Abstract
Objectives
The aim of the study was to examine the hypothesis that MMR exposure has a negative influence on cognitive development in children. Furthermore, MMR was compared to single measles vaccine to determine the potential difference of these vaccines safety regarding children’s cognitive development.
Methods
The prospective birth cohort study with sample consisted of 369 infants born in Krakow. Vaccination history against measles (date and the type of the vaccine) was extracted from physicians’ records. Child development was assessed using the Bayley Scales of Infant Development (BSID-II) up to 3rd year of life, Raven test in 5th and 8th year and Wechsler (WISC-R) in 6th and 7th year. Data on possible confounders came from mothers’ interview, medical records and analyses of lead and mercury level at birth and at the end of 5th year of life. Linear and logistic regression models adjusted for potential confounders were used to assess the association.
Results
No significant differences in cognitive and intelligence tests results were observed between children vaccinated with MMR and those not vaccinated up to the end of the 2nd year of life. Children vaccinated with MMR had significantly higher Mental BSID-II Index (MDI) in the 36th month than those vaccinated with single measles vaccine (103.8±10.3 vs. 97.2±11.2, p=0.004). Neither results of Raven test nor WISC-R were significantly different between groups of children vaccinated with MMR and with single measles vaccine. After standardization to child’s gender, maternal education, family economical status, maternal IQ, birth order and passive smoking all developmental tests were statistically insignificant.
Conclusion
The results suggest that there is no relationship between MMR exposure and children’s cognitive development. Furthermore, the safety of triple MMR is the same as the single measles vaccine with respect to cognitive development.
Keywords: children, MMR vaccine, cognitive development
Introduction
Despite the fact that a number of epidemiological studies failed to show any association between MMR vaccine and autism, the controversy over the vaccine safety still exists [1,2,3]. The anti-vaccine organizations and websites that portray themselves as official resources for credible data on vaccines continue to provide flawed or biased information about MMR [4,5]. It serves to fuel public concern regarding the safety of MMR which leads to increased rates of immunization refusal or delays on-time vaccination, and consequently causes a significant risk of outbreaks of measles in many European countries and the United States [6,7,8].
To counter these anti-vaccination advocates and to promote greater acceptance of vaccination (not exclusively MMR) the evidence-based information concerning the benefits and the risk of immunization is required [9–12]. The hypothesis that MMR as a triple live vaccine is more detrimental for children’s neurodevelopment in comparison to single measles vaccine was developed in the past [13–15]. Nevertheless, the studies have not provided evidence against MMR immunization [16–24]. While earlier studies focused on more advanced health problems like autism [18,21,22], currently epidemiological studies look for more subtle neurodevelopmental outcomes that could be potentially linked to vaccines exposure. Those can be detected by psychological tests being sufficiently sensitive to monitor even minor, subclinical disorders in children. Additionally essential is inclusion of a wide range of potential confounders that may have an impact on children’s neurodevelopment, like maternal age, education and IQ, mercury and lead exposure during pregnancy and other prenatal and postnatal factors.
During the last years in Poland there was a good opportunity to conduct the studies on the MMR safety because the population of children was diversified in terms of vaccination history against measles. The part of infants was vaccinated with MMR as a voluntary option (charged extra money) and some of them were vaccinated only with single measles vaccine which was used according to the national mandatory immunization schedule up to 2004. Obviously, some children for different reasons have not been vaccinated against measles at all.
The aim of this study was to examine the hypothesis that MMR exposure has a negative influence on cognitive development in children. Furthermore, MMR was assessed in comparison to single measles vaccine exposure, to determine the potential difference of these vaccines safety regarding children’s cognitive development.
Materials and Methods
This is a prospective cohort study, combining environmental monitoring and molecular approaches with comprehensive neurodevelopment assessments. In the analysis we used data from an earlier established Krakow birth cohort of children, being part of ongoing, collaborative study with Columbia University in New York, on the vulnerability of fetus and child to environmental factors. The study has received the approval of the Jagiellonian University Ethical Committee.
The enrolment (November 3, 2000 - August 22, 2003) included only non-smoking women, aged 18–35 years, with singleton pregnancy without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension and residing in Krakow for at least one year prior to pregnancy. The infants were followed up to 8th year of life. Each year mothers were asked to provide information on infants’ health and household characteristics by trained interviewers, who carried out detailed, face-to-face standardized interviews. The Test of Nonverbal Intelligence, Third edition (TONI-3) was administered to mothers. We have included this instrument to adjust to the maternal contribution to child cognitive development.
