Abstract
Over the past decade, ideas and experimental support for the hypothesis that G protein–coupled receptors may exist as dimeric or oligomeric complexes moved initially from heresy to orthodoxy, to the current situation in which the capacity of such receptors to interact is generally accepted but the prevalence, maintenance, and relevance of such interactions to both pharmacology and function remain unclear. A vast body of data obtained following transfection of cultured cells is still to be translated to native systems and, even where this has been attempted, results often remain controversial and contradictory. This review will consider approaches that are currently being applied and why these might be challenging to interpret, and will suggest means to overcome these limitations.
Introduction
In the last 10 years, the question of whether G protein–coupled receptors (GPCRs) exist as monomers, dimers, or oligomers has been a substantial component of many studies on members of this family of transmembrane signaling proteins (Milligan, 2004, 2008; Gurevich and Gurevich, 2008; Pétrin and Hébert, 2012). Until recently, these were often considered to be mutually exclusive scenarios, but the coexistence and potential interchange between such forms, based in part simply upon mass action, has resulted in a more textured view (Calebiro et al., 2013; Patowary et al., 2013). It is clear that stable heterocomplexes formed by interactions between polypeptide products of distinct genes encoding members of the class C, metabotropic glutamate–related GPCRs define the pharmacology and function of certain receptors (Maurel et al., 2008; Pin et al., 2009; Kniazeff et al., 2011). These include interactions between the GABAB1 and GABAB2 polypeptides to generate the GABAB receptor (Kniazeff et al., 2011). Similarly, coexpression and interactions between the Taste (TAS) 1R1 and TAS1R3 polypeptides result in perception of savory or umami flavors, whereas similar coexpression and interactions between TAS1R2 and TAS1R3 polypeptides are required for identification of sweet tastes (Chandrashekar et al., 2006; Palmer, 2007). Furthermore, although members of distinct subgroups of metabotropic glutamate receptors appear unable to generate heteromeric interactions with each other (Doumazane et al., 2011), this does occur between more closely related polypeptides within the same subgroup, and each individual member of the metabotropic glutamate receptor family is able to generate homomers, an organizational structure that is integral to function (Doumazane et al., 2011).
Despite these clear examples, all of which meet the broad guidelines proposed by the International Union of Basic and Clinical Pharmacology for acceptance of GPCR complexes as homomers and/or heteromers (Pin et al., 2007), the situation for the numerically much larger class A of rhodopsin-like receptors is far more complex. This article will attempt to appraise why this is so.
Monomeric Class A GPCRs Are Functional
In recent times, a series of studies have purified class A GPCRs and, following insertion as monomers into various forms of phospholipid bilayers, have shown the capacity of these to interact productively with appropriate heterotrimeric G proteins. For example, Kuszak et al. (2009) took this strategy and used a form of the μ-opioid receptor with yellow fluorescent protein and a series of epitope tags linked to the N terminus. Addition of heterotrimeric Gi2 to the system resulted in both the opioid alkaloid morphine and the μ-opioid receptor-selective synthetic enkephalin peptide [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) displaying biphasic competition binding curves with the antagonist [3H]diprenorphine, including a high-affinity component that was not present in the additional presence of a poorly hydrolyzed analog of GTP. Furthermore, in these studies, DAMGO promoted binding of guanosine 5′-O-(3-[35S]thio)triphosphate to the G protein in a concentration-dependent manner (Kuszak et al., 2009). Along with similar experiments using the β2-adrenoceptor (Whorton et al., 2007) and rhodopsin (Whorton et al., 2008), these studies provided firm evidence that a number of class A GPCRs can function effectively as monomers. Furthermore, although there have also been suggestions that dimeric forms of GPCRs might be required for interactions with non–G protein effectors and adaptors, at least in the case of rhodopsin and interactions with arrestin-1 (Bayburt et al., 2011); again, this seems to be accommodated by a monomeric GPCR.
Even GPCRs That Are Capable of Acting as Monomers May Exist as Dimers or Higher Oligomers
Interestingly, the three class A GPCRs noted previously as being functional when forced to be strict monomers have each been shown to have the capacity to exist as dimers or higher oligomers. Indeed, in the case of the organization of rhodopsin, observation via atomic force microscopy of the receptor as paracrystalline arrays of dimers in mouse disc membranes (Fotiadis et al., 2003) was a key observation in providing evidence of physiologically relevant receptor-receptor interactions. Although there were immediate suggestions of concerns about technical details of these studies and that other evidence is consistent with rhodopsin acting as a monomer (Chabre et al., 2003), this remains a landmark study. In the case of the β2-adrenoceptor, alongside rhodopsin, the most extensively studied GPCR, it was one of the first GPCRs in which potential “dimerization” was explored. Building on a series of coimmunoprecipitation studies (see Milligan and Bouvier, 2005, for review), Angers et al. (2000) were instrumental in promoting the use of resonance energy transfer techniques to probe potential oligomeric organization of receptors in intact cells, and showed such interactions for the β2-adrenoceptor. These studies and the rapid adoption of both bioluminescence resonance energy transfer (BRET)- and fluorescence resonance energy transfer (FRET)-based approaches by many groups (Alvarez-Curto et al., 2010a) have generated a mini-industry on assessing GPCR-GPCR interactions, despite concerns that limited understanding of the limitations and caveats of such biophysical methodologies may compromise analytical analysis and result in overinterpretation of the observations (James et al., 2006). Although specific aspects of these criticisms were rebutted rapidly (Salahpour and Masri, 2007), the seemingly basic question of the size of GPCR complexes remains a core question. Importantly, both fluorescence recovery after photobleaching studies (Dorsch et al., 2009) and cointernalization in response to addition of the β-adrenoceptor agonist isoprenaline of coexpressed wild-type and chemically engineered variants of the β2-adrenoceptor that are unable to bind this ligand (Sartania et al., 2007) have provided evidence for oligomerization of this receptor via distinct methodologies. Indeed, quantitative analysis of the fluorescence recovery after photobleaching studies suggested that the β2-adrenoceptor may exist as a stable oligomeric, rather than strictly dimeric, complex (Dorsch et al., 2009). In support of this, Fung et al. (2009) used FRET on purified β2-adrenoceptors reconstituted into model lipid bilayers, and obtained data consistent with the receptor existing predominantly in a tetrameric form.
The widespread interest in efforts to develop nonaddictive analgesics that function via the μ-opioid receptor has also extended to studies on potential homomeric interactions in this receptor. Thus, the recent crystal structure of this receptor showing a clear dimeric interface based on a four-helix bundle with contributions from at least 28 residues along the length of transmembrane helices V and VI provides clear support for a dimeric model (Manglik et al., 2012). The involvement of so many residues, providing a buried footprint of nearly 1500 A2 on each monomer, also suggests that it is likely to be a high-affinity interaction that may be difficult to disassemble in a physiologic setting, although this was obviously achieved by detergent solubilization to produce the monomeric μ-opioid receptor for the G protein–interaction studies of Kuszak et al. (2009) discussed earlier. Furthermore, the crystal structure also demonstrated the potential for a second dimeric interface involving residues from transmembrane helices I and II as well as the intracellular sequence usually referred to as “helix VIII” (Manglik et al., 2012). This interface was not nearly as extensive as the one involving residues from helices V and VI, and is at least compatible with the idea that this might provide a lower-affinity interface that would allow dynamic interchange between dimeric and tetrameric forms of this receptor. Experimentally observed coexistence and the potential for interchange between dimeric and tetrameric forms of a GPCR at the plasma membrane (Patowary et al., 2013) support this concept. Furthermore, the proportions of different oligomeric states might vary for the same receptor in cells and tissues that express a GPCR at markedly different levels. The implications of this potential for the analysis of ligand binding studies have also been considered in a theoretical context (Rovira et al., 2009). The structures of the μ-opioid receptor were obtained in the presence of an irreversibly bound antagonist, and therefore, although the general significance is currently unclear, the fact that Fung et al. (2009) observed that the addition of an inverse agonist to lipid bilayer–reconstituted β2-adrenoceptors seemed to enhance the organization of a tetrameric structure is certainly fascinating. Interestingly, although displaying a more limited interface, a number of individual crystal structures of the chemokine CXCR4 receptor bound by a small molecule antagonist also display a dimeric form, with the interface again defined by residues from transmembrane helices V and VI (Wu et al., 2010), whereas crystals of the κ-opioid receptor also show a parallel dimeric interface involving residues from transmembrane domains I, II, and VIII (Wu et al., 2012).
Is a Monomer More Effective Than a Dimer for G Protein Activation?
If certain GPCR monomers can couple effectively to heterotrimeric G proteins to initiate signal transduction upon binding of an agonist ligand, and the same receptors can exist as dimers or higher-order complexes, what are the implications for signaling, and is one form more efficient than the other? Results on this topic are interesting but do not yet provide clarity. For example, for the leukotriene BLT2 receptor, expression in Escherichia coli followed by refolding and purification of dimers arranged with parallel organization suggested that the dimer activated purified G protein less effectively than receptor monomers (Arcemisbéhère et al., 2010). Although this is a technically exacting and precise study, it is difficult to be sure that the dimer interface in such studies equates fully to what might be found in a cell expression system. By contrast, when using expression of various forms of the serotonin 5-HT4 receptor in COS7 cells, activation of both elements of the dimeric complex was shown to result in greater activation of G protein than of a single protomer of such a complex (Pellissier et al., 2011).
