Abstract
Extracellular calcium (Ca2+e)-induced relaxation of isolated, phenylephrine (PE)-contracted mesenteric arteries is dependent on an intact perivascular sensory nerve network that expresses the Ca2+-sensing receptor (CaSR). Activation of the receptor stimulates an endocannabinoid vasodilator pathway, which is dependent on cytochrome P450 and phospholipase A2 but largely independent of the endothelium. In the present study, we determined the role of nitric oxide (NO) in perivascular nerve CaSR-mediated relaxation of PE-contracted mesenteric resistance arteries isolated from mice. Using automated wire myography, we studied the effects of NO synthase (NOS) gene knockout (NOS−/−) and pharmacologic inhibition of NOS on Ca2+e-induced relaxation of PE-contracted arteries. Endothelial NOS knockout (eNOS−/−) upregulates but neuronal NOS knockout (nNOS−/−) downregulates CaSR expression. NOS−/− reduced maximum Ca2+e-induced relaxation with no change in EC50 values, with eNOS−/− having the largest effect. The responses of vessels to calindol and Calhex 231 indicate that the CaSR mediates relaxation. l-N5-(1-iminoethyl)-ornithine reduced Ca2+e-induced relaxation of PE-contracted arteries from C57BL/6 control mice by ≈38% but had a smaller effect in vessels from eNOS−/− mice. 7-Nitroindazole had no significant effect on relaxation of arteries from NOS−/− mice, but both NG-nitro-l-arginine methylester and NG-monomethyl-l-arginine significantly reduced the relaxation maxima in all groups. Interestingly, the nNOS-selective inhibitor S-methyl-l-thiocitrulline significantly increased the EC50 value by ≈60% in tissues from C57BL/6 mice but reduced the maximum response by ≈80% in those from nNOS−/− mice. Ca2+-activated big potassium channels play a major role in the process, as demonstrated by the effect of iberiotoxin. We conclude that CaSR signaling in mesenteric arteries stimulates eNOS and NO production that regulates Ca2+e-induced relaxation.
Introduction
The mesenteric bed is innervated by sensory nerve fibers that have their cell bodies located in the dorsal root ganglia. The perivascular nerve (PvN) of mesenteric arteries expresses a Ca2+-sensing receptor (CaSR) that is functionally different from the parathyroid gland receptor (Awumey et al., 2007, 2008). Extracellular calcium (Ca2+e)-induced relaxation of isolated mesenteric arteries from rats is dependent on an intact PvN network and mediated by a hyperpolarizing endocannabinoid vasodilator transmitter (Ishioka and Bukoski, 1999). The relaxation occurs through pathways dependent on cytochrome P450 (P450) and cytosolic phospholipase A2 (Awumey et al., 2008). Specifically, our studies indicate that vasodilators resulting from the metabolism of 2-arachidonoylglycerol, which is catalyzed by P450, trigger relaxation by activating Ca2+-activated big potassium (BK) channels. A component (≈20%) of the relaxation, however, is dependent on the endothelium, suggesting that endothelium-derived relaxation factors such as nitric oxide (NO) may also play a role.