Vaccination data
The data on infants’ vaccination history (date of vaccination and type of vaccine) were extracted from the physician’s records. The vaccination status was based on measles vaccination during the second year of life.
Biological samples and analysis
Concentrations of cotinine and heavy metals (mercury, lead) were examined in Cord blood (at delivery) and capilary blood (5-year-old children).. Whole blood lead concentrations were determined using inductively coupled plasma mass spectrometry CLIA’88 method “Blood lead cadmium mercury ICPMS_ITB001A”. This multi-element analytical technique is based on quadruple ICP-MS technology [25]. Mercury levels were measured at the CDC by Zeeman graphite furnace atomic absorption spectrometry, using a phosphate/Triton X-100/nitric acid matrix modifier. Cold vapor atomic spectrometry following chemical reduction of mercury compounds was used to measure total mercury in whole blood. More details on blood sample collection and analysis were presented in earlier publications [26,27].
Infants neurodevelopment testing
The Fagan Test of Infant Intelligence (FTII) was conducted in the 6th month of life. The Bayley Scales of Infants Development, second edition (BSID-II), was administered in the 12th, 24th and 36th months of life. The Mental Scale of that test includes items that assess memory, habituation, problem solving, early number concepts, generalization, classification, vocalization, language, and social skills [28]. Test scores are adjusted to child’s age to obtain the Mental Development Index (MDI). Test results are in one of four categories: 1) accelerated performance (score > 115), 2) within normal limits (score 85 to 114), 3) mildly delayed performance (score 70 to 84), and 4) significantly delayed (score < 69). The outcomes range is from 50 to 150.
The test of Raven’s Colored Progressive Matrices (Raven) was administered twice, in 5th and 8th year of life. The outcomes of the test were measured in terms of centiles. Because the results of this test were generally high, the cut point of poor result category was 74th percentile, which means middle intelligence outcomes. Output scale was presented in centiles standardized to age groups.
The Wechsler Intelligence Scale for Children (WISC-R) was administered in 6th and 7th year of life, and generated verbal, nonverbal and total IQ for evaluated children. Category with IQ <100 was considered as the poorer outcomes. The outcomes range is from 40 to 160.
All neurodevelopment tests were conducted in the Department of Epidemiology and Preventive Medicine by carefully trained examiners being unaware of the child’s exposure. Bayley Scales as well as Raven test both have well defined criteria and were considered as fully consent between different examiners. In order to provide fully comparable assessment of WISC-R test, one psychologist rated performed answers for all children.
Statistical analysis
In the descriptive analysis, difference in the distribution of women and newborns’ parameters grouped by measles vaccination status were tested using χ2 (for nominal variables) and Mann-Whitney and Kruskal-Wallis tests (for continuous variables).
The comparison of the tests outcomes according to the exposure to the type of vaccine (MMR vs. monovalent vaccine and MMR vs. unvaccinated group) was studied using multivariate linear models. As well the logistic models were used to assess risk of developmental delay (MDI <85, Raven<74, IQ<100).
All variables from table 1 which showed a probable association with measles vaccination status (p<0.1) were included in statistical multivariable models. Blood lead level at the age of 5 was used as confounder in models for 5-year-old and older children. Additionally, the child’s gender was added to all models as it is highly associated with developmental tests’ performance.
Table 1.
Cohort characteristics of children vaccinated and unvaccinated against measles up to 2nd year of life.