An extension of this question and these studies is whether each element of a dimer or higher-order complex of a class A GPCR is able to bind a molecule of agonist (at least with similar affinity), and if so, whether this influences the function of the partner protomer(s). Based on the studies by Pellissier et al. (2011), this is clear for the 5-HT4 receptor. Moreover, studies such as those by Herrick-Davis et al. (2005) on the serotonin 5-HT2C receptor that used combinations of a wild-type and a variant receptor unable to bind serotonin are also at least consistent with a need to bind agonist to both protomers of a dimer to generate maximal function. Moreover, kinetic analysis of how the rate of dissociation of a fluorescent agonist from the adenosine A3 receptor expressed in Chinese hamster ovary (CHO) K1 cells is increased markedly in the presence of both agonists and antagonists known to also bind the same, orthosteric site on the receptor is not consistent with ligand dissociation occurring from a monomer (where the ligand dissociation rate should be unaffected by the presence of a second ligand), and has been interpreted as evidence for a dimeric receptor (May et al., 2011) in which ligand binding to one protomer generates a co-operative allosteric effect on ligand binding to the other protomer. The extent of such effects may vary substantially between even closely related receptors. In equivalent studies using the adenosine A1 receptor, much smaller effects on the ligand dissociation rate were recorded, although other evidence also indicated that the receptor formed dimeric complexes (May et al., 2011), indicating that allosteric effects within different receptor dimers may not be sensed or transmitted to the same extent. A further approach that has recently been applied is to examine the effect of unmodified receptors on the behavior of a coexpressed receptor capable of reporting conformational change and activation. Using the angiotensin AT1 receptor as a model, Szalai et al. (2012) first used a form of this receptor which, although still able to bind the endogenous peptide ligand, was unable to bind the small-molecule antagonist candesartan. This allowed selective activation of the antagonist-resistant form in the presence of a combination of the antagonist and angiotensin II. Using BRET to examine either β-arrestin-2 binding to the receptor or the activation of an intramolecular BRET sensor form of the receptor that was not blocked by the presence of candesartan allowed these authors to observe effects also consistent with allosteric effects within a dimer (Szalai et al., 2012). Furthermore, these effects were lacking when a mutant was used in the receptor in which the highly conserved DRY domain at the bottom of transmembrane domain III was mutated. Such a concept of asymmetry of the individual protomers of a dimeric or oligomeric complex is not new and has been discussed previously in detail (e.g., Maurice et al., 2011), but does provide a basis to probe the presence of homodimers. Clear asymmetry of function is integral for the individual protomers within receptor heteromers (see later) and the class C GABAB receptor in which the GABAB1 subunit binds the orthosteric agonist ligand, whereas the partner GABAB2 polypeptide that communicates this to G protein activation is both the prototypic and most fully analyzed example of this to date (see Pin et al., 2009, for review).
Oligomeric Organization of Other Class A GPCRs
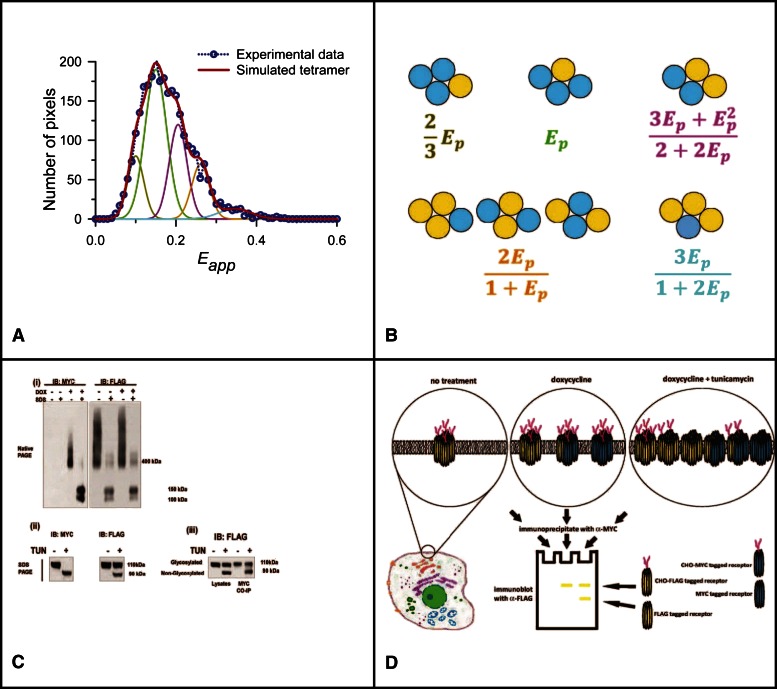
Although a potential dimeric form of rhodopsin was also identified via a crystal structure, for other GPCRs, evidence for oligomeric organization has, to date, been derived from other approaches. Perhaps the most wide-ranging group of studies have been performed on muscarinic acetylcholine receptor subtypes. Beginning with conventional pharmacological ligand binding (Park et al., 2002) and biochemical approaches (Ma et al., 2007), over a number of years, Wells and colleagues have provided a portfolio of data on this topic for the muscarinic M2 receptor that are consistent with the potential for tetrameric organization. Most recently, this has derived from application of quantitative FRET (Pisterzi et al., 2010). Once again, these studies provided evidence of tetrameric organization of the receptor, potentially with a rhombic or parallelogram shape, at least in transiently transfected CHO cells. A further refinement of these techniques has recently been used to explore the oligomeric organization of the muscarinic M3 receptor (Patowary et al., 2013). Herein, a human embryonic kidney 293–based cell line was established in which an energy acceptor–tagged form of the human muscarinic M3 receptor was expressed stably and constitutively, and in which a corresponding energy donor–tagged form of the receptor was harbored at an inducible locus (Alvarez-Curto et al., 2010b). This allowed varying amounts of the energy donor to be expressed in the presence of a constant level of energy acceptor. Quantitative FRET studies with spectral unmixing were then performed on these cells at the level of the plasma membrane. Mathematical analysis of the broad range of FRET efficiencies obtained from these complexes was compatible with the receptor existing within rhombus-shaped tetramers (Patowary et al., 2013) (Fig. 1, A and B). However, detailed analysis of the various FRET efficiency peak heights indicated the coexistence of a dimeric population of the M3 receptor alongside the tetrameric form, and coimmunoprecipitation studies indicated that there was dynamic interchange of units between these species (Patowary et al., 2013) (Fig. 1, C and D).
Fig. 1.
Spectrally resolved FRET and coimmunoprecipitation studies define the complex organization and dynamics of the muscarinic M3 receptor quaternary complex at the surface of cells. Spectrally resolved FRET was used to investigate the organization of the muscarinic M3 receptor at the surface of Flp-In T-REx 293 cells that constitutively express a form of the muscarinic M3 receptor that contains an N-terminal FLAG epitope tag and with the fluorescent protein citrine linked-in frame to the C terminus (the energy acceptor) and harbor at the inducible Flp-In T-REx locus, a variant of this receptor containing an N-terminal MYC epitope and C-terminal cerulean fluorescent protein (the energy donor). Expression from the Flp-In T-REx locus is controlled by the addition of various concentrations of doxycycline (DOX) (A). A broad range of FRET efficiencies were measured at the surface of cells induced to express varying amounts of the energy donor. This is not consistent with the receptor existing simply as a dimer because this scenario would be anticipated to result in a single FRET efficiency peak. Analysis of the experimental data based on the receptor existing, at least partially, as a rhombic tetramer with individual species containing varying numbers of acceptor (yellow) and donor (blue) species (B) showed that the experimentally derived and simulated results were highly similar (A). (C) Lysates from cells as described earlier were resolved by either Native-PAGE (i) or SDS-PAGE (ii–iii). Immunoblots of Native-PAGE resolved samples confirmed doxycycline-induced expression of the MYC-tagged protein and both constitutive and maintained expression of the FLAG-tagged form, whereas addition of SDS to samples prior to addition to the native gel resulted in production of two, potentially differentially N-glycosylated, variant monomer forms of each (i). Treatment of cells with PNGaseF confirmed this (not shown). Maintenance of the cells in the presence of the de novo N-glycosylation inhibitor tunicamycin (TUN) during the period of induction of the MYC-tagged energy donor resulted in all cell surface anti-MYC reactivity lacking carbohydrate (90 kDa), whereas only the proportion of the FLAG-tagged energy acceptor synthesized during this period lacked N-linked glycosylation (ii). Anti-MYC immunoprecipitates performed on lysates from cells treated with both doxycycline and tunicamycin resulted in the coimmunoprecipitation of both N-glycosylated (110 kDa) and nonglycosylated (90 kDa) FLAG-tagged energy acceptor (iii). This must reflect the presence of FLAG-tagged proteins both made before and after addition of tunicamycin in complex with the MYC-tagged variant, and therefore dynamic interactions between the forms. Results are adapted from Patowary et al. (2013). (D) An illustration representation of the experiments illustrated in (C), based on a tetrameric organization of the M3 receptor. For “no treatment,” i.e., in the absence of doxycycline, only the energy acceptor species (yellow) is present and all copies are N-glycosylated. Following addition of doxycycline, a mixture of energy donors (blue) and acceptors are present, and all are N-glycosylated and exist as a mixture of homo- and heterotetramers. With cotreatment of the cells with doxycycline and tunicamycin, a more complex pattern is predicted in which all energy donors lack N-glycosylation, whereas, based upon their time of synthesis, the energy acceptor species may be either N-glycosylated or nonglycosylated. As shown in (Ciii), immunoprecipitation of the MYC-labeled energy donor results in the coimmunoprecipitation of both N-glycosylated and nonglycosylated versions of the energy acceptor, and these are resolved in SDS-PAGE. CHO, carbohydrate; CO-IP, co-immunoprecipitation; IB, immunoblot.
