Abstract
Neurosteroids are endogenous allosteric modulators of GABAA receptors (GABARs), and they enhance GABAR-mediated inhibition. However, GABARs expressed on hippocampal dentate granule neurons of epileptic animals are modified such that their neurosteroid sensitivity is reduced and δ subunit expression is diminished. We explored the molecular mechanisms triggering this GABAR plasticity. In the cultured hippocampal neurons, treatment with N-methyl-D-aspartic acid (NMDA) (10 μM) for 48 hours reduced the surface expression of δ and α4 subunits but did not increase the expression of γ2 subunits. The tonic current recorded from neurons in NMDA-treated cultures was reduced, and its neurosteroid modulation was also diminished. In contrast, synaptic inhibition and its modulation by neurosteroids were preserved in these neurons. The time course of NMDA’s effects on surface and total δ subunit expression was distinct; shorter (6 hours) treatment decreased surface expression, whereas longer treatment reduced both surface and total expression. Dl-2-amino-5-phosphonopentanoic acid (APV) blocked NMDA’s effects on δ subunit expression. Chelation of calcium ions by 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM) or blockade of extracellular signal-regulated kinase (ERK) 1/2 activation by UO126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene) also prevented the effects of NMDA. Thus, prolonged activation of NMDA receptors in hippocampal neurons reduced GABAR δ subunit expression through Ca2+ entry and at least in part by ERK1/2 activation.
Introduction
Neurosteroids are potent endogenous modulators of GABAA receptors (GABARs) (Lambert et al., 2009). These compounds exert a strong anticonvulsant action, and the rank order potency of anticonvulsant action is similar to their potency at GABARs (Kokate et al., 1994, 1996). Neurosteroids convert GABA from a partial to a full agonist at δ subunit–containing GABARs (GABAR-δ) (Bianchi and Macdonald, 2003). GABAR-δs have a high affinity for GABA and desensitize slowly and incompletely. Thus, once activated, these receptors remain open longer (Saxena and Macdonald, 1994; Haas and Macdonald, 1999; Bianchi et al., 2002; Bianchi and Macdonald, 2003). GABAR-δs are localized to extrasynaptic membranes and mediate a major fraction of tonic inhibition in hippocampal dentate granule neurons (DGCs) (Nusser et al., 1998; Wei et al., 2003; Glykys et al., 2007). In contrast γ2 subunit–containing receptors (GABAR-γ2s) are localized to synaptic and extrasynaptic membrane and have a lower affinity for GABA as well as neurosteroids (Saxena and Macdonald, 1994; Belelli et al., 2002).
In animal models of temporal lobe epilepsy, GABAR-δ expression is diminished in the hippocampal DGCs (Peng et al., 2004; Zhang et al., 2007; Rajasekaran et al., 2010). In contrast, the expression of α4 subunits, which usually assemble with δ subunits, is increased (Peng et al., 2004; Rajasekaran et al., 2010). Reduction in GABAR-δ is associated with diminished neurosteroid modulation of tonic currents; however, total tonic current is preserved (Zhang et al., 2007; Rajasekaran et al., 2010). Upregulation of the γ2 subunit–containing receptors maintained tonic current and enhanced synaptic inhibition of the DGCs of epileptic animals, although neurosteroid modulation of synaptic currents is also diminished (Sun et al., 2007; Zhang et al., 2007; Zhan and Nadler, 2009; Rajasekaran et al., 2010). The molecular mechanisms that trigger these changes are not known.
Prolonged seizures or status epilepticus (SE) will often lead to the development of temporal lobe epilepsy in experimental animals (Turski et al., 1987; Cronin and Dudek, 1988; Lothman et al., 1990). One or more of the mechanisms activated during SE may downregulate the expression of GABAR-δ and upregulate the expression of GABAR-γ2. N-methyl-D-aspartic acid (NMDA) receptors (NMDARs) are activated during prolonged SE. Their blockade protects animals from excitotoxic cell death and development of spontaneous seizures (Fujikawa, 1995; Rice and DeLorenzo, 1998; Prasad et al., 2002; Brandt et al., 2003). Furthermore, NMDAR antagonists in combination with benzodiazepines can terminate SE (Rice and DeLorenzo, 1999; Borris et al., 2000; Martin and Kapur, 2008).
We tested whether NMDAR activation reduced GABAR-δ expression and upregulated that of GABAR-γ2. Our findings suggest that NMDAR activation reduced the expression of GABAR-δ via extracellular signal-regulated kinase (ERK) 1/2 signaling. However an increase in the expression of GABAR-γ2 did not occur in NMDA-treated neurons. NMDA treatment led to a reduction in total GABAR-mediated tonic current and its augmentation by neurosteroids.
Materials and Methods
Materials.
All common chemicals were obtained from the Sigma-Aldrich (St. Louis, MO). Sulfosuccinimidyl-6-(biotin-amido) hexanoate (sulfo-NHS-LC-biotin) and neutravidin agarose beads were obtained from Pierce Biotechnology (Rockford, IL). Allopregnanolone was procured from Steraloids Inc. (Newport, RI). Mitogen-activated protein kinase enzyme inhibitors UO126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene) and 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM) were from Calbiochem (Darmstadt, Germany). Human tropomyocin receptor kinase B (TrkB)-Fc chimera was from R&D Systems (Minneapolis, MN).
Antibodies.
The mouse monoclonal anti-γ2 subunit antibody 10F10-C1-B8 was prepared and characterized in our laboratory (Joshi et al., 2011; Rannals and Kapur, 2011). The anti-δ subunit antibody (clone N151/3.3) was prepared in our laboratory in cooperation with Neuromab (Davis, CA). The antibody was synthesized at the Lymphocyte Culture Center, University of Virginia. This antibody reacted with a protein of approximately 50 kDa (Supplemental Fig. 1), similar to that observed using previously characterized Millipore anti-δ subunit antibody (Joshi and Kapur, 2009; Rajasekaran et al., 2010). Antibody N151/3.3 also reacted with rat δ subunit expressed in HEK293 cells (Supplemental Fig. 1). In some experiments, rabbit polyclonal antibodies against GABAR γ2 and δ subunits (Millipore, Billerica, MA) were also used (Joshi and Kapur, 2009; Rajasekaran et al., 2010). The results obtained using monoclonal antibodies were similar to those obtained with polyclonal antibodies. Anti-α4 subunit antibody was also from Millipore. Anti pERK1/2 and ERK1/2 antibodies were from Cell Signaling Technology, Inc. (Danvers, MA).
Hippocampal Neuronal Cultures.
All animals were handled according to a protocol approved by the University of Virginia Animal Care and Use Committee, and efforts were made to minimize animal stress and discomfort. Hippocampal neuron-glia cocultures were prepared from newborn rat pups of both sexes, as described previously (Ming et al., 2006; Rannals and Kapur, 2011). Briefly, hippocampi from newborn pups were dissected free of meninges and treated with trypsin (0.125%) for 15 minutes at 37°C. After denaturation of trypsin with fetal bovine serum, hippocampi were suspended in a medium containing Dulbecco’s modified Eagle’s medium and F-12 supplement (1: 1), 10% fetal bovine serum (heat-inactivated), 2 mM l-glutamine (Sigma-Aldrich), and penicillin (100 U/ml)-streptomycin (100 U/ml); cells were separated by trituration. Neurons were plated at a density of 1,00,000/35 mm on poly-l-lysine–coated culture plates at 37°C in a humidified incubator with 95% oxygen 5% CO2. Twenty-four hours after plating, the medium was changed to a serum-free medium Dulbecco’s modified Eagle’s medium containing 2% B27 and 2 mM L-glutamine). Cultures were fed every 2 days. Cultures grown in vitro for 10–14 days were used for the experiments.
Organotypic Hippocampal Slice Cultures.
Organotypic hippocampal slice cultures were prepared from 3- to 5-day-old rat pups of both sexes, as described previously (Stoppini et al., 1991; Joshi and Kapur, 2009). Cultures grown in vitro for 8 days were treated for 2 days and used in the experiments.
Treatment of Cultures.