| MMR vaccine | Monovalent vaccine | Non-vaccinated | p | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | N | % | ||
| Child’s gender: | |||||||
| boys | 160 | 52.1 | 18 | 56.2 | 15 | 50.0 | 0.968 |
| girls | 147 | 47.9 | 14 | 43.8 | 15 | 50.0 | |
| Maternal education | |||||||
| primary/vocational school | 57 | 18.6 | 15 | 46.9 | 9 | 30.0 | 0.001 |
| high school | 18 | 25.4 | 10 | 31.2 | 6 | 20.0 | |
| university | 172 | 56.0 | 7 | 21.9 | 15 | 50.0 | |
| Maternal marital status | |||||||
| married | 290 | 94.5 | 30 | 93.8 | 28 | 93.3 | 0.730 |
| not married | 17 | 5.5 | 2 | 6.2 | 2 | 6.7 | |
| Poor economical status | 18 | 5.9 | 6 | 18.8 | 4 | 13.3 | 0.016 |
| Birth order | |||||||
| 1 | 214 | 69.7 | 9 | 28.1 | 10 | 33.3 | <0.001 |
| 2 | 83 | 27.0 | 16 | 50.0 | 18 | 60.0 | |
| ≥3 | 10 | 3.3 | 7 | 21.9 | 2 | 6.7 | |
| Birth weight < 2500grams | 10 | 3.3 | 1 | 3.1 | 1 | 3.3 | 1.000 |
| Gestational age <37 weeks | 15 | 4.9 | 0 | - | 3 | 10.0 | 0.148 |
| Virus infection during pregnancy | 74 | 24.1 | 5 | 15.6 | 10 | 33.3 | 0.265 |
| Passive tobacco smoking during pregnancy | |||||||
| non-exposed | 231 | 75.2 | 11 | 34.4 | 20 | 66.7 | <0.001 |
| up to 5 cigarettes/day | 57 | 18.6 | 11 | 34.4 | 3 | 10.0 | |
| above 5 cigarettes/day | 19 | 6.2 | 10 | 31.2 | 7 | 23.3 | |
| Cord blood mercury level >0.9 μg/L | 107 | 46.5 | 9 | 42.9 | 10 | 43.5 | 0.920 |
| Cord blood lead level >1.2 μg/L | 143 | 51.3 | 18 | 62.1 | 15 | 53.6 | 0.536 |
| Fish consumption during pregnancy above 100g per week | 81 | 26.4 | 11 | 34.4 | 6 | 20.0 | 0.434 |
|
| |||||||
| mean | SD | mean | SD | mean | SD | ||
| Mother’s age at 2nd trimester | 27.7 | 3.5 | 27.7 | 4.5 | 27.7 | 3.8 | 0.971 |
| maternal nonverbal intelligence (TONI-3) | 109.3 | 16.8 | 101.0 | 18.2 | 110.3 | 22.8 | 0.091 |
| Maternal depression scale during pregnancy | 27.4 | 0.6 | 28.0 | 2.5 | 28.8 | 2.3 | 0.811 |
| Cord blood mercury level (μg/L) | 1.08 | 0.05 | 0.95 | 0.13 | 1.01 | 0.15 | 0.561 |
| Cord blood lead level (μg/L) | 1.43 | 0.04 | 1.48 | 0.09 | 1.61 | 0.21 | 0.439 |
| Blood mercury level at age of 5 years (μg/L) | 0.57 | 0.32 | 0.52 | 0.23 | 0.44 | 0.26 | 0.438 |
| Blood lead level at age of 5 years (μg/L) | 2.16 | 0.78 | 2.53 | 0.70 | 2.27 | 0.65 | 0.063 |
| Peak blood mercury level (μg/L) | 1.09 | 0.68 | 0.88 | 0.57 | 0.99 | 0.68 | 0.133 |
| Peak blood lead level (μg/L) | 1.94 | 0.88 | 1.96 | 0.78 | 2.03 | 1.03 | 0.811 |
| Fish consumption during pregnancy(g/week) | 79.9 | 63.8 | 78.8 | 67.7 | 75.3 | 54.4 | 0.901 |
Statistical analyses were performed using STATA software version 8.0.
Results
Study population
The analyzed population consisted of 369 children: 52.3% boys and 47.7% girls. From that group 10 children (2,7%) were absent during BSID-II test in 24th month. Retention rate in that group during psychological tests in further years was respectively: 94.1% in 3rd, 72.6% in 5th, 58.5% in 6th, 60.2% in 7th and 51.2% in 8th. During the second year of life (period of exposure included to analysis) 83.2% of children were exposed to MMR, 8.7% to single measles vaccine and 8.1% were unvaccinated. Only two children with known vaccination history were non-vaccinated against measles up to the 6th year of life.
Children vaccinated with MMR were more frequently the first child in the family than those either vaccinated with monovalent vaccine or unvaccinated up to the end of the 2nd year of life (69.7%,28.1% and 33.3%, p<0.001) (table 1). Mothers of children vaccinated with monovalent vaccine had less frequently university degree than those vaccinated with MMR or unvaccinated (21.9%, 56.0% and 50%, p=0.001) and were in higher percentage both in poor economical situation (18.8%, 5.9%, 13.3% respectively, p=0.016) and exposed to passive tobacco smoking during pregnancy (65.6%, 24.8%, 33.3%, p<0.001). No statistically significant differences were observed in other variables taken into consideration (table 1).