Although clearly able to demonstrate, as anticipated, that the proportion of tetramers containing three energy donor–linked protomers and a single energy acceptor–linked one increased as the fraction of energy donor molecules was increased, the ability to vary the relative levels of energy donor and acceptor species in these studies was insufficient to define unambiguously whether the proportion of tetramer to dimer increased as total expression levels increased, as predicted by mass action. The capacity to control donor-to-acceptor ratios across a much broader scale, within such a regulated system rather than via transient transfection, will be required to clarify this question fully. It also remains uncertain if different tetrameric organizations of various receptors might exist, e.g., squares and parallelograms, but conclusions based on combinations of direct experimental and modeling studies on potential interfaces and stability of δ-opioid receptor dimers suggest at least two possible, distinct orientations that might generate forms with different FRET efficiency signals (Johnston et al., 2011). The dopamine D2 receptor has also been used widely to explore aspects of the organizational structure of class A GPCRs (Han et al., 2009). Using combinations of bioluminescence/fluorescence complementation and energy transfer to allow detection of complexes that are larger than dimers, Guo et al. (2008) showed that this receptor could exist as a tetrameric complex based on symmetrical interfaces involving transmembrane domains I and IV. This expanded and built on a potential “daisy-chain” model of the quaternary structure of the α1b-adrenoceptor developed by Lopez-Gimenez et al. (2007) in which symmetrical interfaces provided by residues in transmembrane domains I and IV left the potential for further symmetrical interactions to extend the size of the complex. Indeed, in the analyses of muscarinic M3 receptor organization by Patowary et al. (2013), a model based on a hexamer was also consistent with (but not required and therefore excluded on the principle of Occam’s razor) the experimental data. Despite these results, a number of other studies are compatible with GPCR oligomers being restricted to dimers. These include studies in which the neurotensin 1 receptor behaved as a dimer when reconstituted in polar lipid bilayers (Harding et al., 2009), and fluorescence correlation spectroscopy studies on the brightness of complexes of the serotonin 5-HT2C receptor when tagged with a yellow fluorescent protein (Herrick-Davis et al., 2012). Although this review is centered on rhodopsin-like, class A GPCRs, information on this topic is currently more advanced for the class C receptors. Herein, directly comparable studies indicate that metabotropic glutamate receptors are restricted to dimeric pairs (Maurel et al., 2008), whereas the GABAB receptor, long appreciated as a heteromer containing both GABAB1 and GABAB2 subunits, is clearly able to exist as a tetramer (Maurel et al., 2008) and potentially even as an octamer (Calebiro et al., 2013). Interestingly, efforts to assess subunit exchange within such heteromers have indicated the complex to be stable (Maurel et al., 2008), but this seems unlikely if dimers and tetramers as well as further higher-order complexes can coexist. FRET efficiency measurements using suitably labeled GABAB1 and GABAB2 subunits suggested that the GABAB1 subunits are closer together in the tetramer than the GABAB2 subunits (Maurel et al., 2008), suggesting direct interactions between GABAB1 subunits but not between GABAB2 subunits. These observations could be accommodated in either a “linear” or a “rhombic” model. However, despite the data discussed previously, recent studies have shown the capacity of the large extracellular domain sections of GABAB1 and GABAB2 to interact directly with each other (Geng et al., 2012), and this may provide the additional binding energy to stabilize the tetrameric (or even larger) complex.
Are Dimers/Oligomers of Class A GPCRs Stable Complexes?
A wide range of studies have provided evidence that class A GPCRs initially generate quaternary structure at an early stage of biosynthesis (Salahpour et al., 2004; Wilson et al., 2005; Herrick-Davis et al., 2006; Lopez-Gimenez et al., 2007; Canals et al., 2009; Kobayashi et al., 2009; Cunningham et al., 2012). This has implications for function, as many GPCR mutants (Pidasheva et al., 2006) and nonsynonymous single-nucleotide polymorphisms (Leskela et al., 2012) may interact with the corresponding wild-type receptor at this level and, by forming quaternary interactions and acting as dominant negatives, limit cell surface delivery. It has been suggested that early-stage dimerization may encourage or be required for effective folding and maturation of the receptor. Studies of this type contributed to a view that GPCR dimers/oligomers would likely be stable complexes and might exist as such until turnover and destruction. Furthermore, a series of studies have suggested that such interactions can be measured across a wide range of expression levels (e.g., Guo et al., 2008), observations that are certainly consistent with such complexes being stable, long-lived entities. However, as well as a range of studies that indicate either the detailed organizational structure or indeed the extent of oligomerization can be modified upon ligand binding, a number of papers have suggested that oligomeric organization may alter over time or with cellular location. A key set of studies in this regard were performed by Dorsch et al. (2009). Although antibody-mediated immobilization of a defined proportion of cell surface β2-adrenoceptors markedly restricted lateral mobility of other copies of this receptor, this was not observed in equivalent studies on the β1-adrenoceptor. These results were interpreted to suggest that interactions between β1-adrenoceptor monomers were limited and/or transitory, whereas the β2-adrenoceptor formed a stable oligomeric complex. However, in contrast to the studies of Sartania et al. (2007), which indicated that dimers/oligomers of the β2-adrenoceptor internalized from the surface of cells as a maintained and presumably stable complex, recent studies from Lan et al. (2011), despite using the same mutational approach to ensure that only a fraction of the protomers were able to bind the agonist ligand isoprenaline, were unable to observe a similar cointernalization of agonist-binding competent and incompetent forms, and concluded that β2-adrenoceptors either associate transiently with each other in the plasma membrane, or the complexes are actively disrupted during internalization. Similarly, by using an immobilization strategy conceptually similar to that of Dorsch et al. (2009), Fonseca and Lambert (2009) concluded that the dopamine D2 receptor also produced complexes that were transitory in nature, again in marked contrast to the observations and conclusions of Guo et al. (2008). Similar conclusions as to the transitory nature of at least certain family A GPCR oligomers were reached for the M1 muscarinic receptor using a combination of imaging and potential single-molecule tracking via total internal reflection fluorescence microscopy (Hern et al., 2010). These studies concluded, at least when expressed in CHO cells, that at steady state, some 30% of the receptor was present as a dimer with no detectable presence of higher-order oligomers. However, these conclusions rest entirely on the view that the ligand used to label the receptor was monitoring monomer-dimer transitions rather than, as suggested by Patowary et al. (2013) for the closely related M3 receptor, potential dimer-tetramer transitions. Equally, single-molecule imaging has been used to conclude that monomers and dimers of the chemoattractant N-formyl peptide receptor undergo rapid, subsecond interconversions (Kasai et al., 2011), and further, very recent, single-molecule studies also favor rapid transitions between monomers and dimers of β-adrenoceptor subtypes (Calebiro et al., 2013). This appears to contrast with recent studies on the serotonin 5-HT2C receptor where, using combinations of fluorescence correlation spectroscopy and photon counting histogram analysis, it was concluded that all of the cell surface receptor was dimeric (Herrick-Davis et al., 2012). A similar conclusion was recently reached for the orexin OX1 receptor based on combinations of energy transfer and biochemical studies (Xu et al., 2011), and evidence based on the use of fluorescence lifetime measurements indicates that the serotonin 5-HT1A receptor exists as an oligomer (Ganguly et al., 2011). However, it may be that a number of the approaches used are unable to resolve fluctuations in receptor interactions occurring on a time scale of seconds. It will be interesting, therefore, to begin to see results that provide interaction affinities for different GPCR protomers, and the results of single-molecule tracking after GPCR protomers are linked together via cleavable cross-linkers. Modeling approaches have also been applied to efforts to predict whether such interactions might be transitory (Provasi et al., 2010). If GPCRs routinely alternate between monomers and dimers/oligomers, it should be possible to detect evidence of this biochemically. Indeed, for the muscarinic M3 receptor, analysis of interactions between recently synthesized, nonglycosylated, and fully N-glycosylated forms of the receptor that had been synthesized previously showed the presence of these two forms in the same molecular complex, arguing that there must be dynamic exchange between complexes (Patowary et al., 2013). The studies by Calebiro et al. (2013) are of particular interest for the range and selection of controls used. These included the use of a previously well characterized monomeric, single-transmembrane domain polypeptide CD86, to which either a single or two copies of the “SNAP” tag (Maurel et al., 2008; Alvarez-Curto et al., 2010a; Ward et al., 2011) was attached to the extracellular domain. This allowed labeling of the monomer with either one or two molecules of fluorophore for calibration purposes. Based on these measurements, studies using transiently transfected SNAP-tagged forms of the β1- and β2-adrenoceptors indicated that both were able to form dimers, but that the proportion of such complexes was greater for the β2-adrenoceptor at a similar receptor density, and that, in both cases, this increased with receptor density (Calebiro et al., 2013).