Cultured hippocampal pyramidal neurons or organotypic hippocampal slice cultures were treated with 10 μM NMDA or 2 μM tetrodotoxin (TTX). The regular growth medium was exchanged with the growth medium containing NMDA or TTX, and cultures were incubated for 48 hours. At the end of treatment, cultures were washed twice with regular growth medium to remove NMDA and used for experiments. To record tonic or synaptic currents, cultures were placed in an external recording solution and used for a maximum period of 3 hours after removing them from the treatment medium. Biotinylation assay was performed immediately after the cultures were removed from the treatment medium. For cotreatment of NMDA (10 μM) and different inhibitors such as dl-2-amino-5-phosphonopentanoic acid (APV) (50 μM), BAPTA-AM (10 μM), UO126 (10 μM), KN93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine)) (10 μM), KN62 (1-[N,O-bis-(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine) (5 μM), or TrkB-Fc chimera (2 μg/ml), the inhibitors were added to the culture medium 30 minutes before the addition of NMDA, and cultures were incubated for 48 hours. To study whether NMDA’s effect on δ subunit expression was reversible, cultures were incubated in the medium containing NMDA (10 μM) for 48 hours, washed twice with regular growth medium, and incubated in it for additional 48 hours.
Biotinylation and Western Blotting.
Surface proteins were biotinylated in organotypic hippocampal slice cultures as described previously (Joshi and Kapur, 2009). The experimental and control samples were processed simultaneously for all experiments at the end of treatment. Proteins from eight cultured slices were pooled for each treatment and constituted a single replicate. To determine the surface expression of GABAR subunits, tissue lysates corresponding to 100–200 μg of protein were incubated with neutravidin agarose beads, and the pulled-down proteins were loaded onto the gels. The total expression of the subunits was determined using 10–20 μg of protein. Standard electrophoresis, transfer, and Western blotting techniques were used to determine the expression of δ, α4, and γ2 subunits. Anti-δ (5 μg/ml N151/3.3 antibody or 1:500 dilution of Millipore antibody), anti-γ2 (2 μg/ml) or anti-α4 (1:250) antibodies were used. Western blots were imaged on a Kodak gel Logic 2200 imaging system (Carestream Health Molecular Imaging, New Haven, CT). The blots were reprobed with a mouse monoclonal anti-β-actin antibody (1:1000 dilution; Sigma-Aldrich) to confirm the purity of surface samples. Signal intensity was determined by densitometric scanning of the Western blots. The total expression of the GABAR subunits was normalized to the expression of β-actin. The ratio of surface to total protein in the control and experimental treatment groups was compared.
Electrophysiology.
Whole-cell patch-clamp technique was used to record GABAR-mediated currents from cultured hippocampal pyramidal neurons (Rajasekaran et al., 2010; Rannals and Kapur, 2011). All recordings were performed at room temperature. Cultured hippocampal neurons were maintained in an external solution consisting of (in millimolars) 142 NaCl, 1 CaCl2, 2.5 KCl, 6 MgCl2, 10 glucose, 10 HEPES (pH 7.4), and 310–320 mOsm. The neurons were visualized using an inverted Nikon Eclipse Ti microscope (Nikon Instruments, Inc., Melville, NY) under a 40× objective lens (NA 0.8). Cultured hippocampal slices were incubated in oxygenated artificial cerebrospinal fluid containing (in millimolars), 127 NaCl, 2 KCl, 1.5 MgSO4, 1.1 KH2PO4, 25.7 NaHCO3, 10 dextrose, and 1.5 CaCl2, 300 mOsm, in a recording chamber mounted on the stage of an Olympus BX51 microscope (Olympus Corporation, Center Valley, PA) equipped with a 40× water-immersion objective (NA 0.8), infrared differential interference constrast (Olympus) optics, and video. External solutions were perfused using a peristaltic pump at a flow rate of 2–3 ml/min. Glutamatergic currents were blocked by adding dl-2-amino-5-phosphonopentanoic acid (D, L-APV; 50 μM) and 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM) to the external solution. Three micromolar GABA and 10 μM 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid (NO711) were also included in the external solution in some experiments to measure tonic current. To record the miniature inhibitory postsynaptic currents (mIPSCs), TTX (1 μM) was added to the external medium.
Patch pipettes (resistance 5–8 MΩ) were pulled on a P-97 Flaming/Brown puller (Sutter Instrument Company, Novato, CA) using a three-stage pull protocol and filled with a recording solution containing (in millimolars) 153 CsCl, 1 MgCl2, 10 HEPES, and 5 EGTA (pH 7.3). Mg2+-ATP (5 mM), and 5 lidocaine-N-methyl bromide (10 μM) were added to the internal solution immediately before use (280–290 mOsm).
The neurons were voltage-clamped to −60 mV with an Axopatch 1D (cultured hippocampal neurons) or 200B (organotypic hippocampal slice cultures) amplifier (Molecular Devices, Sunnyvale, CA). The currents were low-pass filtered at 5 kHz and digitized at 10 kHz using a 1322A Digidata A/D converter and acquired using Axoscope 10.2 software (Molecular Devices). Access resistance was monitored with a 10-millisecond 5-mV test pulse. It was 10.4 ± 0.6 mΩ (n = 38) in the dissociated cultured hippocampal neurons and 22.7 ± 2.2 mΩ (n = 28) in the DGCs of organotypic hippocampal slice cultures. The recordings were terminated if access resistance changed more than 25% at any time.
Analysis of Currents.
The current required to clamp neurons to −60 mV (Ihold) was measured. Ihold was determined by averaging the mean current in a 250-ms epoch sampled every 2500 ms using MiniAnalysis (Synaptosoft, Fort Lee, NJ). Immediately before drug application and 5 minutes after drug application, when a steady-state response was observed, 30 to 50 of these epochs were collected. The contribution of synaptic currents to Ihold measures was eliminated by removing epochs containing synaptic events. The drug effects on individual neurons were assessed by comparing the mean holding current before and after drug application using a paired t test. The shift in the mean Ihold (ΔIhold) after drug application relative to the baseline was calculated (Supplemental Fig. 2). At the end of recording, total GABAR-mediated tonic current was determined by applying picrotoxin to block all GABARs. The Ihold recorded from control (226 ± 20 pA, n = 10) and NMDA-treated cultured hippocampal neurons (224 ± 19, n = 12) was similar (P > 0.05, t test). The baseline current recorded from DGCs of untreated (97 ± 13 pA, n = 19) or NMDA-treated (74 ± 8 pA, n = 18) organotypic hippocampal slice cultures was also similar (P > 0.05, t test).
The mIPSCs were analyzed using MiniAnalysis software as before (Sun et al., 2007). The detection threshold was set at 5 times the root mean square noise. After detection, decay time constants and peak amplitude were analyzed in individual mIPSCs. The decay was analyzed in 10–90% of the peak amplitude, and 100 iterations were used for each event. The mIPSCs could be fitted with double-exponential decay time constants. The weighted decay time constant (τw) was then calculated with the following equation: τw = τ1 A1 + τ2A2/ (A1A2), where τ1 and τ2 are fast and slow decay time constants (first and second exponential functions), and A1 and A2 represent the magnitude of fast and slow components, respectively.
Statistical Analysis.
The results are presented as the mean ± S.E.M. Data were compared using either a t test or a one-way analysis of variance followed by Dunnett’s multiple comparison post hoc test. In the voltage-clamp recordings, n represents number of neurons, whereas it represents number of replicates in the biochemical experiments.
Results
NMDA Treatment Reduced GABAR-δ Expression in Cultured Hippocampal Neurons.
The effect of prolonged NMDA treatment on the surface and total expression of δ, α4, and γ2 subunits was studied in organotypic hippocampal slice cultures. The cultures were maintained in a medium supplemented with 10 μM NMDA for 48 hours (NMDA-treated neurons) or in the regular culture medium (control neurons), and GABAR surface expression was studied by a biotinylation assay.