Children’s cognitive development in pre-exposure period
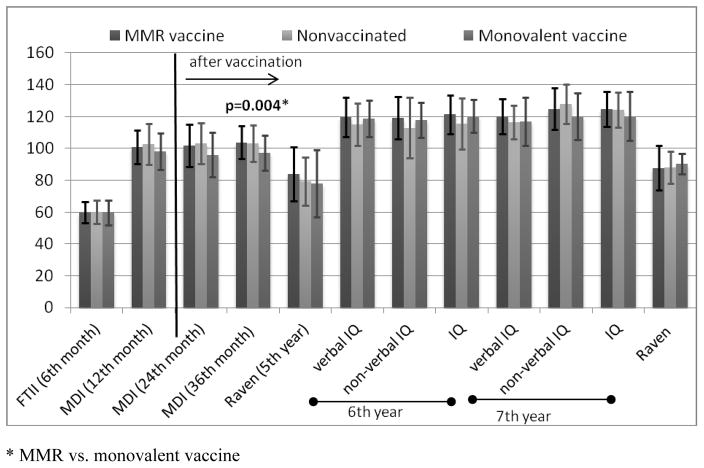
There were no significant differences in tests scores that were performed during pre-exposure period. The average outcomes of the Fagan test, administered in the 6th month of life, amounted to about 60 points in all three groups under analysis. The average scores of MDI in the 12th month of life were also on the similar level (from 98.3 to 102.7 point) (Figure 1). In 1-year-old infants categories: “Mildly Delayed” or “Significantly Delayed” (MDI<85) were reached by about 10% of children and differences were not statistically significant between studied groups (Table 3).
Figure 1.
Average tests scores in MMR or monovalent vaccine exposed and non-exposed groups (with standard deviation).
* MMR vs. monovalent vaccine
Table 3.
Percentage of children with mild or significant developmental delay according to the tests outcomes.
| MMR | Non-vaccinated | Monovalent vaccine | p | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| MDI of BSID II | 12th month of life | 23 | 7.5 | 3 | 10.7 | 4 | 12.9 | ns |
| MDI of BSID II | 24th month of life | 24 | 8.2 | 3 | 11.1 | 8 | 26.7 | 0.009 |
| MDI of BSID II | 36th month of life | 10 | 3.5 | 1 | 4.2 | 6 | 20.7 | 0.003 |
| Raven (centilles) | 5th year of life | 44 | 19.3 | 5 | 26.3 | 7 | 35.0 | ns |
| WISC-R | 6th year of life | |||||||
| verbal IQ | 13 | 7.1 | 2 | 14.3 | 2 | 10.5 | ns | |
| non-verbal IQ | 12 | 6.6 | 2 | 14.3 | 2 | 10.5 | ns | |
| IQ | 10 | 5.5 | 1 | 7.1 | 1 | 5.3 | ns | |
| WISC-R | 7th year of life | |||||||
| verbal IQ | 5 | 2.6 | 1 | 7.1 | 3 | 20.0 | 0.012 | |
| non-verbal IQ | 8 | 4.2 | 0 | 0.0 | 1 | 6.7 | ns | |
| IQ | 8th year of life | 5 | 2.6 | 0 | 0.0 | 1 | 6.7 | ns |
| Raven (centilles) | 8th year of life | 18 | 10.8 | 0 | 0.0 | 0 | 0.0 | ns |
MMR and cognitive tests outcomes
No significant differences of cognitive and intelligence tests results were observed between children vaccinated with MMR and unvaccinated in univariable analysis. Their outcomes were on similar level (Fig. 1). After standardization to child’s gender, maternal education, family economical status, maternal IQ, birth order and passive tobacco smoking (as well as lead level in cord blood in the end of 5th year of life for 5-year-old and older children) none of the tests outcomes of cognitive development or intelligence tests results were statistically significant (table 2).
Table 2.
Standardized test scores in MMR and monovalent exposed and non-exposed groups (multivariable linear regression models).