What Are the Dimer/Oligomer Interfaces?
As noted earlier, for both the μ-opioid receptor (Manglik et al., 2012) and the chemokine CXCR4 receptor (Wu et al., 2010), crystals have been obtained in which dimeric interfaces provided by residues from transmembrane domains V and VI have been highlighted. Such an interface is consistent with some previous biochemical studies, but less so with other data sets. These crystal structures were all obtained with the receptor occupied by small-molecule antagonists/inverse agonists. One feature of class A GPCR activation that is consistent across approaches, ranging from atomic level structures (Rasmussen et al., 2011), via biophysical analyses on purified proteins following insertion of conformational sensors into the sequence of various receptors (Rosenbaum et al., 2009), to studies using GPCRs engineered to act as intramolecular FRET sensors (Ambrosio et al., 2011; Xu et al., 2012), is that agonist occupation and activation is associated with substantial movement of transmembrane domain VI. Assuming such a movement occurs within a dimer, then this might, at least in part, account for the agonist-induced intradimer communication reported in a wide range of studies (e.g., May et al., 2011). However, the potentially high-affinity interaction produced by the extensive transmembrane domain V/VI interactions observed in the μ-opioid receptor crystal structure (Manglik et al., 2012) might be anticipated to limit helix movement in this region. It is, therefore, interesting to note, although only a conceptual model at this point, that Manglik et al. (2012) have suggested a model in which a tetrameric form of the μ-opioid receptor could interact with two heterotrimeric G proteins. Clearly, many more examples will be required before patterns emerge, but it is interesting to note that May et al. (2011) observed a much smaller effect of orthosteric ligands on dissociation of a fluorescent ligand from a potential adenosine A1 receptor dimer than at the closely related adenosine A3 receptor. The other obvious feature of the μ-opioid receptor crystal structure was the additional presence of a second set of contacts provided by residues from transmembrane domains I and II as well as from helix VIII. This second interface both allows for the presence of higher-order oligomers and, perhaps, may indicate why a number of efforts to define the structural basis of GPCR dimers have resulted in evidence for contributions from a number of distinct elements. Although it is clearly not impossible to envisage that different GPCRs dimerize in very different ways, the overall structural conservation of the transmembrane helix bundle and the restricted number of ways in which the large family of class A GPCRs are likely to engage a limited population of heterotrimeric G proteins make this an intellectually unappealing hypothesis, and suggest that there are likely to be general features that define or favor the stability of the GPCR quaternary structure.
Although certain studies, not least a very early study on the β2-adrenoceptor (Hebert et al., 1996), provided support for a role of transmembrane VI in dimer interactions and for the dimer as the functional signal transducer, a wide range of more recent studies have attributed key roles in dimer contacts to residues within other transmembrane domains, including I (Wang and Konopka, 2009), IV (Lopez-Gimenez et al., 2007; de la Fuente et al., 2012), and V (Hu et al., 2012) in different receptors. Furthermore, despite the bulk of studies concentrating on the contribution of transmembrane domains to dimeric interfaces, a number of studies have also suggested key roles of intracellular loops (Navarro et al., 2010), with particular attention being given to potential electrostatic interactions between groups of positively and negatively charged residues and of the extracellular N-terminal domain (Uddin et al., 2012). Modeling approaches have also contributed suggestions to the ways in which GPCRs may organize (Casciari et al., 2008; Johnston et al., 2012), but, although intrinsically of interest, such studies need to be linked to direct experimental analysis to maximize their impact. There are, of course, a number of potential limitations in studies that rely extensively on mutagenesis to attempt to define protein-protein interaction interfaces, not least, as has also been an issue for mutagenic approaches to define ligand binding sites, that loss of function may reflect protein misfolding rather than reflecting the anticipated endpoint, and such potential effects must be considered as a realistic scenario.
Physiology and Pharmacology of Class A GPCR Homomers
The equivalence of the protomers in class A GPCR homomers has meant that it is challenging to identify the presence of such complexes in native systems. By far the most enterprising, but also most challenging, route was taken by Rivero-Müller et al. (2010). Building on the concept that distinct nonfunctional forms of the glycoprotein hormone receptors can be generated by mutations that prevent either ligand binding or G protein activation, they generated knock-in lines of mice in which either of two distinct, inactive forms of the luteinizing hormone receptor was introduced into luteinizing hormone receptor knockout animals. Although neither of these was able to restore function, crossing of the lines to produce coexpression of the two individually inactive forms did result in rescue of function, implying a need for complementation between the two forms and, by extension, their dimerization. Nothing akin to this has been attempted in vivo for any other class A GPCR. Despite the apparent clarity of these results, even the basic premise of the mode of action of such complementation has recently been questioned, and the interpretation challenged (Zhang et al., 2012).
Within these studies, Zhang et al. (2012) reported that the mutated variants of the receptor used by Rivero-Müller et al. (2010) may not be entirely lacking in function and that, in part, the reported lack of function might relate to limited cell surface delivery. Although certainly providing a series of challenging questions for previous interpretation, the studies by Zhang et al. (2012) were entirely limited to heterologous cell expression. This dichotomy clearly requires further study, but the commitment to production of transgenic animals to address these issues is not likely to be undertaken lightly.
Other approaches that might have value in either ex vivo or in vivo studies remain challenging. Conceptually, detailed analysis of the dissociation kinetics of receptor ligands may provide evidence in favor of models that are incompatible with the GPCR in question acting as a single noninteracting species (Albizu et al., 2010; May et al., 2011) or of the behavior of certain ligands observed in vitro that can best be described in terms of receptor homomers (Brea et al., 2009) can be replicated ex vivo. Perhaps the most promising approach takes advantage of time-resolved FRET (Albizu et al., 2010; Cottet et al., 2011). Here, using FRET-competent ligands with affinity for the oxytocin receptor, FRET signals consistent with receptors with a quaternary structure were detected in mammary gland patches known to express high levels of this receptor, but not in the brain. Even here, however, the expression levels would probably have to be substantially higher than are known to be the case for many GPCRs to produce acceptable signal to background ratios.
There has also been interest in the idea that certain ligands or other receptor regulators may bind selectively to a receptor dimer. Perhaps the best example of this to date is not for a small-molecule ligand, but for the snake toxin MT7 (Marquer et al., 2011). Here, both extensive mutagenesis studies and a series of studies that indicated that the toxin favors either the formation or stability of such a complex of the M1 muscarinic receptor have been produced. Even for this reagent, however, the suggestion that it favors the production of a M1 muscarinic receptor quaternary structure might result in an overestimate of the presence of such complexes in native tissues.
GPCR Heteromers
Beyond the obvious examples of heteromeric GPCR complexes provided by the class C taste and GABAB receptors described earlier, there is much literature on the ability of coexpressed class A GPCRs to form heteromers. Such interactions frequently result in markedly distinct receptor pharmacology and function, at least as defined in transfected cell systems.