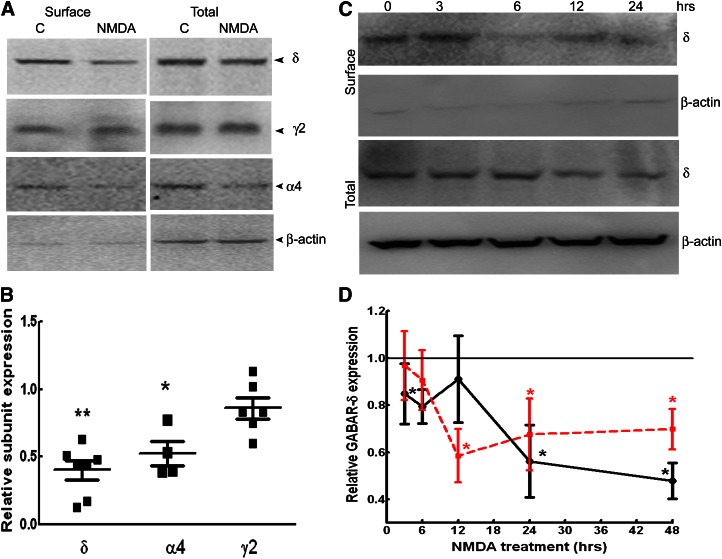
Surface expression of δ and α4 subunits was reduced in NMDA-treated cultures, and that of γ2 subunits was unaltered (Fig. 1, A and B). In NMDA-treated cultures, surface δ subunit expression was only 40% ± 7% of that in controls (n = 7, P < 0.05). Total δ subunit expression was also diminished in NMDA-treated cultures (70% ± 7%, n = 7, P < 0.05). Surface expression of α4 subunits was also less in NMDA-treated cultures (52% ± 9%, n = 4, P < 0.05). In contrast, the total expression and surface expression of the γ2 subunit were unchanged in NMDA-treated cultures (86% ± 8%, n = 6, P > 0.05). Together, these studies revealed that NMDA treatment reduced the expression of α4δ subunit–containing GABARs, but it did not upregulate GABAR-γ2 expression.
Fig. 1.
NMDA treatment reduced surface expression of GABAR-δ. (A) Representative Western blots showing the surface and total expression of the δ, α4, and γ2 subunits in organotypic hippocampal slice cultures incubated in regular growth medium or medium supplemented with NMDA (10 μM) for 48 hours The expression of β-actin is shown to demonstrate the purity of the surface samples. Rabbit polyclonal antibodies against the δ, γ2, and α4 subunits (Millipore) were used in these experiments. (B) Relative surface expression of the δ, α4, and γ2 subunits of GABARs in NMDA-treated cultures. n = 4–6 experiments. * P < 0.05; ** P < 0.005. (C) A representative Western blot demonstrating the time course of changes in δ subunit surface expression in NMDA-treated slice cultures. Mouse monoclonal anti-δ subunit antibody (N151/3.3) was used in these experiments. (D) Time course of change in GABAR-δ surface (black solid line) and total expression (red dotted line) after NMDA treatment relative to that at time 0. Data represent average and standard error from five experiments. * P < 0.05.
The time course of NMDA effects was characterized by treating cultures for 3–24 hours. NMDA had distinct effects on both total and surface δ subunit expression (Fig. 1, C and D). Reduced surface and total δ subunit expression was evident in cultures treated for 24 and 48 hours (n = 5, P < 0.05). In contrast, after 12 hours of NDMA treatment, although total δ subunit expression was decreased (n = 5, P < 0.05), its surface expression was unaltered (n = 5, P > 0.05). Furthermore, 6 hours of NMDA treatment reduced surface δ subunit expression without altering its total expression (n = 5, P < 0.05).
The effect of various NMDA concentrations on δ subunit expression was also determined. In cultures treated with 3 μM NMDA, δ subunit expression was 118% ± 30% (n = 4, P > 0.05), similar to that in untreated cultures; however, expression was lower in the cultures treated with 7.5 μM NMDA (64% ± 17%, n = 3, P < 0.05).
NMDA induces excitotoxic damage in hippocampal slice cultures (Sakaguchi et al., 1997), which may play a role in the downregulation of δ subunit expression; however, such effects should not be reversible. Hence, the effect of washout of NMDA was determined. In the slice cultures exposed to NMDA (10 μM) for 48 hours, followed by incubation in NMDA-free medium for an additional 48 hours, surface δ subunit expression showed a trend toward recovery. In four replicates, surface δ subunit expression was 74% ± 17%, which was similar to that in controls (P > 0.05) and slightly higher than δ subunit expression in NMDA-treated cultures without washout (38% ± 18%).
NMDA Treatment Also Reduced Tonic Current and its Neurosteroid Modulation.
GABAR-δs are localized to the extrasynaptic membrane and mediate tonic current and its neurosteroid modulation (Nusser et al., 1998; Wei et al., 2003; Glykys and Mody, 2007). Hence, the physiologic significance of NMDA-induced downregulation of GABAR-δ expression was determined by measuring tonic current and its modulation by neurosteroids. Tonic current was the change in holding current induced by blocking GABARs with picrotoxin, a noncompetitive antagonist. In the dissociated cultured hippocampal pyramidal neurons, tonic current and its neurosteroid modulation were diminished after NMDA treatment (Fig. 2, A and B). Tonic current recorded from NMDA-treated neurons was only 18 ± 3 pA (n = 6 neurons from five cultures, P < 0.05), significantly smaller than that recorded from untreated neurons (28 ± 4 pA, n = 7 neurons from four cultures). Furthermore, allopregnanolone (10 nM) enhanced tonic current recorded from NMDA-treated neurons only minimally compared with control neurons (8 ± 3 pA, n = 6 neurons compared with 23 ± 3 pA, n = 7 neurons, P < 0.05).
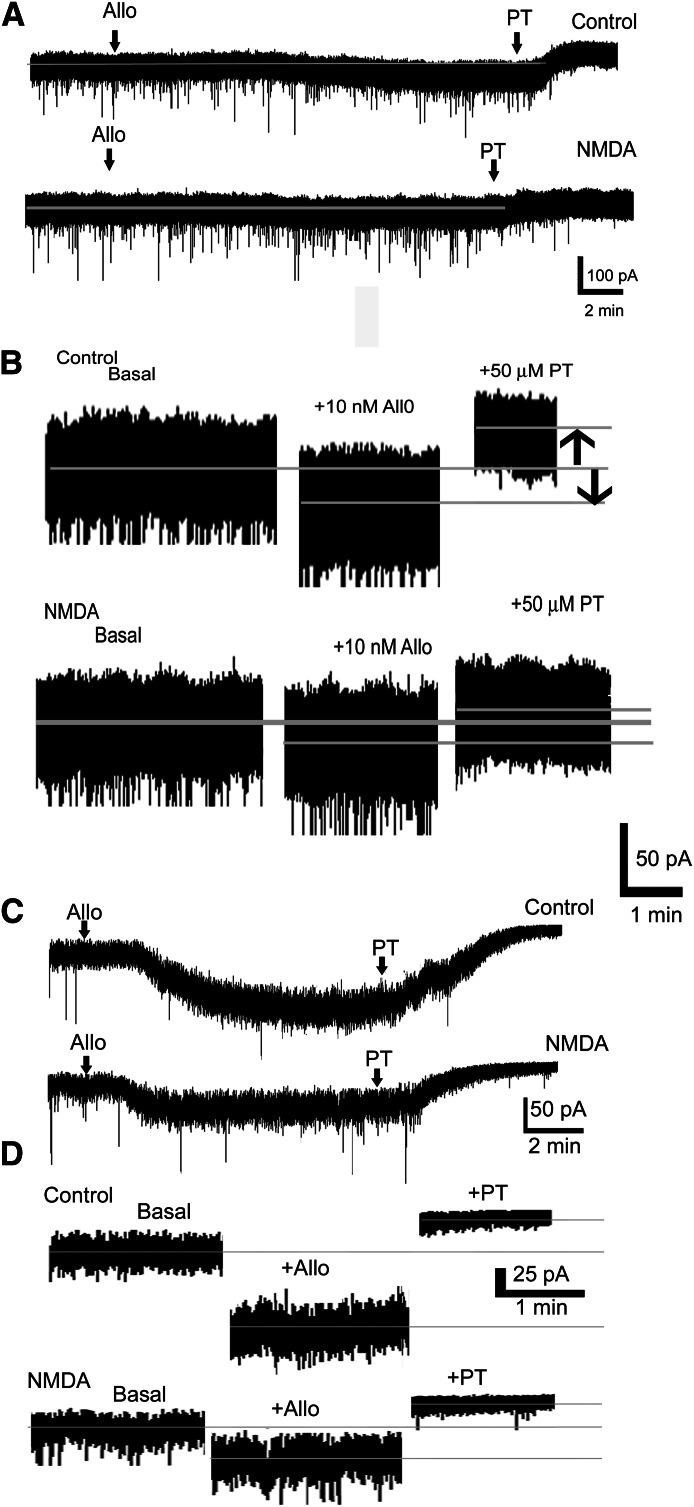
Fig. 2.