| test | age | MMR vs. non-vaccinated | MMR vs. monovalent vaccine | ||||
|---|---|---|---|---|---|---|---|
| β* | 95% CI | p | β* | 95% CI | p | ||
| MDI of BSID II | 24th month of life | −3.7 | −9.6; 2.1 | 0.212 | 1.0 | −5.1; 7.0 | 0.749 |
| MDI of BSID II | 36th month of life | −3.4 | −8.2; 1.5 | 0.172 | 4.7 | −0.1; 9.5 | 0.056 |
| Raven (centilles) | 5th year of life | −3.0 | −13.5; 7.5 | 0.574 | 3.1 | −7.8; 14.0 | 0.572 |
| WISC-R | 6th year of life | ||||||
| verbal IQ** | −2.5 | −11.1; 6.1 | 0.568 | −3.5 | −11.8; 4.7 | 0.394 | |
| non-verbal IQ** | −2.8 | −11.1; 5.5 | 0.502 | −0.3 | −8.4; 7.7 | 0.936 | |
| IQ** | −3.0 | −11.0; 5.1 | 0.464 | −2.23 | −10.1; 5.6 | 0.572 | |
| WISC-R | 7th year of life | ||||||
| verbal IQ** | 3.5 | −4.2; 11.2 | 0.370 | −0.7 | −9.6; 8.1 | 0.867 | |
| non-verbal IQ** | −0.9 | −10.1; 8.2 | 0.843 | 3.4 | −7.2; 14.1 | 0.523 | |
| IQ** | 1.5 | −6.0; 9.1 | 0.688 | 1.3 | −7.4; 10.1 | 0.761 | |
| Raven (centilles)** | 8th year of life | −2.3 | −13.9; 9.3 | 0.694 | 0 | −12.8; 12.7 | 0.997 |
standardized to child’s gender, maternal education, maternal IQ, maternal economical status, birth order (further child vs. first one) and exposure to environmental tobacco smoke during pregnancy(yes vs. no);
additional standardization to blood lead level at the age of 5
Children vaccinated with MMR had significantly higher mental BSID-II scores in the 36th month than those vaccinated with single measles vaccine (103.8±10.3 vs. 97.2±11.2, p=0.004) (Figure 1). Neither results of Raven test nor WISC-R were significantly different between groups of children exposed to MMR and single measles vaccine. The results of MDI in the 36th month in children vaccinated with MMR vs. vaccinated with single measles vaccine became non-significant (β=4.7, p=0.056) after standardization to child’s gender, maternal education, family economical status, maternal IQ, birth order and passive tobacco smoking. Results of MDI in the 24th month as well as WISC-R and Raven in MMR and monovalent group didn’t differ significantly.
Subjects exposed to monovalent vaccine had a higher percentage of “Mildly Delayed” or “Significantly Delayed” (MDI<85) outcomes in the 24th month and the 36th month of life in comparison with exposed to MMR or unvaccinated children (26.7% vs. 8.2% and 11.1%, p=0,009 and 20.7% vs. 3.5% and 4.1%, p=0.023, respectively). The differences between groups related to delayed tests outcomes in children in the 5th, 6th, and 8th years of life were not statistically significant. The percentage of ”delayed” verbal IQ results in 7 year-old children was significantly higher in group vaccinated with monovalent vaccine compared to MMR and unvaccinated subjects (20% vs. 2.6% and 7.1%, p=0.012) (Table 3).
After adjusting to possible confounders, MMR exposure didn’t affect the risk of delayed cognitive development compared to neither unvaccinated children nor those vaccinated with monovalent measles vaccine. The odds ratio of delayed cognitive development was even significantly lower in MMR than in single measles vaccine group among 3-year-old children (OR=0.18, 95%CI: 0.03–0.91) (Table 4).
Table 4.
The risk of delayed development in MMR vs. monovalent vaccine exposed or non-exposed groups (multivariate logistic regression models).
| MMR vs. non-vaccinated | MMR vs. monovalent vaccine | ||||||
|---|---|---|---|---|---|---|---|
| test | age | OR | 95% CI | p | OR | 95% CI | p |
| MDI of BSID II ** | 24th month of life | 1.35 | 0.15; 12.0 | 0.786 | 0.35 | 0.09; 1.37 | 0.132 |
| MDI of BSID II ** | 36th month of life | 0.37 | 0.03; 4.02 | 0.414 | 0.18 | 0.03; 0.91 | 0.038 |
| Raven (centilles)* | 5th year of life | 1.22 | 0.23;6.55 | 0.820 | 0.45 | 0.11; .1.81 | 0.261 |
| WISC-R | 6th year of life | ||||||
| verbal IQ* | 1.23 | 0.09; 17.03 | 0.875 | - | - | - | |
| non-verbal IQ* | - | - | - | 1.04 | 0.09; 11.78 | 0.973 | |
| IQ | - | - | - | - | - | - | |
OR standardized to child’s gender, maternal education, maternal IQ, maternal economical status, birth order (further child vs. first one) and exposure to environmental tobacco smoke during pregnancy(yes vs. no);
additional standardization to blood lead level at the age of 5;
standardization without maternal economical status
In children older than 6 year-old it was impossible to build up logistic regression models due to the lack of subjects with developmental delay in monovalent vaccine and unvaccinated group.
Discussion
The study addresses the association between MMR and cognitive development in children during the eight-year observation since the exposure. Because the population of children under study was diversified in terms of vaccination history, we concentrated on the safety of MMR vs. single measles vaccine. No other country in Europe and the USA had similar opportunity to conduct such an analysis as MMR vaccine was introduced there to the immunization calendar much earlier than in Poland, and single measles vaccine has not been administered since over 20 years there [29,30].