A number of studies have shown that CaSR is also expressed in vascular endothelium (Berra Romani et al., 2009) and smooth muscle cells (Wonneberger et al., 2000; Molostvov et al., 2008; Alam et al., 2009), suggesting that CaSR-mediated relaxation of mesenteric arteries may involve multiple components within the vasculature. Thus, activation of the PvN CaSR may be coupled to increased intracellular Ca2+ concentration ([Ca2+]i) and activation of neuronal and/or endothelial NO synthase (nNOS and eNOS, respectively) and synthesis of NO. The NO can then diffuse into the vascular smooth muscle to contribute to or modulate relaxation. NO is important in the maintenance of vascular tone, and genetic deletions of NOS isoforms are used to determine the importance of these isoforms to specific processes. The main source of NO in the vasculature is the microvascular endothelium, from where it can contribute to cGMP activation in vascular smooth muscle cells and thus influence vascular reactivity (McCarron et al., 2006; Berra Romani et al., 2009). However, NO can also be produced from nNOS activation in the peripheral nerve terminals on blood vessels (Toda and Okamura, 2011). In the present study, we tested the hypothesis that NOS knockout modulates Ca2+e-induced relaxation of mesenteric arteries as a compensatory mechanism to counteract the effects of reduced NO synthesis and to confirm that the relaxation is mediated by the CaSR. We employed nNOS−/− and eNOS−/− mice; the BK channel blocker iberiotoxin (IbTX); and the NOS inhibitors NG-nitro-l-arginine methylester (l-NAME; nonselective), NG-monomethyl-l-arginine (l-NMMA; competitive, nonselective) l-N5-(1-iminoethyl)-ornithine (l-NIO; nonselective), 7-nitroindazole (7-NI; highly selective for the neuronal enzyme), and S-methyl-l-thiocitrulline (S-MeTC; selective for rat nNOS) to determine the role of nNOS and eNOS in Ca2+e-induced vascular relaxation.
Materials and Methods
Animals.
All procedures using animals were carried out according to protocols approved by the North Carolina Central University Institutional Animal Care and Use Committee.
Chemicals.
l-NAME, l-NMMA, l-NIO, 7-NI, and S-MeTC were obtained from Calbiochem (La Jolla, CA). Phenylephrine (PE) and IbTX were from Sigma-Aldrich (St. Louis, MO). Affinity-purified rabbit polyclonal antibodies raised against synthetic peptides corresponding, to human nNOS (Cat. #4234), to residues around Ser600 of human eNOS (Cat. #9572) and Ser100 of human iNOS (Cat. # 2877) were from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal CaSR antibodies, raised against a synthetic polypeptide corresponding to the N terminus of rat CaSR, were from Pierce Biotechnology (Rockford, IL). Calindol, Calhex 231, and glyceraldehyde-3-phosphate dehydrogenase antibody (sc-25778) raised against the full-length glyceraldehyde-3-phosphate dehydrogenase of human origin were from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals used were of the purest grade available commercially.
Expression Analysis of NOS Isoforms and the CaSR in Mesenteric Arteries.
Total proteins were extracted from mesenteric arcades isolated from C57BL/6, nNOS−/−, and eNOS−/− mice and analyzed for NOS isoforms and CaSR protein by Western blotting. Briefly, mesenteric arcades were isolated from anesthetized animals, trimmed of fat, and stored at −80°C until used. Tissues were homogenized in ice-cold Tris buffer (10 mM Tris, pH 7.5; 0.25 M sucrose and 3 mM MgCl2) with freshly added Halt Protease Inhibitor cocktail (Pierce Biotechnology). Homogenates were centrifuged at 800g for 10 minutes and the supernatant fractions analyzed by electrophoretic separation on 8% sodium dodecyl sulfate polyacrylamide gel, transferred onto polyvinylidene difluoride (Immuno-Blot polyvinylidene difluoride; Bio-Rad, Hercules, CA) membranes, and blotted with polyclonal NOS (nNOS, eNOS, and iNOS) or CaSR antibodies. The blots were then incubated with horseradish peroxidase–conjugated anti-rabbit IgG and visualized using the enhanced chemiluminescence method.
Vessel Isolation.
Mesenteric arteries were dissected from mice under deep anesthesia with isoflurane and sacrificed by open-chest cardiac puncture as previously described (Bridges et al., 2011). Briefly, the small intestine and all vessels feeding it were removed in block and placed in physiological salt solution (PSS) (mM: NaCl, 115; KCl, 4.7; MgSO47H2O, 1.4; NaHCO3, 5; KH2PO4, 1.2; Na2HPO4, 1.1; CaCl2, 1.0; HEPES, 20; and glucose, 5; pH 7.4). Branch I and II mesenteric arteries were carefully dissected from the surrounding fat and mesenterium, taking care to leave a portion of the omental membrane attached to the vessel. A 40-μm-diameter stainless steel wire was inserted into the lumen to remove blood and to serve as a handle for moving the vessel segment between solutions.