NMDA treatment reduced tonic current and its neurosteroid modulation. (A) Representative voltage-clamp recordings from control and NMDA-treated cultured hippocampal pyramidal neurons show the effect of the bath application of allopregnanolone (10 nM, allo) and picrotoxin (50 μM, PT). Neurons were incubated in normal growth medium (control) or in a medium containing 10 μM NMDA for 48 hours. Gray line running through the trace indicates basal holding current. (B) Magnified portion of traces from (A) showing tonic current before and after drug application, when a steady response was obtained. Gray lines through the trace mark the position of mean holding current. Upward arrow indicates tonic current, and downward arrow indicates neurosteroid modulation of tonic current. (C) Representative voltage-clamp recordings demonstrating the effect of bath application of 10 nM allopregnanolone (arrow, allo) on tonic current recorded from DGCs of untreated (control) and NMDA-treated (10 μM, 48 hours) organotypic hippocampal slice cultures. Tonic current was determined by bath application of picrotoxin (50 μM, PT, arrow) after allopregnanolone application. The external solution contained GABA (1 μM) and NO711 (10 μM). (D) Magnified portions of traces from (A) to demonstrate the effect of allopregnanolone and picrotoxin on holding current.
In some cells, the effect of allopregnanolone was studied in the presence of GABAR blocker picrotoxin (50 μM). Allopregnanolone did not enhance Ihold in these neurons (Ihold −83 ± 22 pA versus −82 ± 23 pA, n = 6 neurons from three cultures, P > 0.05) and confirmed that the observed effect of allopregnanolone was through the modulation of GABARs.
NMDA treatment could alter GABA release or uptake, which could influence allopregnanolone’s modulation of tonic current. Hence, in some recordings, external solution was supplemented with GABA (1 μM) and GABA uptake blocker NO711 (10 μM). Tonic current recorded from NMDA-treated neurons under the condition of artificially increased GABA concentration was also less sensitive to modulation by allopregnanolone (7 ± 1 pA, n = 4 versus 34 ± 7 pA, n = 5 neurons from four cultures, P < 0.05).
Changes in cell volume and expression of ion channels in NMDA-treated neurons may alter whole-cell capacitance, which could influence neurosteroid modulation of tonic current. Therefore, the change in tonic current was normalized to whole-cell capacitance. Allopregnanolone modulation of tonic current was significantly smaller in NMDA-treated neurons (1.6 ± 0.6 pA/pF, n = 6 versus 6.4 ± 0.7 pA/pF, n = 7, P < 0.05). This finding indicated that the observed reduction in neurosteroid modulation of tonic current was not due to changes in capacitance.
NMDA-induced reduction in tonic current and its neurosteroid modulation was also confirmed in DGCs of organotypic hippocampal slice cultures. Similar to cultured hippocampal pyramidal neurons, tonic current (54 ± 7 pA, n = 18 compared with 84 ± 12 pA, n = 18 neurons from five to seven cultures, P < 0.05) and its allopregnanolone (10 nM) modulation (25 ± 4 pA, n = 8 compared with 47 ± 6 pA, n = 8 neurons from four or five, P < 0.005) were diminished in DGCs of NMDA-treated slices (Fig. 2, C and D). Together, these studies revealed that NMDA-induced downregulation of GABAR-δ was associated with reduced tonic current and its neurosteroid modulation.
Allopregnanolone Modulation of mIPSCs Was Preserved in NMDA-Treated Neurons.
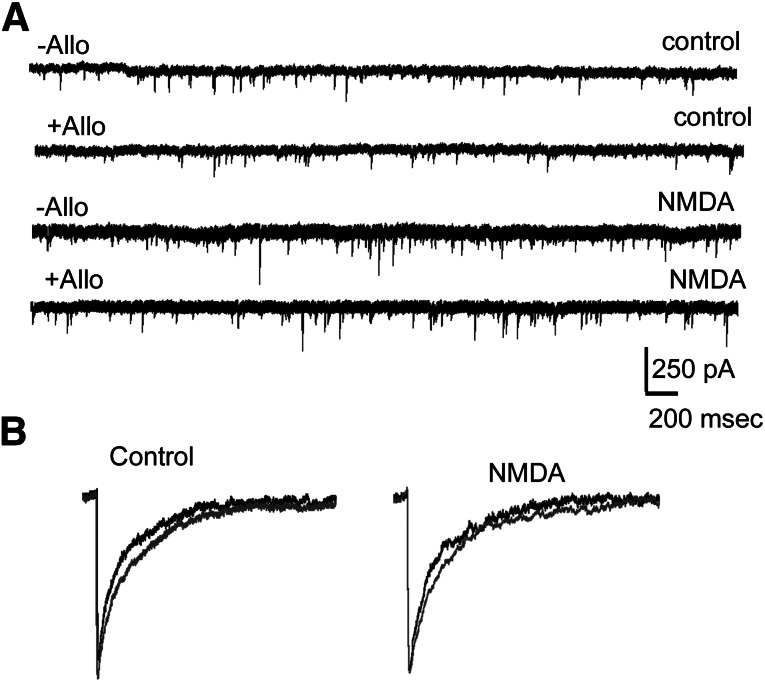
Synaptic inhibition is augmented in epileptic animals; however, its neurosteroid modulation is reduced likely as a result of the presence of α4 subunit containing receptors at the synapses (Sun et al., 2007). We measured synaptic currents and their neurosteroid modulation in NMDA-treated dissociated hippocampal neuronal cultures (Fig. 3, A and B). Synaptic currents recorded from NMDA-treated neurons tended to be more frequent (0.51 ± 0.2 Hz compared with 0.3 ± 0.12 Hz, n = 7 neurons from four cultures) and with larger amplitudes (73 ±7 pA compared with 63 ± 3 pA, n = 7 neurons from four cultures) compared with those recorded from untreated neurons; however, these differences were not statistically significant (P > 0.05). The 10–90% rise time (2.5 ± 0.4 milliseconds compared with 2.6 ± 0.4 milliseconds, n = 7) and decay (39 ± 7 ms compared with 31 ± 4 ms, n = 5–7) of mIPSCs recorded from NMDA-treated and -untreated neurons were comparable (P > 0.05). Furthermore, allopregnanolone (10 nM) prolonged the decay of mIPSCs recorded from NMDA-treated and -untreated neurons to a similar extent (change in the decay: 18 ± 4 milliseconds, n = 7 compared with 16 ± 2 milliseconds, n = 5 neurons, P > 0.05) without altering other parameters of mIPSCs (unpublished data). Thus, NMDA treatment neither altered GABAR-γ2 expression nor changed the characteristics of mIPSCs.
Fig. 3.
NMDA treatment did not affect synaptic currents or their neurosteroid modulation. (A) Representative voltage clamp recordings showing effect of allopregnanolone (10 nM) on cultured dissociated hippocampal neurons either untreated or treated with NMDA (10 μM, 48 hours). The external solution contained TTX. (B) Averaged currents from representative control and NMDA-treated neurons before (black) and after application of allopregnanolone (gray).
Activity Blockade by TTX Increased GABAR-δ subunit Expression.
