death risk [10, 11].
Another plausible hypothesis invokes the potentially causal role of arrhythmias in cachectic conditions [12]. This pathway may explain why beta-blockers can improve survival in patients with chronic heart failure, where cardiac cachexia is quite common. It is still not clear what exact mechanisms, mediators, or pathways may lead to higher occurrence rate of arrhythmia-related death in cachectic conditions (if they indeed are present) [13].
Endothelial dysfunction may be another important causal pathway in the cachexia–death association. Progressive wasting leads to fat loss, leading to lower level of anti-inflammatory cytokines such as interleukin 10 and adiponectin, along with the decline in circulating level of lipoproteins. According to the endotoxin–lipoprotein hypothesis by Rauchhaus et al. [14], cachectic patients may be subject to enhanced endotoxin bioactivity in the circulation. Lipoproteins are known to have the ability to block endotocin bioactivity in vivo [14]. The lack of neutralizing circulating lipoproteins may explain why patients in chronic illness often show better survival when lipoprotein levels are high [15, 16]. In hypocholesterolemic patients, the lack of an adequate lipoprotein pool leads to free circulating endotoxins, which per se results in the generation of a pro-inflammatory and oxidative cascade with subsequent endothelial dysfunction and cardiovascular events [17]. Some traditional hypotheses pertaining to the pathophysiologic link between wasting and cachexia are based on the suppressive effect of wasting on the immune system, leading to a propensity for infectious diseases and deaths caused by infectious events. Some hypotheses pertaining to the cause of death in protein–energy wasting of CKD patients are listed in Table 2.
Conclusions
Cachexia is a major public health issue [18]. Millions of people with chronic disease states are more likely to die when they lose weight and develop progressive cachexia. There are opposing views as to whether cachexia is a biologically plausible cause of increased death risk in patients with wasting disorders or whether this is a mere epiphenomenon. The pathophysiologic pathways whereby cachexia leads to death are not clear. Increases in platelet numbers and activation pathways, and arrhythmias appear to be causes of sudden cardiovascular events. The field of applied cachexia will remain in its millennium-old infancy as long as there is no major advancement in disclosing the true etiology of death in cachexia.
Acknowledgments
The study was supported by the following grants for the authors: KK-Z is supported in part by NIH grants K24-DK091419 and R01-DK078106 and R01-DK095668 and R01-DK096920 and R13-DK094686 2011. KKZ was additionally supported by a philanthropist grant from Mr. Harold Simmons. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, et al. J Cachexia Sarcopenia Muscle 2010; 1:7–8).
Conflict of interest
KKZ has received honoraria and/or research grants from Abbott, Shire, Takeda, Gambro, Amgen, and Baxter.
References
- 1.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Farkas J, von Haehling S, Kalantar-Zadeh K, Morley JE, Anker SD, Lainscak M. Cachexia as a major public health problem: frequent, costly, and deadly. J Cachexia Sarcopenia Muscle. 2013. doi:10.1007/s13539-013-0105-y [DOI] [PMC free article] [PubMed]
- 3.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Kalantar-Zadeh K. Observational studies versus randomized controlled trials: avenues to causal inference in nephrology. Adv Chronic Kidney Dis. 2012;19:11–18. doi: 10.1053/j.ackd.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95:S144–S150. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 6.Molnar MZ, Streja E, Kovesdy CP, Budoff MJ, Nissenson AR, Krishnan M, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94:945–954. doi: 10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52:727–736. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keung YK, Owen J. Iron deficiency and thrombosis: literature review. Clin Appl Thromb Hemost. 2004;10:387–391. doi: 10.1177/107602960401000412. [DOI] [PubMed] [Google Scholar]
- 9.Beyan C, Kaptan K. Reactive thrombocytosis accompanying subclinical hypothyroidism due to Hashimoto’s thyroiditis. Blood Coagul Fibrinolysis. 2013. doi:10.1097/MBC.0b013e32836069f5 [DOI] [PubMed]
- 10.Lee PS, Sampath K, Karumanchi SA, Tamez H, Bhan I, Isakova T, et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J Am Soc Nephrol. 2009;20:1140–1148. doi: 10.1681/ASN.2008091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Kalantar-Zadeh K. Why is protein–energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb JG, Kiess MC, Chan-Yan CC. Malnutrition and the heart. CMAJ. 1986;135:753–758. [PMC free article] [PubMed] [Google Scholar]
- 13.Doehner W, Anker SD. Beta blockers and glucose metabolism in chronic heart failure: friend or foe? Clin Res Cardiol. 2008;97:21–23. doi: 10.1007/s00392-008-0609-1. [DOI] [PubMed] [Google Scholar]
- 14.Rauchhaus M, Koloczek V, Volk H, Kemp M, Niebauer J, Francis DP, et al. Inflammatory cytokines and the possible immunological role for lipoproteins in chronic heart failure. Int J Cardiol. 2000;76:125–133. doi: 10.1016/S0167-5273(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 15.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Pedroso FE, Spalding PB, Cheung MC, Yang R, Gutierrez JC, Bonetto A, et al. Inflammation, organomegaly, and muscle wasting despite hyperphagia in a mouse model of burn cachexia. J Cachexia Sarcopenia Muscle. 2012;3:199–211. doi: 10.1007/s13539-012-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschirner A, von Haehling S, Palus S, Doehner W, Anker SD, Springer J. Ursodeoxycholic acid treatment in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3:31–36. doi: 10.1007/s13539-011-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle. 2012;3:245–251. doi: 10.1007/s13539-012-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]