GPCR Quaternary Structure: Relevance to Physiology, Disease, and Drug Design
A major challenge has been and remains in defining the presence of GPCR heteromers in native tissues and either replicating or extending the functional and pharmacological sequelae noted following cotransfection of pairs of GPCRs into simple model cell systems. Despite these challenges, significant progress is being made. One of the key examples has been to define the contribution of a potential 5-HT2A-metabotropic glutamate reception subtype 2 (mGluR2) heteromer to both pharmacological and behavioral responses to a group of 5-HT2A agonists that generate hallucinogenic effects. Intriguingly, although drugs such as 2,5-dimethoxy-4-iodoamphetamine are able to induce a head-twitch response in mice that requires expression of the 5-HT2A receptor in cortical pyramidal neurons (Gonzalez-Maeso et al., 2007), this effect also requires expression of the mGluR2 receptor because it is also lacking in mGluR2 knockout animals (Moreno et al., 2011b). Moreover, this effect is restored in such animals by virally mediated reintroduction of the mGluR2 receptor (Moreno et al., 2012). A series of studies have shown the capacity of these two otherwise unrelated GPCRs to interact and form a functional complex in both cellular systems and the brain (González-Maeso et al., 2008; Delille et al., 2012), whereas a range of other studies have also shown pharmacological interactions between these receptors (e.g., Molinaro et al., 2009). A key element of the studies by González-Maeso et al. (2008), which was extended by further studies by Fribourg et al. (2011), was the alteration in signal transduction pathways produced via this heteromer. It must be noted that not all studies have been able to replicate the reported changes in ligand pharmacology and signaling (Delille et al., 2012), but further recent studies from González-Maeso and colleagues (Moreno et al., 2012) used chimeric mGluR2/mGluR3 constructs and variation in sequence at the cytoplasmic end of transmembrane domain IV between these closely related receptors to define a small number of residues of mGluR2 that, when mutated to the equivalent sequence of mGluR3, greatly reduced interactions with the 5-HT2A receptor. When a mutant mGluR2 containing three such amino acid changes was introduced into mGluR2 knockout mice, unlike wild-type mGluR2, this construct was unable to restore the head-twitch response produced by 2,5-dimethoxy-4-iodoamphetamine. It is well established that there is functional cross-talk between the glutamate and serotonergic systems in the brain (e.g., Benneyworth et al., 2007), and the studies by Moreno et al. (2012) now appear to link the capacity of coexpressed 5-HT2A and mGluR2 receptors to form a heteromeric complex to a behavioral response to a hallucinogenic drug. It remains possible, however, that observations consistent with altered function arising from a potential heteromer may instead reflect interactions between coexpressed but noninteracting receptors via signaling pathway cross-talk. For example, although careful not to imply that previously reported heteromerization between the cannabinoid CB1 and orexin OX1 receptors (Ward et al., 2011) might not be the molecular explanation for previously reported allosteric effects between selective ligands at these two receptors, Jantti et al. (2013) recently highlighted that autocrine regulation of endocannabinoid generation can produce similar pharmacological effects. Moreover, although certain studies have indicated the capacity of coexpressed cannabinoid CB1 and μ-opioid receptors to interact directly (Rios et al., 2006), other studies suggest that pharmacological variation in the function of the opioid peptide DAMGO produced in the presence of the CB1 receptor reflect constitutive activity of this receptor. Evidence in favor of this model included that activity of DAMGO was restored by coaddition of the CB1 receptor inverse agonist 5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (SR141716; rimonabant) but not by the CB1 receptor neutral antagonist (6aR,10aR)-3-(1-methanesulfonylamino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran (O-2050), and that a mutationally modified form of the CB1 receptor lacking constitutive activity was unable to mimic the effects of the wild-type receptor (Canals and Milligan, 2008).
The capacity of heteromers to alter the G protein–coupling selectivity compared with those of the corresponding monomers/homomers has also been a central element in defining specific roles for the dopamine D1-D2 receptor heteromer (Ng et al., 2010). This pairing has been indicated to form a specific heteromer in parts of the striatum (Hasbi et al., 2011) and to mediate a group of distinct signals through engagement of Ca2+-mediated signaling rather than the regulation of cAMP levels most normally anticipated to result from either dopamine D1 or D2 receptor activation. Recent work from a number of teams has implicated charged residues in the third intracellular loop of the D2 receptor and in the carboxyl terminal tail of the D1 receptor in defining this interaction, rather than the transmembrane domains (Pei et al., 2010; O'Dowd et al., 2012a), and in this regard has similarities to the studies by Navarro et al. (2010) described earlier. Furthermore, this had been predicted in earlier studies from Łukasiewicz et al. (2009), although these authors were careful to note mis-localization of mutants of the D2 receptor as providing a possible contribution to lack of heteromer formation rather than a direct role for specific amino acids. Pei et al. (2010) have suggested a key role for this complex in major depression, because higher levels of D1 receptor were immunoprecipitated by an anti-D2 receptor antibody from postmortem human striatal tissue from individuals suffering major depression than from controls and, subsequent to demonstrating that a peptide corresponding to part of the third intracellular loop of the D2 receptor was able to interfere with interactions with the D1 receptor, infused such a peptide into the frontal cortex of rats. Remarkably, this was as effective as treatment with the antidepressant drug imipramine in reducing immobility in forced swim tests (Pei et al., 2010). It will be interesting to discover if other heteromers between dopamine receptor subtypes also are stabilized by interactions involving elements of the intracellular loops. Other heteromers between dopamine receptor subtypes have, indeed, been reported, including D1-D3 (Marcellino et al., 2008; Ferre et al., 2010; Moreno et al., 2011a), D2-D3 (Pou et al., 2012), and D2-D4 (González et al., 2012) complexes, but the basis for their interactions and potential functional sequelae have not yet been explored in the level of detail of the D1-D2 heteromer. Interestingly, although it is widely accepted that the detection of receptor heteromers is likely to be associated with the concurrent presence of the corresponding homomers, this is difficult to assess in ex vivo situations, and even in vitro this has only recently been explored in cells coexpressing varying amounts of the dopamine D2 and D3 receptors to show that the expectation of a mixture of homomers and heteromers was likely to be correct (Pou et al., 2012).
Even at the level of dopamine receptor homomers, Wang et al. (2010) have suggested that there might be a marked shift in the balance between monomers and dimers of the D2 receptor in disease conditions highly linked to function of this receptor, including schizophrenia. Reviews on this topic have also highlighted the potential contribution of dopamine-containing heteromers (e.g., Maggio and Millan, 2010; Perreault et al., 2011). Although it is too early to draw general conclusions, the concept discussed earlier, that interconversion between quaternary structure states of GPCRs may be rapid and extensive, linked to the idea that different organizational states of receptors might differentially affect the capacity for signal transduction, means that assessment of how the proportion of monomers, dimers, and higher-order oligomers might be regulated and potentially vary with disease is an area ripe for reinvestigation.
A number of other GPCR heteromers appear to play important roles in brain function. A recent study highlighted the potential importance of interactions between the dopamine D2 receptor and the ghrelin receptor (Kern et al., 2012). These workers showed coexpression of the two receptors via a combination of stained brain sections from mice in which green fluorescent protein was driven from the ghrelin receptor protomer with an anti-D2 receptor antibody and subsequently noted an alteration in signaling pathways for D2 receptor agonists in cells engineered to coexpress the two GPCRs that was recapitulated in primary cultures of hypothalamic neurons. Time-resolved FRET studies using SNAP- and CLIP-tagged (Maurel et al., 2008) forms of the two receptors were compatible with direct interactions between the two, as were allosteric effects of receptor selective ligands (Kern et al., 2012). Such an interaction was also detected in native tissue using a ligand-based time-resolved FRET concept and approach. Herein, a red fluorophore-coupled form of ghrelin was used to label and bind to the ghrelin receptor and to act as an energy acceptor, whereas an antibody against the D2 receptor was labeled with an energy donor, cryptate-labeled fluorophore secondary antibody. Time-resolved FRET signals were detected in membranes from the hypothalamus of wild-type but not ghrelin receptor knockout animals (Kern et al., 2012). Furthermore, physiologic relevance of the heteromer was assessed in a model of anorexia in wild-type animals. Here a selective ghrelin receptor antagonist was shown to block cabergoline (an ergot-related D2 receptor agonist)-induced anorexia (Kern et al., 2012). The authors suggested that inhibiting dopamine D2 receptor signaling in subsets of neurons with a ghrelin receptor antagonist would provide much greater pharmacological selectivity than the use of a direct dopamine D2 receptor antagonist, as the effect would be restricted to cells expressing the heteromer. This concept of tissue selectivity provided by targeting GPCR heteromers has been discussed extensively as a potential therapeutic approach (Milligan and Smith, 2007; Casado et al., 2009; Del Burgo and Milligan, 2010; Smith and Milligan, 2010). Observations of allosteric effects between highly selective ligands for pairs of receptors coexpressed in the same cell have become something of a mainstay in providing pharmacological support for the presence of receptor heteromers. Examples relating to opioid receptor subtypes are considered later, but examples supporting interactions between chemokine receptor pairs have been perhaps the most numerous and best studied (e.g., Sohy et al., 2007). These can potentially involve more than two coexpressed receptor subtypes (Sohy et al., 2009), and can be observed in both native and transfected cells (Sohy et al., 2009). As a complex and wide ranging topic, this area has been reviewed extensively, and readers are guided toward some recent viewpoints (Wang and Norcross, 2008; Appelbe and Milligan, 2009; Salanga et al., 2009; Thelen et al., 2010).