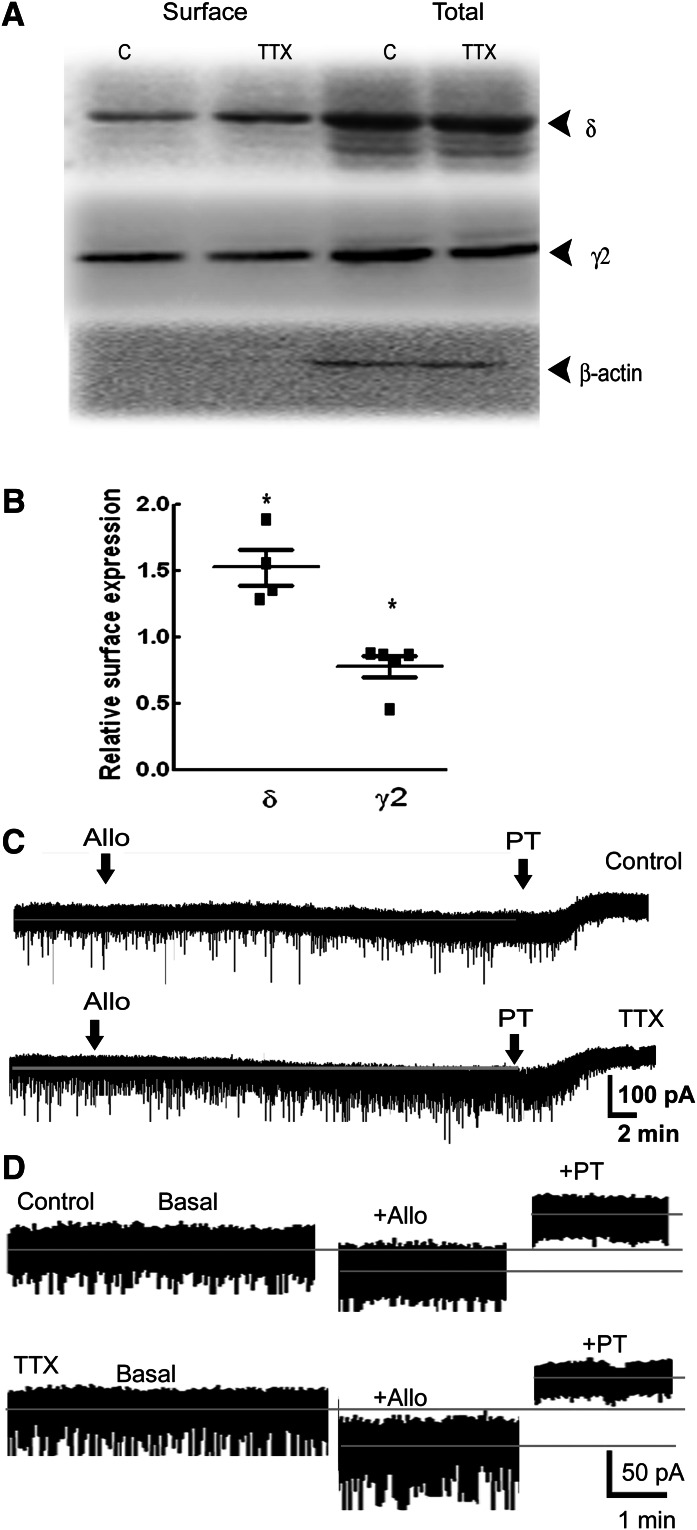
In subsequent experiments, the effect of activity blockade on GABAR-δ expression was determined. TTX treatment of slice cultures (2 μM, 48 hours) increased GABAR-δ surface expression (Fig. 4, A and B) (152% ± 13% n = 4, P < 0.05), whereas surface γ2 subunit expression was decreased (78% ± 8%, n = 4, P < 0.05). Consistent with the biochemical findings, tonic current recorded from TTX-treated neurons was unaltered (36 ± 6 pA, n = 5 compared with 25 ± 4 pA, n = 7 neurons from four cultures), but its modulation by allopregnanolone was enhanced (38 ± 5 pA, n = 5, compared with 24 ± 3 pA, n = 7, P < 0.05) (Fig. 4, C and D).
Fig. 4.
Activity blockade by TTX increased GABAR-δ expression. (A) A representative Western blot demonstrating surface and total expression of δ and γ2 subunits of GABARs in organotypic hippocampal slice cultures treated with TTX (2 μM, 48 hours). Expression of β-actin demonstrates purity of the surface samples. Millipore anti-δ subunit antibody was used in these experiments. (B) Relative surface expression of δ and γ2 subunits of GABARs in TTX-treated cultures, n = 4 experiments, *P < 0.05. (C) Representative current traces demonstrating the effect of allopregnanolone (10 nM, allo, arrow) on tonic current in cultured hippocampal pyramidal neurons treated with TTX (2 μM, 48 hours). Picrotoxin (50 μM, PT) was applied after recording the effect of allopregnanolone to determine tonic current. Gray line running through the trace represents the position of the basal holding current. (D) Magnified portions of traces from (A) showing the effect of allopregnanolone and picrotoxin.
NMDAR Activation and Calcium Influx Were Necessary for NMDA-Induced Downregulation of δ Subunit Expression.
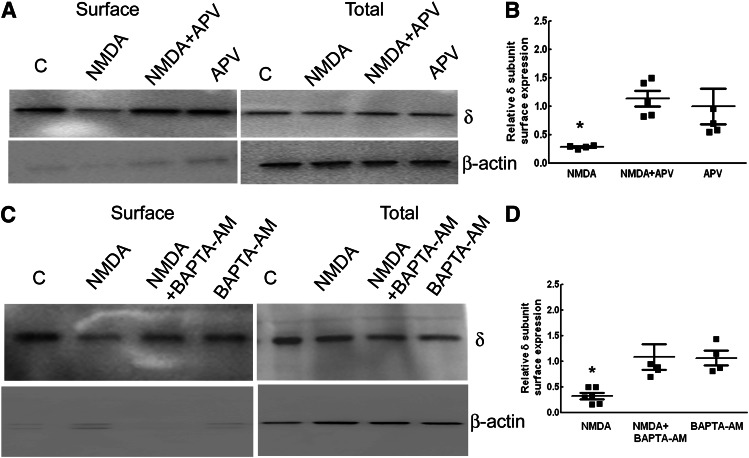
To confirm that NMDA-induced NMDAR activation led to downregulation of δ subunit expression, cultures were cotreated with NMDA and APV. APV blocked NMDA-induced reduction in surface GABAR-δ expression without altering its expression under basal conditions (Fig. 5, A and B). Surface δ subunit expression in the neurons cotreated with NMDA and APV was (113% ± 14%, n = 5) greater than that in slices treated with NMDA alone (30% ± 11%, n = 5, P < 0.05) but similar to that in cultures treated with only APV (100% ± 31%, n = 5). Tonic current and its neurosteroid modulation were also preserved in the DGCs of organotypic slice cultures cotreated with APV and NMDA (Table 1). Thus, the NMDA effects were mediated via NMDAR activation.
Fig. 5.
NMDA effects on the δ subunit expression were mediated by activation of NMDARs and calcium flux. (A) A representative Western blot showing surface and total expression of the δ subunit in NMDA-treated (for 48 hours) slices in the presence or absence of APV (50 μM). (B) Relative surface δ subunit expression. n = 5, * P < 0.05. (C) A representative Western blot demonstrating δ subunit surface and total expression in slices treated with NMDA in the presence or absence of BAPTA-AM (10 μM) for 48 hours. Expression of β-actin demonstrates the purity of the surface samples. Anti-δ subunit antibody (N151/3.3) was used in these studies. (D) Bar graph showing the relative surface δ subunit expression. n = 5 experiments, *P < 0.05.
TABLE 1.
Effect of NMDA receptor activation on tonic currents and their neurosteroid modulation is mediated at least in part by ERK1/2 activation
Organotypic hippocampal slice cultures were treated with NMDA (10 μM) without or with APV (50 μM), BAPTA-AM (10 μM), KN62 (5 μM), UO126 (10 μM), or TrkB-Fc (2 μg/ml) for 48 hours. Tonic current and its neurosteroid modulation were recorded from dentate granule neurons. Cultures without any treatment grown in parallel were used as controls. Data represent mean ± S.E.M.
| Treatment | Tonic Current | Neurosteroid Modulation of Tonic Current |
|---|---|---|
| Controls | 84 ± 12 pA (n = 18) | 47 ± 6 pA (n = 8) |
| NMDA | 54 ± 7 pA* (n = 18) | 25 ± 4 pA* (n = 8) |
| NMDA + APV | 98 ± 17 pA (n = 7) | 37 ± 8 pA (n = 5) |
| NMDA + BAPTA-AM | 93 ± 14 pA (n = 5) | 24 ± 4 pA* (n = 5) |
| NMDA + UO126 | 72 ± 14 pA (n = 11) | 39 ± 6 pA (n = 8) |
| NMDA + KN62 | 63 ± 9 pA (n = 7) | 16 ± 7 pA* (n = 5) |
| NMDA + TrkB-Fc | 90 ± 22 pA (n = 4) | 15 ± 5 pA* (n = 4) |
P < 0.05.
NMDAR activation leads to calcium flux, and the role of calcium in NMDA-induced downregulation of δ subunit expression was determined by chelating intracellular calcium by BAPTA-AM. Calcium ion chelation blocked NMDA-induced downregulation of δ subunit expression without affecting basal δ subunit expression (Fig. 5, C and D). Surface δ subunit expression in cultures cotreated with NMDA and BAPTA-AM was 109% ± 24% (n = 5), higher than that in cultures treated with NMDA alone (33% ± 6%, n = 5, P < 0.05), but similar to that in cultures treated with BAPTA-AM alone (107% ± 14%, n = 4). The tonic current recorded from DGCs of NMDA and BAPTA-AM cotreated cultures was comparable to that in controls; however, its neurosteroid modulation remained diminished (Table 1).