The studies on the safety of both MMR and single measles vaccine and their link with the risk of cognitive development disorders in children have significant value, especially that the growing number of parents are opting out of the MMR vaccination or at least substituting it with a single vaccinations [31]. The result is that the MMR vaccination rate has fallen, causing a significant risk of outbreaks of measles in many countries [7,8]. Our findings are supporting commonly accepted immunization program against measles and rubella which allows to replace single vaccines with MMR vaccine, if adequately controlled [32].
The results of the studies published over the last 12 years on the association between MMR vaccine and autism and our observations have found no evidence for such causal links [17–24]. Despite adverse opinions and pressures, the WHO has not withdrawn the recommendations for MMR vaccine, and measles and rubella prevention programs have been continued, though with some difficulties, being a part of the MMR mass immunization policy [33].
The study also deals with the association between MMR immunization and development of the vaccinated and unvaccinated children, which was possible only in the early exposed infants, as some of the children from this cohort were vaccinated with delay, over the age of two. Having in mind potentially significant role of the time of exposure, we concentrated on the effects of different vaccination status in children at the age of two. Until this age most children in Poland became vaccinated according to the mandatory immunization program for preventing measles and rubella, and as many as 95% of children should have already received the first dose of MMR. Therefore, the analysis of MMR vaccine safety related to the infants exposure time is crucial for finding the link between the vaccination and child development. If there were no evidence for the harmful effect of MMR vaccine on the development of early exposed infants, it would be hard to anticipate that the children vaccinated with different time delays are at risk. In this study authors have not concentrated on the causal link between MMR and autism although this hypothesis caused high level of anxiety around the MMR vaccine. There is sufficient epidemiologic evidence that failed to show any link between MMR and autism [17–23]. At generally low incidence rates of autism, we should not anticipate high rates of autism in a prospective study of the cohort consisting of 500 children. During a few-year observation there was only a single case of autism that corresponded to the overall average incidence of autism. Still, the size of the cohort was big enough to observe the dynamics of health outcomes, such as disorders of cognitive development, psychomotor activity or behavior. Assuming a power level 0.8 and α=0.05 and the small number of children unvaccinated or vaccinated with monovalent vaccine, our population was big enough to find possible differences in neurodevelopment outcomes, e.g. 6-point difference for MDI outcomes or 8-point for WISCR IQ.
The main purpose of the study was to establish whether there is an association between MMR and early developmental delays of milder intensity. This is the strong point of the study because most of the epidemiologic analyses concentrate on the links between MMR and more serious post -vaccination side effects in children. In our opinion, the analyses should also cover those mild side effects or disorders, to be able to either find evidence for or against the causal relationship between MMR and other less serious health outcomes. Similar issues have not already been analyzed in clinical studies conducted so far and epidemiological surveys do not provide information on adverse post -vaccination effects and their influence on child development. All developmental tests conducted within the study provided consistent results that failed to show any link between MMR and increased risk of cognitive development delays in children. The analyses of child development over the period of several years also did not provide the evidence for the association of tests scores and the type of exposure, MMR or single vaccine. The children vaccinated with MMR had even slightly higher scores of infant development in BSID-II tests in 24th and 36th month of life and in Raven at the age of five. Higher scores obtained by the vaccinated children can in no way link MMR with higher intellectual outcomes, as this effect is most likely associated with the parents’ education, intelligence or material status. During the time of the study MMR was a recommended vaccine, though it was charged extra, and maybe for this reason it was chosen by better educated and well-off parents. Therefore to avoid the bias, associated with social and economical inequalities, we included available factors such as maternal education, marital status and family economical status in final statistical models.
Wakefield’s hypothesis stated that MMR vaccine causes a series of events including intestinal inflammation, loss of intestinal barrier function, entrance into the bloodstream of encephalopathic proteins and consequent development of autism [15]. Though it has been challenged many times, there are still doubts as to MMR safety in terms of child development [11,12]. We estimate that our study is the first one that addresses MMR safety in wider sense beyond autism, and therefore it could be very considerable for public acceptance of immunization. The weak point of our research is that the results cannot be compared to any other findings reported in the literature. Only certain, limited number of quality categories can be compared and the results of these observations are compatible with the findings of other authors in providing strong evidence against association between MMR and developmental delay in children with autism [34].
An important advantage of the study is that it compares the results of developmental tests before MMR exposure (two independent tests assessing cognitive development in children administered in the 6th and 12th month of life) and after MMR immunization. No signs of cognitive developmental delay were found after MMR exposure compared to children who received single measles vaccines and those unvaccinated. All results of the different developmental tests used in the study were consistent. The tests administered in our study are highly reliable and validated, the methodology was carefully selected in all cases. The study was blinded during the collection of questionnaires and therefore the interpretation of the results was objective. Long observation period (8 years) further increased reliability of the obtained results.