A further heteromer involving the dopamine D2 receptor that has been studied extensively is the potential adenosine A2A–dopamine D2 complex. Functional interactions between these two receptors have been studied for many years, with the work of Fuxe and collaborators playing a central role. This team has written and speculated on the role of this and other potential heteromers so extensively (e.g., Filip et al., 2012; Fuxe et al., 2012) that it is almost impossible to summarize the available information and possibilities within a short review. However, in addition to the usual range of transfected cell, coimmunoprecipitation, and energy transfer approaches used to attempt to define the potential existence of such heteromers, it is noteworthy that recent proximity ligation studies that use immunohistochemical antibody detection of each partner followed by amplification of specific nucleotide sequences linked to secondary antibodies have provided evidence for proximity and, therefore, potential direct identification of an adenosine A2A–dopamine D2 heteromer in the striatum (Trifilieff et al., 2011). Although technically challenging, not least because of wide-ranging concerns over the specificity of many of the available GPCR antibodies (Beermann et al., 2012) and the modest expression levels of many GPCRs, approaches such as this offer the opportunity to begin to examine potential colocalization of GPCRs without resorting to more traditional methods based on, e.g., immunoelectron microscopy (Moreno et al., 2012). Interestingly, each of large-scale GPCR protein production for crystallography trials, novel approaches to GPCR antigen presentation (Larsson et al., 2011), and indications that anti-GPCR–directed “biologicals” may have clinical utility (Harris et al., 2012) hint at ways forward in providing a wider range of more specific and high-affinity immunologic reagents. Furthermore, despite some of the concerns noted earlier about antibody specificity, in relation to the identification of heteromers, a small number of heteromer-specific antibodies have been described (Gupta et al., 2010; Rozenfeld et al., 2011) that potentially identify such complexes directly. Rozenfeld and colleagues (2011) used an antibody reported to selectively detect angiotensin AT1 receptor–cannabinoid CB1 receptor heteromers to detect higher levels of such a complex in hepatic stellate cells from ethanol-administered rats, whereas Gupta et al. (2010) used a potentially δ-μ–opioid receptor heteromer-specific antibody to detect higher levels of this complex in chronic morphine-treated animals in areas of the central nervous system important for pain processing. It would be interesting to see the behavior of this antibody in cells coexpressing forms of these two receptors reported to disrupt heteromer formation (O’Dowd et al., 2012b).
Interactions between opioid receptor subtypes and the implications of this for both pharmacology and function have been studied perhaps more widely than in any other group of class A GPCRs. As noted earlier, crystals of the μ-opioid receptor formed with a strong potential dimeric interface involving many residues from transmembrane helices V and VI and a more limited second interface involving residues from helices I and II as well as the intracellular helix VIII (Manglik et al., 2012). Similarly, crystals of the κ-opioid receptor containing a bound antagonist also display a parallel dimeric interface involving residues of helices I, II, and VIII (Wu et al., 2012). By contrast, no parallel interaction interfaces were observed in crystals of the δ-opioid receptor bound by naltrindole (Granier et al., 2012). Despite this, reported heteromeric opioid receptors are not restricted to the κ-μ–receptor pairing. Indeed, for the potential δ-μ–receptor pairing, it has been suggested that each individual receptor may exist as a homodimer that can combine to form a heterotetramer (Golebiewska et al., 2011). Interactions between δ- and μ-receptors have been widely implicated in efforts to understand specific features of the action of morphine, and to consider if targeting such a heteromer might overcome aspects of the development of tolerance to this clinically important drug (Berger and Whistler, 2010; Costantino et al., 2012). Interestingly, given the potential role of transmembrane domains in such heteromers, it is noteworthy that alteration of a few key residues of the intracellular loops of these receptors is reported to interfere with heteromer formation (O'Dowd et al., 2012b). On this basis, it should be possible to disrupt such interactions using either cell permeable peptides or peptidomimetic small molecules and hence assess functional significance as for the dopamine D1-D2 heteromer (Pei et al., 2010). This, however, remains to be tested. The δ-μ–opioid receptor heteromer is also reported to display unique ligand pharmacology (e.g., Kabli et al., 2010; Gomes et al., 2011). The opioid receptors have been particularly amenable to such studies because of the complex pharmacology of opioid receptors that has been described, which is not easily defined or replicated by studies performed on cells expressing only one of the cloned subtypes, as well as the vast array of both peptide- and alkaloid-based opioid ligands that have been generated to explore the function of these receptors. Given the vast literature around this topic, only an expert on opioid receptor pharmacology could provide a balanced short overview. However, among key observations are those that have reported marked variation in, and often unique, ligand pharmacology upon coexpression of pairs of opioid receptors (e.g., Kabli et al., 2010; Gomes et al., 2011). A key question that remains to be established firmly is the extent and distribution of this heteromer. Detailed studies by Scherrer et al. (2009) indicated a limited overlap of δ- and μ-opioid receptors expressing neurones and, if confirmed, this would indicate, at most, a limited distribution of the heteromer.
In addition to heteromers incorporating only opioid receptor subtypes, reports of heteromers in which an opioid receptor interacts with a less-related GPCR are widespread. Coexpression of such pairs, e.g., the cannabinoid CB1 receptor with either the δ- (Bushlin et al., 2012; Rozenfeld et al., 2012) or μ- (Rios et al., 2006) opioid receptor, also results in substantial alterations in ligand pharmacology and function. Furthermore, when the μ-opioid receptor was coexpressed with a form of the α2A-adrenoceptor engineered to function as an intramolecular FRET sensor, coaddition of opioid ligands was able to modify the FRET response to the presence of agonists of the α2A-adrenoceptor (Vilardaga et al., 2008). Although potentially a powerful approach, other examples have not yet been reported as suitable FRET sensors are not generally available. A further example of a potentially heteromeric interaction involving an opioid receptor with implications for physiology and behavioral response was described by Liu et al. (2011). Herein, interactions between the gastrin-releasing peptide receptor and different μ-opioid receptor splice variants had distinct functional sequelae. Opioid-induced itch is linked to use of morphine in pain management, and Liu et al. (2011) demonstrated that interaction of the gastrin-releasing peptide receptor specifically with the MOR1D splice isoform was required to cause morphine-induced scratching, whereas the MOR1 receptor was key for morphine-induced analgesia. These two splice variants of the μ-opioid receptor displayed limited overlap of distribution, but strong coexpression of the MOR1D and the gastrin-releasing peptide receptor was observed. Equally, and rather like the loss of the behavioral function of 5-HT2A receptor-directed hallucinogenic agonists in mGluR2 knockout mice, morphine-induced scratching, but not morphine-induced analgesia, was almost lacking in gastrin-releasing peptide receptor knockout mice (Liu et al., 2011), and a gastrin-releasing peptide receptor antagonist greatly reduced morphine-induced scratching, but not analgesia, in wild-type mice. As these slice variants of the μ receptor differ only in the intracellular C-terminal domain, a peptide containing the sequence variance between the two μ receptor forms was introduced into the spinal cord of mice. This markedly reduced both the ability of the gastrin-releasing peptide receptor and MOR1D isoform to be coimmunoprecipitated and the extent of morphine-induced scratching (Liu et al., 2011).
Conclusions
Although clearly requiring substantial physiologic insight as well as high-level technical skills, a number of recent examples of disruption of GPCR heteromers, based partly on phenotypes of GPCR knockout mice, both provide increasing support of the presence of such complexes in native cell and tissue settings and confirm their potential as distinct therapeutic targets. Despite the fascinating physiologic effects noted to be associated with understanding the presence of such heteromers and targeting them either pharmacologically or via transgenic or other interventions, there remains an enormous lack of knowledge of even the basic principles of interactions between class A GPCRs, the overall size of such complexes, and their stability and dynamics. A clear understanding of all of these features will be required before a systematic means to target them therapeutically will become a reality.
Abbreviations
- BRET
bioluminescence resonance energy transfer
- CHO
Chinese hamster ovary
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- FRET
fluorescence resonance energy transfer
- GPCR
G protein–coupled receptor
- mGluR2
metabotropic glutamate reception subtype 2
- O-2050
(6aR,10aR)-3-(1-methanesulfonylamino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran
- rimonabant/SR141716
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- TAS
Taste
Authorship Contribution
Wrote or contributed to the writing of the manuscript: Milligan.
Footnotes
Work in the Milligan laboratory on this topic was supported by the Medical Research Council UK [Grant G0900050].