ERK1/2 Activation Was Associated with NMDA-Induced Downregulation of δ Subunit Expression.
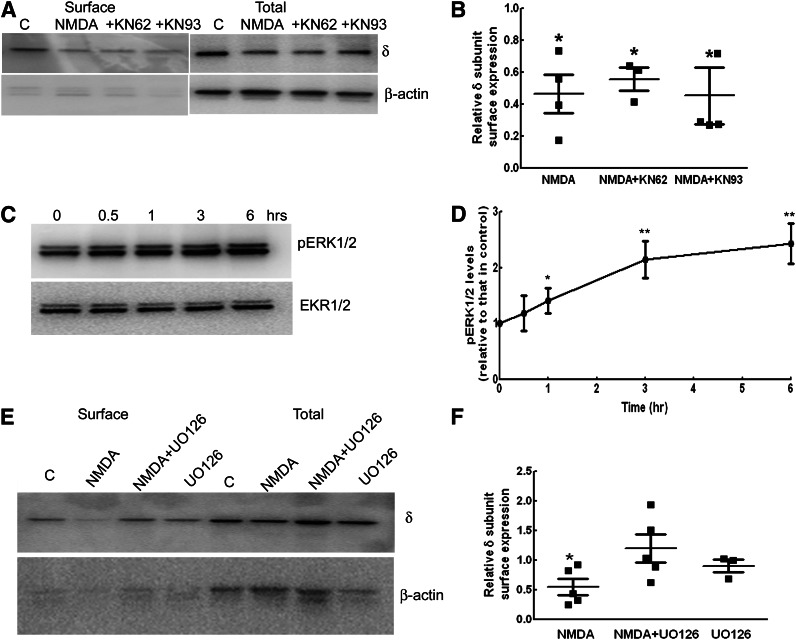
Calcium flux after NMDAR activation could activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Fukunaga and Soderling, 1990; Fukunaga et al., 1992). We determined whether activation of CaMKII was necessary for NMDA-induced downregulation of GABAR-δ expression by cotreating cultures with NMDA and KN62 (5 μM) or KN93 (10 μM), which are known to block CaMKII activity (Tokumitsu et al., 1990; Sumi et al., 1991). However cotreatment of cultures with KN62 or KN93 did not prevent NMDA-induced downregulation of GABAR-δ expression (Fig. 6, A and B). Surface δ subunit expression in slices cotreated with NMDA and KN62 or KN93 was 55% ± 7% and 45% ± 17%, respectively (n = 4, P < 0.05), which was similar to surface δ subunit expression in slices treated with NMDA alone (54% ± 16%, n = 4). CaMKII blockade did not alter basal GABAR-δ subunit expression (unpublished data). Thus, activation of CaMKII did not appear to mediate NMDA effects. In accordance with the biochemical studies, allopregnanolone did not enhance tonic current recorded from the DGCs of slice cultures treated with NMDA and KN62 (Table 1).
Fig. 6.
Blockade of ERK1/2 activation prevented NMDA-induced downregulation of the δ subunit expression. (A) A representative Western blot showing the surface and total δ subunit expression in slice cultures treated with NMDA in the presence or absence of CaMKII inhibitor KN62 (5 μM) or KN93 (10 μM) (+KN62, +KN93). (B) Graph shows the relative surface expression of the δ subunit. n = 4, * P < 0.05. (C) A representative Western blot demonstrating the time course of ERK1/2 phosphorylation after NMDA treatment. (D) The ratio of pERK1/2 to total ERK1/2 relative to that in controls was plotted as a function of time, n = 4, *P < 0.05; **P < 0.005. (E) Blocking ERK1/2 activation prevented NMDA effects on δ subunit surface expression. UO126 (10 μM), an inhibitor of MEK1/2, was added 30 minutes before NMDA, and the surface δ subunit expression was determined after 48 hours. Mouse monoclonal anti-δ subunit antibody (N151/3.3) was used in the experiments in (A) and (D). (F) Bar graph showing relative surface expression of δ subunit. n = 5, * P < 0.05.
Activity of ERK1/2 is increased in the neurons during SE, which could involve NMDAR activation (Bading and Greenberg, 1991; Kim et al., 1994; Garrido et al., 1998; Berkeley et al., 2002). ERK1/2 phosphorylation was studied after NMDA treatment, and it appeared to increase as early as 1 hour after NMDA treatment and was sustained even after 6 hours of NMDA treatment (Fig. 6, C and D). Levels of pERK1/2 were 140% ± 22% (n = 5, P < 0.05) of that in controls after 1 hour of NMDA treatment, increased to 214% ± 33% and 243% ± 35% after 3 and 6 hours of NMDA treatment, respectively (n = 4, P < 0.05). We then determined whether blockade of ERK1/2 activation prevented NMDA-induced downregulation of δ subunit expression. Protein kinases mitogen-activated protein kinase enzyme 1/2 phosphorylate activate ERK1/2, and their inhibition by UO126 prevents activation of ERK1/2 (Zheng and Guan, 1993; Favata et al., 1998; Roskoski, 2012). Cotreatment of NMDA and UO126 (10 μM) prevented δ subunit downregulation (Fig. 6, E and F). Surface expression of the δ subunit in NMDA- and UO126-treated cultures was 120% ± 23% that of control cultures (n = 5), greater than that in cultures treated with NMDA alone (55% ± 13%, n = 5, P < 0.05). Blockade of ERK1/2 under basal conditions did not affect δ subunit expression (85% ± 16% of that in controls, n = 3, P > 0.05). Tonic current recorded from the DGCs of NMDA- and UO126-treated slice cultures was also comparable to that recorded from control neurons and was enhanced by allopregnanolone (Table 1). Thus, the NMDA effects were at least in part mediated by the activation of ERK1/2.
Activation of TrkB Receptors Did Not Mediate NMDA Effects on δ Subunit Expression.
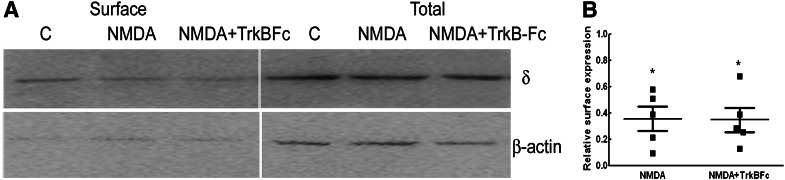
Activation of TrkB receptors is an important epileptogenic stimulus and could play a role in NMDA-induced downregulation of GABAR-δ expression (Binder et al., 2001; He et al., 2004; Kotloski and McNamara, 2010). The role of TrkB receptors in mediating the NMDA effect was determined in cultures cotreated with NMDA and TrkB-Fc (2 μg/ml, 48 hours), which is known to block biologic activity of brain-derived neurotrophic factor (BDNF). Surface expression of GABAR-δ was lower in cultures treated with NMDA in the presence or absence of TrkB-Fc (Fig. 7, A and B). The δ subunit surface expression in cultures treated with NMDA without or with TrkB-Fc was 47% ± 6% and 38% ± 8% of that in controls, respectively (n = 4, P < 0.05). Neurosteroid modulation of tonic current was also diminished in DGCs of cultures treated with NMDA or TrkB-Fc (Table 1). Hence, NMDA’s effects did not require activation of TrkB receptors.
Fig. 7.
Activation of TrkB receptors was not necessary for NMDA effects. (A) A representative Western blot demonstrating the surface and total δ subunit expression in slice cultures treated with NMDA without or with TrkB-Fc chimera (2 μg/ml) for 48 hours. The expression of β-actin demonstrates the purity of the surface samples. Millipore anti-δ subunit antibody was used in these experiments. (B) Relative surface δ subunit expression from four experiments. *P < 0.05.
Discussion
Key findings of this study were 1) 48-hour NMDA treatment of hippocampal neuronal cultures reduced δ and α4 subunit expression but did not change the expression of γ2 subunits; 2) NMDA treatment also reduced tonic current and its neurosteroid modulation, but synaptic currents and their neurosteroid modulation were preserved; 3) activity blockade by TTX induced changes opposite those triggered by NMDA; 4) NMDA’s effects were mediated via NMDAR activation and required calcium flux; and 5) the NMDA effects were mediated at least in part by ERK1/2 phosphorylation.