The study is a prospective cohort observation, which is the most powerful tool in terms of formulating conclusions. Study design covered assessment of multiple agents that might potentially influence child development. Wide variety of available data made possible taking into consideration multiple potential confounders, which is a great benefit of the study. The obtained results had as well high level of internal agreement. No evidence was found for the links between MMR and developmental delay in the children from the cohort. The great advantage of the study is that it is at low risk of bias due to MMR vaccine manufacturers. All MMR vaccines have been registered for the use in Poland and there was no preference for any of the vaccines.
In conclusion, our results suggest that there is no relationship between MMR exposure and children cognitive development. Furthermore, the safety of triple MMR is similar to single measles vaccine with respect to cognitive development. However, as the results are of the first epidemiological study regarding that issue, the interpretation of the effects requires careful assessment.
Highlights.
Potential effect of MMR vaccine on cognitive development was examined.
There is no relationship between MMR exposure and children cognitive development
The MMR is as safe as the single measles vaccine focusing on cognitive development
Acknowledgments
The study received funding from a R01 grant entitled “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (R01ES010165-01 NIEHS) and from The Lundin Foundation, The John and Wendy Neu Family Foundation, and The Gladys and Roland Harriman Foundation. Principal investigator: Prof. FP Perera; co-investigator: Prof. W Jedrychowski.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dorota Mrozek-Budzyn, Email: dorota.mrozek-budzyn@uj.edu.pl.
Agnieszka Kiełtyka, Email: mykielty@cyf-kr.edu.pl.
Renata Majewska, Email: rmajewska@cm-uj.krakow.pl.
Małgorzata Augustyniak, Email: malgorzata.augustyniak@uj.edu.pl.
References
- 1.Casiday R, Cresswell T, Wilson D, Panter-Brick C. A survey of UK parental attitudes to the MMR vaccine and trust in medical authority. Vaccine. 2006;24(2):177–84. doi: 10.1016/j.vaccine.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 2.Elliman D, Bedford H. MMR: where are we now? Arch Dis Child. 2007;92(12):1055–7. doi: 10.1136/adc.2006.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillaume L, Bath PA. A content analysis of mass media sources in relation to the MMR vaccine scare. Health Informatics J. 2008;14(4):323–34. doi: 10.1177/1460458208096654. [DOI] [PubMed] [Google Scholar]
- 4.Casiday R. Uncertainty, decision-making and trust: lessons from the MMR controversy. Community Pract. 2006;79(11):354–7. [PubMed] [Google Scholar]
- 5.Brown KF, Kroll JS, Hudson MJ, Ramsay M, Green J, Long SJ, Vincent CA, Fraser G, Sevdalis N. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine. 2010;28(26):4235–48. doi: 10.1016/j.vaccine.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. [accessed May 2011];WHO vaccine-preventable diseases: monitoring system –2010 global summary. Available from http://whqlibdoc.who.int/hq/2010/WHO_IVB_2010_eng_p32-R242.pdf.
- 7.World Health Organization Regional Office for Europe. [accessed May 2011];WHO Epidemiological Briefing No 14. 2011 May; Available from http://www.euro.who.int/_data/assets/pdf_file/0003/142176/WHO_EPI_Brief__May_2011e.pdf.
- 8.US Centers for Disease Control and Prevention. [accessed May 2011];Morbidity and Mortality Weekly Report: Measles – United States. 2011 Jan-May; Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm60e0524a1.htm?s_cid=mm60e0524a1_w.
- 9.Skea ZC, Entwistle VA, Watt I, Russell E. ‘Avoiding harm to others’ considerations in relation to parental measles, mumps and rubella (MMR) vaccination discussions - an analysis of an online chat forum. Soc Sci Med. 2008;67(9):1382–90. doi: 10.1016/j.socscimed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Health Protection Agency Centre for Infections. Protecting the health of England’s children: the benefit of vaccines. First national report on the current status of the universal vaccine programmes from the Centre for Infections. London: Health Protection Agency Centre for Infections; May, 2005. [accessed July 2010]. Available from http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1203064757607. [Google Scholar]
- 11.Porter-Jones G, Williams S, Powell C, Pusey L, Roberts RJ. Impact of a novel way to communicate information about MMR on uptake of MMR vaccine: a randomized controlled trial. Public Health. 2009;123(1):78–80. doi: 10.1016/j.puhe.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician-gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med. 2009;37(3):231–4. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Pearce A, Law C, Elliman D, Cole TJ, Bedford H Millennium Cohort Study Child Health Group. Factors associated with uptake of measles, mumps, and rubella vaccine (MMR) and use of single antigen vaccines in a contemporary UK cohort: prospective cohort study. BMJ. 2008;336(7647):754–7. doi: 10.1136/bmj.39489.590671.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer JR, Muller CP. Measles in Europe--there is room for improvement. Lancet. 2009;373(9661):356–8. doi: 10.1016/S0140-6736(08)61850-4. [DOI] [PubMed] [Google Scholar]
- 15.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–41. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 16.General Medical Council. [accessed May 2011];Drs Wakefield, Professor Walker-Smitch, Professor Murch – Determination on Findings of Fact. 2010 Jan 28; Available from http://www.gmc-uk.org/static/documents/content/Wakefield_Smitch_Murch.pdf.