References
- Albizu L, Cottet M, Kralikova M, Stoev S, Seyer R, Brabet I, Roux T, Bazin H, Bourrier E, Lamarque L, et al. (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol 6:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Curto E, Pediani JD, Milligan G. (2010a) Applications of fluorescence and bioluminescence resonance energy transfer to drug discovery at G protein coupled receptors. Anal Bioanal Chem 398:167–180 [DOI] [PubMed] [Google Scholar]
- Alvarez-Curto E, Ward RJ, Pediani JD, Milligan G. (2010b) Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET. J Biol Chem 285:23318–23330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio M, Zürn A, Lohse MJ. (2011) Sensing G protein-coupled receptor activation. Neuropharmacology 60:45–51 [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. (2000) Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci USA 97:3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbe S, Milligan G. (2009) Chapter 10. Hetero-oligomerization of chemokine receptors. Methods Enzymol 461:207–225 [DOI] [PubMed] [Google Scholar]
- Arcemisbéhère L, Sen T, Boudier L, Balestre MN, Gaibelet G, Detouillon E, Orcel H, Mendre C, Rahmeh R, Granier S, et al. (2010) Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J Biol Chem 285:6337–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayburt TH, Vishnivetskiy SA, McLean MA, Morizumi T, Huang CC, Tesmer JJ, Ernst OP, Sligar SG, Gurevich VV. (2011) Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem 286:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann S, Seifert R, Neumann D. (2012) Commercially available antibodies against human and murine histamine H₄-receptor lack specificity. Naunyn Schmiedebergs Arch Pharmacol 385:125–135 [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72:477–484 [DOI] [PubMed] [Google Scholar]
- Berger AC, Whistler JL. (2010) How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol 67:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea J, Castro M, Giraldo J, López-Giménez JF, Padín JF, Quintián F, Cadavid MI, Vilaró MT, Mengod G, Berg KA, et al. (2009) Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine(2A) receptor homodimers. Mol Pharmacol 75:1380–1391 [DOI] [PubMed] [Google Scholar]
- Bushlin I, Gupta A, Stockton SD, Jr, Miller LK, Devi LA. (2012) Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain. PLoS ONE 7:e49789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Rieken F, Wagner J, Sungkaworn T, Zabel U, Borzi A, Cocucci E, Zürn A, Lohse MJ. (2013) Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci USA 110:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Lopez-Gimenez JF, Milligan G. (2009) Cell surface delivery and structural re-organization by pharmacological chaperones of an oligomerization-defective alpha(1b)-adrenoceptor mutant demonstrates membrane targeting of GPCR oligomers. Biochem J 417:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Milligan G. (2008) Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem 283:11424–11434 [DOI] [PubMed] [Google Scholar]
- Casadó V, Cortés A, Mallol J, Pérez-Capote K, Ferré S, Lluis C, Franco R, Canela EI. (2009) GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol Ther 124:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciari D, Dell’Orco D, Fanelli F. (2008) Homodimerization of neurotensin 1 receptor involves helices 1, 2, and 4: insights from quaternary structure predictions and dimerization free energy estimations. J Chem Inf Model 48:1669–1678 [DOI] [PubMed] [Google Scholar]
- Chabre M, Cone R, Saibil H. (2003) Biophysics: is rhodopsin dimeric in native retinal rods? Nature 426:30–31, discussion 31 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. (2006) The receptors and cells for mammalian taste. Nature 444:288–294 [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. (2012) Opioid receptor heteromers in analgesia. Expert Rev Mol Med 14:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottet M, Albizu L, Comps-Agrar L, Trinquet E, Pin JP, Mouillac B, Durroux T. (2011) Time resolved FRET strategy with fluorescent ligands to analyze receptor interactions in native tissues: application to GPCR oligomerization. Methods Mol Biol 746:373–387 [DOI] [PubMed] [Google Scholar]
- Cunningham MR, McIntosh KA, Pediani JD, Robben J, Cooke AE, Nilsson M, Gould GW, Mundell S, Milligan G, Plevin R. (2012) Novel role for proteinase-activated receptor 2 (PAR2) in membrane trafficking of proteinase-activated receptor 4 (PAR4). J Biol Chem 287:16656–16669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente M, Noble DN, Verma S, Nieman MT. (2012) Mapping human protease-activated receptor 4 (PAR4) homodimer interface to transmembrane helix 4. J Biol Chem 287:10414–10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Burgo LS, Milligan G. (2010) Heterodimerisation of G protein-coupled receptors: implications for drug design and ligand screening. Expert Opin Drug Discov 5:461–474 [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. (2012) Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 62:2184–2191 [DOI] [PubMed] [Google Scholar]
- Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bünemann M. (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat Methods 6:225–230 [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. (2011) A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 25:66–77 [DOI] [PubMed] [Google Scholar]
- Ferré S, Lluis C, Lanciego JL, Franco R. (2010) Prime time for G-protein-coupled receptor heteromers as therapeutic targets for CNS disorders: the dopamine D₁-D₃ receptor heteromer. CNS Neurol Disord Drug Targets 9:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K. (2012) The importance of the adenosine A(2A) receptor-dopamine D(2) receptor interaction in drug addiction. Curr Med Chem 19:317–355 [DOI] [PubMed] [Google Scholar]
- Fonseca JM, Lambert NA. (2009) Instability of a class a G protein-coupled receptor oligomer interface. Mol Pharmacol 75:1296–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. (2003) Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature 421:127–128 [DOI] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, et al. (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung JJ, Deupi X, Pardo L, Yao XJ, Velez-Ruiz GA, Devree BT, Sunahara RK, Kobilka BK. (2009) Ligand-regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J 28:3315–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Marcellino D, Romero-Fernandez W, Frankowska M, Guidolin D, Filip M, Ferraro L, Woods AS, Tarakanov A, et al. (2012) GPCR heteromers and their allosteric receptor-receptor interactions. Curr Med Chem 19:356–363 [DOI] [PubMed] [Google Scholar]
- Ganguly S, Clayton AH, Chattopadhyay A. (2011) Organization of higher-order oligomers of the serotonin₁(A) receptor explored utilizing homo-FRET in live cells. Biophys J 100:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Xiong D, Mosyak L, Malito DL, Kniazeff J, Chen Y, Burmakina S, Quick M, Bush M, Javitch JA, et al. (2012) Structure and functional interaction of the extracellular domain of human GABA(B) receptor GBR2. Nat Neurosci 15:970–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S. (2011) Differential response to morphine of the oligomeric state of μ-opioid in the presence of δ-opioid receptors. Biochemistry 50:2829–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. (2011) G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol 79:1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, Borycz J, Ortiz J, Lluís C, Franco R, et al. (2012) Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry 17:650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452 [DOI] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27:2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, et al. (2010) Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 3:ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2008) How and why do GPCRs dimerize? Trends Pharmacol Sci 29:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding PJ, Attrill H, Boehringer J, Ross S, Wadhams GH, Smith E, Armitage JP, Watts A. (2009) Constitutive dimerization of the G-protein coupled receptor, neurotensin receptor 1, reconstituted into phospholipid bilayers. Biophys J 96:964–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GL, Creason MB, Brulte GB, Herr DR. (2012) In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS ONE 7:e35129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. (2011) Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. (1996) A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271:16384–16392 [DOI] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, Molloy JE, Birdsall NJ. (2010) Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA 107:2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. (2005) Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J Biol Chem 280:40144–40151 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Cowan A, Mazurkiewicz JE. (2012) Oligomer size of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor revealed by fluorescence correlation spectroscopy with photon counting histogram analysis: evidence for homodimers without monomers or tetramers. J Biol Chem 287:23604–23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. (2006) Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem 281:27109–27116 [DOI] [PubMed] [Google Scholar]
- Hu J, Thor D, Zhou Y, Liu T, Wang Y, McMillin SM, Mistry R, Challiss RA, Costanzi S, Wess J. (2012) Structural aspects of M₃ muscarinic acetylcholine receptor dimer formation and activation. FASEB J 26:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. (2006) A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods 3:1001–1006 [DOI] [PubMed] [Google Scholar]
- Jäntti MH, Putula J, Turunen PM, Näsman J, Reijonen S, Lindqvist C, Kukkonen JP. (2013) Autocrine endocannabinoid signaling through CB1 receptors potentiates OX1 orexin receptor signaling. Mol Pharmacol 83:621–632 [DOI] [PubMed] [Google Scholar]
- Johnston JM, Aburi M, Provasi D, Bortolato A, Urizar E, Lambert NA, Javitch JA, Filizola M. (2011) Making structural sense of dimerization interfaces of delta opioid receptor homodimers. Biochemistry 50:1682–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Wang H, Provasi D, Filizola M. (2012) Assessing the relative stability of dimer interfaces in g protein-coupled receptors. PLOS Comput Biol 8:e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, O’Dowd BF, George SR. (2010) Agonists at the δ-opioid receptor modify the binding of µ-receptor agonists to the µ-δ receptor hetero-oligomer. Br J Pharmacol 161:1122–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai RS, Suzuki KG, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, Kusumi A. (2011) Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol 192:463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. (2012) Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 73:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Prézeau L, Rondard P, Pin JP, Goudet C. (2011) Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacol Ther 130:9–25 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ogawa K, Yao R, Lichtarge O, Bouvier M. (2009) Functional rescue of beta-adrenoceptor dimerization and trafficking by pharmacological chaperones. Traffic 10:1019–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK. (2009) Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 284:26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan TH, Kuravi S, Lambert NA. (2011) Internalization dissociates β2-adrenergic receptors. PLoS ONE 6:e17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K, Hofström C, Lindskog C, Hansson M, Angelidou P, Hökfelt T, Uhlén M, Wernérus H, Gräslund T, Hober S. (2011) Novel antigen design for the generation of antibodies to G-protein-coupled receptors. J Immunol Methods 370:14–23 [DOI] [PubMed] [Google Scholar]