In the DGCs of epileptic animals, expression of the δ subunit is reduced, whereas that of α4 and γ2 subunits is upregulated (Peng et al., 2004; Sun et al., 2007; Zhang et al., 2007; Rajasekaran et al., 2010). Current study demonstrated that NMDAR activation diminished δ subunit expression but did not increase the expression of α4 and γ2 subunits. BDNF-activated TrkB signaling increases the α4 subunit expression (Roberts et al., 2006). In contrast, the downregulation of δ subunit expression in NMDA-treated hippocampal slice cultures was independent of BDNF signaling. Thus, the signaling pathways underlying a reduction in δ subunit expression and upregulation of α4 and γ2 subunits expression appear to be distinct.
Hippocampal slice cultures were used to determine the expression of δ and α4 subunits because they yield sufficient protein for biochemical analysis. In the hippocampus, the δ subunit is expressed primarily in the DGCs, as well as in hilar interneurons (Sperk et al., 1997; Wei et al., 2003; Glykys et al., 2007). However, in the proteins isolated from hippocampal slice cultures, the fraction of the δ subunit originating from interneurons should be minor compared with that originating from the DGCs. Consistent with this interpretation, reduced δ subunit expression was associated with diminished tonic current recorded from DGCs of NMDA-treated slice cultures. High-affinity, slowly desensitizing receptors mediate tonic inhibition of DGCs (Mtchedlishvili et al. 2006). The properties of δ subunit–containing receptors are uniquely suited for this function (Saxena and Macdonald, 1994; Nusser et al., 1998; Haas and Macdonald, 1999; Bianchi et al., 2002; Bianchi and Macdonald, 2003; Wei et al., 2003).
Neurosteroid modulation of the tonic current recorded from DGCs of NMDA-treated slice cultures was also reduced. GABARs containing a δ subunit have greater neurosteroid sensitivity than do other GABAR subtypes, and neurosteroids convert GABA from a partial to a full agonist at these receptors (Belelli et al., 2002; Wohlfarth et al., 2002; Bianchi and Macdonald, 2003). Animals lacking the δ subunit also have attenuated neurosteroid sensitivity (Mihalek et al., 1999). Increased phosphorylation of the β2/3 subunits of GABARs is also associated with reduced neurosteroid enhancement of synaptic and tonic GABAR currents (Fáncsik et al., 2000; Kia et al., 2011). The role of GABAR phosphorylation was not determined in this study. Pharmacological perturbations that prevented NMDA-induced downregulation of δ subunit expression also blocked diminution in neurosteroid modulation of tonic current. However, in the cultures cotreated with BAPTA-AM and NMDA, neurosteroid modulation of tonic current did not recover. The reason for this discrepancy is unclear. It is possible that calcium-regulated mechanisms play a role in neurosteroid modulation of tonic current under basal conditions.
In a previous study, prolonged NMDA treatment of cultured cerebellar granule neurons did not affect the expression of δ subunit mRNA but increased expression of γ2 subunit mRNA (Memo et al., 1991). These differences could be due to the different neuronal types studied and because mRNA and protein expression may be distinctly regulated. Further studies are needed to determine the effect of NMDA treatment on the expression of mRNA encoding the δ and α4 subunits in hippocampal neurons.
The time course of reduction in surface and total expression of δ subunits in NMDA-treated cultures was distinct; shorter treatment reduced only surface expression of δ subunits, whereas prolonged treatment also decreased total expression. Altered kinetics of receptor trafficking could reduce surface expression of δ subunits after 6 hours of NMDA treatment. Expression of δ subunits in the endoplasmic reticulum fraction is greater in epileptic animals, suggestive of their potential endoplasmic reticulum retention and a likely impairment in surface membrane insertion (Rajasekaran et al., 2010). In contrast, prolonged NMDA treatment likely involved trafficking and genomic changes.
NMDAR activation could induce excitotoxic cell death, which may play a role in the downregulation of δ subunit expression (Rothman, 1985; Rothman and Olney, 1987; Choi et al., 1988). However, previous studies indicated that cornu ammonis (CA)3 and CA1 neurons are predominately susceptible to cell death, whereas DGCs are comparatively resistant (Engel, 1996; Sakaguchi et al., 1997). Furthermore, the time course of NMDA effects on surface and total expression of δ subunits was distinct, which argues against a nonspecific action of NMDA on δ subunit expression. In the cultures in which NMDA was washed out, δ subunit expression showed a trend toward recovery, suggesting that the NMDA effects could be partially reversed. It is important to note that cell death cannot account for diminution in tonic current and its neurosteroid modulation recorded from the DGCs of NMDA-treated slice cultures.
NMDAR activation leads to calcium influx, which could activate CaMKII, PKC, mitogen activated protein kinases, and protein phosphatases (MacDermott et al., 1986; Berridge, 1998; DeLorenzo et al., 1998; Hardingham and Bading, 2003). Phosphorylation of ERK1/2 was increased in the NMDA-treated slice cultures and appeared to play a role in the downregulation of δ subunit expression. Increased ERK1/2 phosphorylation levels could originate from neurons or glia. However, previous immunohistochemical studies in brain slices revealed increased pERK1/2 immunoreactivity in the neurons during SE or after spontaneous seizures (Kim et al., 1994; Garrido et al., 1998; Berkeley et al., 2002; Houser et al., 2008).
This study revealed a connection between NMDAR-induced calcium flux, ERK1/2 activation, and δ subunit expression. An interaction between the NR2B subunit of NMDARs and CaMKII or RasGRF1 may lead to ERK1/2 activation (Kurino et al., 1995; Krapivinsky et al., 2003; El Gaamouch et al., 2012). It is noteworthy that activation of CaMKII was not necessary for the NMDA effects on δ subunit expression. In a previous study, bath application of glutamate reduced CaMKII activity in cultured hippocampal neurons (Churn et al., 1995), which also supports the findings presented here. Future studies addressing the role of Ras, phosphoinositide 3-kinase-Akt, adenylylcyclase, and heterotrimeric G proteins, which may play a role in calcium-induced activation of ERK1/2, could provide additional insight on NMDA-induced ERK1/2 activation (Lopez-Ilasaca 1998; Agell et al., 2002; Holstein et al., 2004; Xia and Storm, 2012). NMDA-induced oxidative stress could also lead to ERK1/2 activation (Lafon-Cazal et al., 1993; Rybakova et al., 2012).
The expression of γ2 subunits was not altered in NMDA-treated hippocampal slice cultures, and synaptic inhibition recorded from NMDA-treated cultured hippocampal pyramidal neurons was unchanged. However, these electrophysiological and biochemical studies cannot be directly correlated at this point because of the use of different culture preparations. Future studies are necessary to address whether synaptic inhibition of DGCs in NMDA-treated slice cultures was also preserved.
The δ subunit–containing GABARs play an important role in regulating neuronal excitability (Stell et al., 2003). In the δ subunit, the knockout mice threshold to pentelenetetrazole-induced seizures is diminished (Spigelman et al., 2002). The dentate gating hypothesis suggests that an impaired inhibitory function of DGCs could allow spread of seizures into CA subfields of the hippocampus (Lothman et al., 1992). DGCs shield excitable pyramidal neurons of CA3 and CA1 regions from spread of the activity from the entorhinal cortex (Stringer and Lothman, 1989; Stringer et al., 1989). However, if the dentate gate is compromised, activity from the entorhinal cortex can spread into the hippocampal CA subfields, forming a re-entrant loop, which could amplify seizure activity. Previous studies revealed a rapid reduction in δ subunit expression after SE, which is not accompanied by increased expression of α4 and γ2 subunits (Peng et al., 2004). The dentate gating function appears to be impaired after SE (Pathak et al., 2007). Hence, future studies in experimental animals will be important to determine whether blocking ERK1/2 activation after SE can prevent downregulation of δ subunit expression, maintain the dentate gating function, and alter the course of epileptogenesis.
Supplementary Material
Acknowledgments
The authors thank Kendra J. Keith for the hippocampal neuronal cultures and Suji Cha for technical assistance.
Abbreviations
- APV
dl-2-amino-5-phosphonopentanoic acid
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid tetrakis (acetoxymethyl ester)
- BDNF
brain-derived neurotrophic factor
- CA
cornu ammonis
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DGC
dentate granule neuron
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- ERK1/2
extracellular signal-regulated kinase 1/2
- GABAR
GABAA receptor
- Ihold
current required to voltage clamp neuron to -60 mV
- KN62
1-[N,O-bis-(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine
- KN93
2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine)
- mIPSC
miniature inhibitory postsynaptic current
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
- NO711
1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid
- SE
status epilepticus
- TrkB
tropomyocin receptor kinase B
- TTX
tetrodotoxin
- UO126
1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene
Authorship Contributions
Participated in research design: Joshi, Kapur.