- 17.DeWilde S, Carey IM, Richards N, Hilton SR, Cook DG. Do children who become autistic consult more often after MMR vaccination? British Journal of General Practice. 2001;51:226–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkelä A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics. 2003;110(5):957–63. doi: 10.1542/peds.110.5.957. [DOI] [PubMed] [Google Scholar]
- 19.Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, Thorsen P, Olsen J, Melbye M. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347(19):1477–82. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- 20.DeStefano F, Bhasin TK, Thompson WW, Yeargin-Allsopp M, Boyle C. Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan atlanta. Pediatrics. 2004;113(2):259–66. doi: 10.1542/peds.113.2.259. [DOI] [PubMed] [Google Scholar]
- 21.Smeeth L, Cook C, Fombonne E, Heavey L, Rodrigues LC, Smith PG, Hall AJ. MMR vaccination and pervasive developmental disorders: a case-control study. Lancet. 2004;364(9438):963–9. doi: 10.1016/S0140-6736(04)17020-7. [DOI] [PubMed] [Google Scholar]
- 22.Mrozek-Budzyn D, Kiełtyka A, Majewska R. Lack of association between measles-mumps-rubella vaccination and autism in children: a case-control study. Pediatr Infect Dis J. 2010;29(5):397–400. doi: 10.1097/INF.0b013e3181c40a8a. [DOI] [PubMed] [Google Scholar]
- 23.Peltola H, Patja A, Leinikki P, Valle M, Davidkin I, Paunio M. No evidence for measles, mumps, and rubella vaccine-associated inflammatory bowel disease or autism in a 14-year prospective study. Lancet. 1998;351:1327–8. doi: 10.1016/S0140-6736(98)24018-9. [DOI] [PubMed] [Google Scholar]
- 24.Patja A, Davidkin I, Kurki T, Kallio MJ, Valle M, Peltola H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatr Infect Dis J. 2000;19(12):1127–34. doi: 10.1097/00006454-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 25.CDC. CLIA methods. Centers for Disease Control and Prevention; Atlanta, GA: 2003. Whole blood lead, cadmium and mercury determined using inductively coupled plasma mass spectrometry, DLS method code: 2003–01/OD. [Google Scholar]
- 26.Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Edwards S, Skarupa A, Lisowska-Miszczyk I. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology. 2009;32(4):270–8. doi: 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, Lisowska-Miszczyk I, Szpanowska-Wohn A, Rauh V, Skolicki Z, Kaim I, Perera F. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16(6):439–47. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. Manual for the Bayley Scales of Infant Development. 2. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 29.Department of Health. [accessed May 2011];Immunisation Against Infectious Disease (The Green Book) Available from http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_116622.pdf.
- 30.Sonnenberg P, Crowcroft NS, White JM, Ramsay ME. The contribution of single antigen measles, mumps and rubella vaccines to immunity to these infections in England and Wales. Arch Dis Child. 2007;92(9):786–789. doi: 10.1136/adc.2006.109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamlin J, Senthilnathan S, Bernstein HH. Update on universal childhood immunizations. Curr Opin Pediatr. 2008;20(4):483–489. doi: 10.1097/MOP.0b013e328306ebd1. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organisation. [accessed May 2011];Measles Mortality Reduction and Regional Elimination: Strategic Plan 2001–2005. Available from http://www.who.int/vaccines-documents/DocsPDF01/www573.pdf.
- 33.Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84(35):349–60. [PubMed] [Google Scholar]
- 34.Jick H, Kaye JA. Epidemiology and possible causes of autism. Pharmacotherapy. 2003;23(12):1524–1530. doi: 10.1592/phco.23.15.1524.31955. [DOI] [PubMed] [Google Scholar]