- Leskelä TT, Lackman JJ, Vierimaa MM, Kobayashi H, Bouvier M, Petäjä-Repo UE. (2012) Cys-27 variant of human δ-opioid receptor modulates maturation and cell surface delivery of Phe-27 variant via heteromerization. J Biol Chem 287:5008–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. (2011) Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. (2007) The alpha1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol Pharmacol 71:1015–1029 [DOI] [PubMed] [Google Scholar]
- Łukasiewicz S, Faron-Górecka A, Dobrucki J, Polit A, Dziedzicka-Wasylewska M. (2009) Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J 276:760–775 [DOI] [PubMed] [Google Scholar]
- Ma AW, Redka DS, Pisterzi LF, Angers S, Wells JW. (2007) Recovery of oligomers and cooperativity when monomers of the M2 muscarinic cholinergic receptor are reconstituted into phospholipid vesicles. Biochemistry 46:7907–7927 [DOI] [PubMed] [Google Scholar]
- Maggio R, Millan MJ. (2010) Dopamine D2-D3 receptor heteromers: pharmacological properties and therapeutic significance. Curr Opin Pharmacol 10:100–107 [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, et al. (2008) Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem 283:26016–26025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C, Fruchart-Gaillard C, Letellier G, Marcon E, Mourier G, Zinn-Justin S, Ménez A, Servent D, Gilquin B. (2011) Structural model of ligand-G protein-coupled receptor (GPCR) complex based on experimental double mutant cycle data: MT7 snake toxin bound to dimeric hM1 muscarinic receptor. J Biol Chem 286:31661–31675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, et al. (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods 5:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Kamal M, Jockers R. (2011) Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol Sci 32:514–520 [DOI] [PubMed] [Google Scholar]
- May LT, Bridge LJ, Stoddart LA, Briddon SJ, Hill SJ. (2011) Allosteric interactions across native adenosine-A3 receptor homodimers: quantification using single-cell ligand-binding kinetics. FASEB J 25:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. (2004) G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol 66:1–7 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2008) A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153 (Suppl 1):S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Bouvier M. (2005) Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J 272:2914–2925 [DOI] [PubMed] [Google Scholar]
- Milligan G, Smith NJ. (2007) Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol Sci 28:615–620 [DOI] [PubMed] [Google Scholar]
- Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G. (2009) Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol 76:379–387 [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepúlveda M, Navarro G, Casadó V, Cortés A, Mallol J, Vignes M, McCormick PJ, Canela EI, et al. (2011a) Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem 286:5846–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. (2011b) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493:76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuéllar F, Mocci G, Seto J, Callado LF, Neve RL, et al. (2012) Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem 287:44301–44319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Ferré S, Cordomi A, Moreno E, Mallol J, Casadó V, Cortés A, Hoffmann H, Ortiz J, Canela EI, et al. (2010) Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J Biol Chem 285:27346–27359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O’Dowd BF, George SR. (2010) Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience 165:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Ji X, Nguyen T, George SR. (2012a) Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem Biophys Res Commun 417:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Ji X, O’Dowd PB, Nguyen T, George SR. (2012b) Disruption of the mu-delta opioid receptor heteromer. Biochem Biophys Res Commun 422:556–560 [DOI] [PubMed] [Google Scholar]
- Palmer RK. (2007) The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol Interv 7:87–98 [DOI] [PubMed] [Google Scholar]
- Park PS, Sum CS, Pawagi AB, Wells JW. (2002) Cooperativity and oligomeric status of cardiac muscarinic cholinergic receptors. Biochemistry 41:5588–5604 [DOI] [PubMed] [Google Scholar]
- Patowary S, Alvarez-Curto E, Xu T-R, Holz JD, Oliver JA, Milligan G, Raicu V. (2013) The muscarinic M3 acetylcholine receptor exists as two differently sized complexes at the plasma membrane. Biochem J 452:303–312 [DOI] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. (2010) Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med 16:1393–1395 [DOI] [PubMed] [Google Scholar]
- Pellissier LP, Barthet G, Gaven F, Cassier E, Trinquet E, Pin JP, Marin P, Dumuis A, Bockaert J, Banères JL, et al. (2011) G protein activation by serotonin type 4 receptor dimers: evidence that turning on two protomers is more efficient. J Biol Chem 286:9985–9997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, O’Dowd BF, George SR. (2011) Dopamine receptor homooligomers and heterooligomers in schizophrenia. CNS Neurosci Ther 17:52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrin D, Hébert TE. (2012) The functional size of GPCRs - monomers, dimers or tetramers? Subcell Biochem 63:67–81 [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. (2006) Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15:2200–2209 [DOI] [PubMed] [Google Scholar]
- Pin JP, Comps-Agrar L, Maurel D, Monnier C, Rives ML, Trinquet E, Kniazeff J, Rondard P, Prézeau L. (2009) G-protein-coupled receptor oligomers: two or more for what? Lessons from mGlu and GABAB receptors. J Physiol 587:5337–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, et al. (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev 59:5–13 [DOI] [PubMed] [Google Scholar]
- Pisterzi LF, Jansma DB, Georgiou J, Woodside MJ, Chou JT, Angers S, Raicu V, Wells JW. (2010) Oligomeric size of the m2 muscarinic receptor in live cells as determined by quantitative fluorescence resonance energy transfer. J Biol Chem 285:16723–16738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou C, Mannoury la Cour C, Stoddart LA, Millan MJ, Milligan G. (2012) Functional homomers and heteromers of dopamine D2L and D3 receptors co-exist at the cell surface. J Biol Chem 287:8864–8878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provasi D, Johnston JM, Filizola M. (2010) Lessons from free energy simulations of delta-opioid receptor homodimers involving the fourth transmembrane helix. Biochemistry 49:6771–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. (2006) mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol 148:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Müller A, Chou YY, Ji I, Lajic S, Hanyaloglu AC, Jonas K, Rahman N, Ji TH, Huhtaniemi I. (2010) Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci USA 107:2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. (2009) The structure and function of G-protein-coupled receptors. Nature 459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira X, Vivó M, Serra J, Roche D, Strange PG, Giraldo J. (2009) Modelling the interdependence between the stoichiometry of receptor oligomerization and ligand binding for a coexisting dimer/tetramer receptor system. Br J Pharmacol 156:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Bushlin I, Gomes I, Tzavaras N, Gupta A, Neves S, Battini L, Gusella GL, Lachmann A, Ma’ayan A, et al. (2012) Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS ONE 7:e29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, Nieto N, Devi LA. (2011) AT1R-CB₁R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J 30:2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagacé M, Marullo S, Bouvier M. (2004) Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem 279:33390–33397 [DOI] [PubMed] [Google Scholar]
- Salahpour A, Masri B. (2007) Experimental challenge to a ‘rigorous’ BRET analysis of GPCR oligomerization. Nat Methods 4:599–600, author reply 601 [DOI] [PubMed] [Google Scholar]
- Salanga CL, O’Hayre M, Handel T. (2009) Modulation of chemokine receptor activity through dimerization and crosstalk. Cell Mol Life Sci 66:1370–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartania N, Appelbe S, Pediani JD, Milligan G. (2007) Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric beta2-adrenoceptor. Cell Signal 19:1928–1938 [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62:701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohy D, Parmentier M, Springael JY. (2007) Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem 282:30062–30069 [DOI] [PubMed] [Google Scholar]
- Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael JY. (2009) Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 284:31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai B, Barkai L, Turu G, Szidonya L, Várnai P, Hunyady L. (2012) Allosteric interactions within the AT₁ angiotensin receptor homodimer: role of the conserved DRY motif. Biochem Pharmacol 84:477–485 [DOI] [PubMed] [Google Scholar]
- Thelen M, Muñoz LM, Rodríguez-Frade JM, Mellado M. (2010) Chemokine receptor oligomerization: functional considerations. Curr Opin Pharmacol 10:38–43 [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch JA. (2011) Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MS, Kim H, Deyo A, Naider F, Becker JM. (2012) Identification of residues involved in homodimer formation located within a β-strand region of the N-terminus of a Yeast G protein-coupled receptor. J Recept Signal Transduct Res 32:65–75 [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. (2008) Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol 4:126–131 [DOI] [PubMed] [Google Scholar]
- Wang HX, Konopka JB. (2009) Identification of amino acids at two dimer interface regions of the alpha-factor receptor (Ste2). Biochemistry 48:7132–7139 [DOI] [PubMed] [Google Scholar]
- Wang J, Norcross M. (2008) Dimerization of chemokine receptors in living cells: key to receptor function and novel targets for therapy. Drug Discov Today 13:625–632 [DOI] [PubMed] [Google Scholar]
- Wang M, Pei L, Fletcher PJ, Kapur S, Seeman P, Liu F. (2010) Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: increased dopamine D2 receptor dimerization. Mol Brain 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Pediani JD, Milligan G. (2011) Heteromultimerization of cannabinoid CB(1) receptor and orexin OX(1) receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem 286:37414–37428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 104:7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem 283:4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Wilkinson G, Milligan G. (2005) The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem 280:28663–28674 [DOI] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, et al. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, et al. (2012) Structure of the human κ-opioid receptor in complex with JDTic. Nature 485:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T-R, Ward RJ, Pediani JD, Milligan G. (2011) The orexin OX(1) receptor exists predominantly as a homodimer in the basal state: potential regulation of receptor organization by both agonist and antagonist ligands. Biochem J 439:171–183 [DOI] [PubMed] [Google Scholar]
- Xu T-R, Ward RJ, Pediani JD, Milligan G. (2012) Intramolecular fluorescence resonance energy transfer (FRET) sensors of the orexin OX1 and OX2 receptors identify slow kinetics of agonist activation. J Biol Chem 287:14937–14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Guan R, Segaloff DL. (2012) Revisiting and questioning functional rescue between dimerized LH receptor mutants. Mol Endocrinol 26:655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]