Conducted experiments: Joshi.
Performed data analysis: Joshi.
Wrote or contributed to the writing of the manuscript: Joshi, Kapur.
Footnotes
This study was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants RO1 NS 040337 and RO1 NS044370 (to J.K.)]. A part of the work was also supported by an American Epilepsy Society postdoctoral fellowship to S.J.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Agell N, Bachs O, Rocamora N, Villalonga P. (2002) Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal 14:649–654 [DOI] [PubMed] [Google Scholar]
- Bading H, Greenberg ME. (1991) Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science 253:912–914 [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. (2002) The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology 43:651–661 [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. (2002) The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem 82:192–201 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (1998) Neuronal calcium signaling. Neuron 21:13–26 [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. (2002) α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the δ subunit. Neuropharmacology 43:492–502 [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. (2003) Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci 23:10934–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. (2001) BDNF and epilepsy: too much of a good thing? Trends Neurosci 24:47–53 [DOI] [PubMed] [Google Scholar]
- Borris DJ, Bertram EH, Kapur J. (2000) Ketamine controls prolonged status epilepticus. Epilepsy Res 42:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Potschka H, Löscher W, Ebert U. (2003) N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience 118:727–740 [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. (1988) Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci 8:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Limbrick D, Sombati S, DeLorenzo RJ. (1995) Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J Neurosci 15:3200–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. (1988) Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res 474:181–184 [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Pal S, Sombati S. (1998) Prolonged activation of the N-methyl-D-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc Natl Acad Sci USA 95:14482–14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gaamouch F, Buisson A, Moustié O, Lemieux M, Labrecque S, Bontempi B, De Koninck P, Nicole O. (2012) Interaction between αCaMKII and GluN2B controls ERK-dependent plasticity. J Neurosci 32:10767–10779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr (1996) Introduction to temporal lobe epilepsy. Epilepsy Res 26:141–150 [DOI] [PubMed] [Google Scholar]
- Fáncsik A, Linn DM, Tasker JG. (2000) Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20:3067–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F,, et al. (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632 [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. (1995) Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia 36:186–195 [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Soderling TR. (1990) Activation of Ca(2+)/calmodulin-dependent protein kinase II in cerebellar granule cells by N-methyl-d-aspartate receptor activation. Mol Cell Neurosci 1:133–138 [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Soderling TR, Miyamoto E. (1992) Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem 267:22527–22533 [PubMed] [Google Scholar]
- Garrido YCS, Sanabria ERG, Funke MG, Cavalheiro EA, Naffah-Mazzacoratti MG. (1998) Mitogen-activated protein kinase is increased in the limbic structures of the rat brain during the early stages of status epilepticus. Brain Res Bull 47:223–229 [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. (2007) Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56:763–770 [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. (2007) A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10:40–48 [DOI] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. (1999) GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol 514:27–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26:81–89 [DOI] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. (2004) Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron 43:31–42 [DOI] [PubMed] [Google Scholar]
- Holstein DM, Berg KA, Leeb-Lundberg LMF, Olson MS, Saunders C. (2004) Calcium-sensing receptor-mediated ERK1/2 activation requires Galphai2 coupling and dynamin-independent receptor internalization. J Biol Chem 279:10060–10069 [DOI] [PubMed] [Google Scholar]
- Houser CR, Huang CS, Peng Z. (2008) Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience 156:222–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. (2009) Slow intracellular accumulation of GABA(A) receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience 164:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sun C, Kapur J. (2011) A mouse monoclonal antibody against the γ2 subunit of GABAA receptors. Hybridoma (Larchmt) 30:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SSG, Poulter MO. (2011) Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem 116:1043–1056 [DOI] [PubMed] [Google Scholar]
- Kim YS, Hong KS, Seong YS, Park JB, Kuroda S, Kishi K, Kaibuchi K, Takai Y. (1994) Phosphorylation and activation of mitogen-activated protein kinase by kainic acid-induced seizure in rat hippocampus. Biochem Biophys Res Commun 202:1163–1168 [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. (1996) Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 35:1049–1056 [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. (1994) Anticonvulsant activity of neurosteroids: correlation with GABA-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229 [PubMed] [Google Scholar]
- Kotloski R, McNamara JO. (2010) Reduction of TrkB expression de novo in the adult mouse impairs epileptogenesis in the kindling model. Hippocampus 20:713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40:775–784 [DOI] [PubMed] [Google Scholar]
- Kurino M, Fukunaga K, Ushio Y, Miyamoto E. (1995) Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem 65:1282–1289 [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. (1993) NMDA-dependent superoxide production and neurotoxicity. Nature 364:535–537 [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D. (2009) Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 34 (Suppl 1):S48–S58 [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M. (1998) Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol 56:269–277 [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Kapur J, Stringer JL. (1990) Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res 6:110–118 [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. (1992) The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl 7:301–313 [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321:519–522 [DOI] [PubMed] [Google Scholar]
- Martin BS, Kapur J. (2008) A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia 49:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memo M, Bovolin P, Costa E, Grayson DR. (1991) Regulation of GABAA receptor subunit expression by activation of NMDA-selective glutamate receptors. Mol Pharmacol 39:599–603 [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG,, et al. (1999) Attenuated sensitivity to neuroactive steroids in GABAA receptor δ subunit knockout mice. Proc Natl Acad Sci USA 96:12905–12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Y, Zhang H, Long L, Wang F, Chen J, Zhen X. (2006) Modulation of Ca2+ signals by phosphatidylinositol-linked novel D1 dopamine receptor in hippocampal neurons. J Neurochem 98:1316–1323 [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. (2001) Diminished allopregnanolone enhancement of GABA(A) receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol 537:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. (2006) High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol 69:564–575 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18:1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. (2007) Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci 27:14012–14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. (2004) Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 24:8629–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Williamson JM, Bertram EH. (2002) Phenobarbital and MK-801, but not phenytoin, improve the long-term outcome of status epilepticus. Ann Neurol 51:175–181 [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. (2010) Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis 40:490–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannals MD, Kapur J. (2011) Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J Neurosci 31:17701–17712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. (1998) NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res 782:240–247 [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. (1999) N-methyl-D-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience 93:117–123 [DOI] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. (2006) Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor α 4 subunits in hippocampal neurons. J Biol Chem 281:29431–29435 [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 66:105–143 [DOI] [PubMed] [Google Scholar]
- Rothman SM. (1985) The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci 5:1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. (1987) Excitotoxity and the NMDA receptor. Trends Neurosci 10:299–302 [DOI] [PubMed] [Google Scholar]
- Rybakova Y, Akkuratov E, Kulebyakin K, Brodskaya O, Dizhevskaya A, Boldyrev A. (2012) Receptor-mediated oxidative stress in murine cerebellar neurons is accompanied by phosphorylation of MAP (ERK 1/2) kinase. Curr Aging Sci 5:225–230 [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Okada M, Kuno M, Kawasaki K. (1997) Dual mode of NMDA-induced neuronal death in hippocampal slice cultures in relation to NMDA receptor properties. Neuroscience 76:411–423 [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. (1994) Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci 14:7077–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. (1997) GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience 80:987–1000 [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. (2002) Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia 43 (Suppl 5):3–8 [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100:14439–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37:173–182 [DOI] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. (1989) Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol 62:136–143 [DOI] [PubMed] [Google Scholar]
- Stringer JL, Williamson JM, Lothman EW. (1989) Induction of paroxysmal discharges in the dentate gyrus: frequency dependence and relationship to afterdischarge production. J Neurophysiol 62:126–135 [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. (1991) The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181:968–975 [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. (2007) Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABA(A) receptors in an animal model of epilepsy. J Neurosci 27:12641–12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. (1990) KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 265:4315–4320 [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Czuczwar SJ, Turski WA, Kleinrok Z. (1987) The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm 39:545–555 [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. (2003) Perisynaptic localization of δ subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23:10650–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the δ subunit. J Neurosci 22:1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Storm DR. (2012) Role of signal transduction crosstalk between adenylyl cyclase and MAP kinase in hippocampus-dependent memory. Learn Mem 19:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV. (2009) Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol 102:670–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. (2007) Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci 27:7520–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. (1993) Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem 268:11435–11439 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.