Wire Myography.
Isometric force generation in isolated arteries was determined as previously described (Awumey et al., 2008; Bridges et al., 2011). Segments (2 mm long) of mesenteric branch arteries were mounted in a small-vessel Mulvany-Halpern 510A Auto Dual Wire Myograph (DMT-USA, Marietta, GA) by means of tungsten-free stainless steel wires inserted through the lumen and maintained in PSS with 1 mM CaCl2 and 100 μM ascorbic acid. When gassed with a mixture of 95% air and 5% CO2, this solution has a pH of 7.4. After 30-minute equilibration at 37°C, vessels were “normalized” by stepwise stretching and challenged with 5 μM PE until reproducible responses were observed. Relaxation was assessed by adding cumulative concentrations of test compounds to vessels that were precontracted to maximal tone. When inhibitors were used, tissues were preincubated with the compounds for 20 minutes before relaxation assays.
Statistical Analysis.
Concentration-response curves were analyzed with SigmaPlot 11.0 statistics programs from Systat Software (Point Richmond, CA). Comparisons between groups and within groups were carried out by one-way analysis of variance and differences with P < 0.05 were considered significant. EC50 values were determined by fitting data to a standard four-parameter logistic function in the Pharmacology menu of the program.
Results
Modulation of CaSR Expression in Mesenteric Arcade by NOS Knockout.
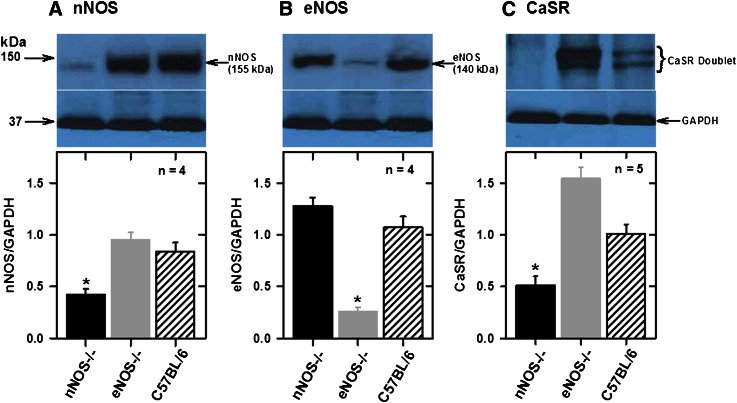
To confirm the knockout of NOS genes in mice used in the present studies and to determine the expression of the CaSR in mesenteric arteries, we analyzed total tissue proteins by Western blotting with NOS and CaSR antibodies. Figure 1A shows that nNOS was highly expressed at similar levels in mesenteric arteries from eNOS−/− and C57BL/6 mice but significantly reduced in tissues from nNOS−/− mice, and eNOS was highly expressed at similar levels in nNOS−/− and C57BL/6 but significantly reduced in eNOS−/− animals (Fig. 1B). Residual eNOS and nNOS were detected in eNOS−/− and nNOS−/−, respectively. No detectable iNOS expression was observed in these tissues (unpublished data). Analysis of CaSR expression showed that nNOS knockout animals (eNOS expression) have lower and eNOS knockout animals (nNOS expression) have higher CaSR expression relative to the C57BL/6 animals in mesenteric arteries (Fig. 1C).
Fig. 1.
Analysis of NOS isoforms and CaSR expressions in mesenteric arcades from C57BL/6 (control) and NOS−/− mice. Western blot analysis of nNOS (A), eNOS (B), and CaSR (C) in tissues from control and NOS−/− mice. Bar charts show individual density values of protein bands expressed as ratios of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. nNOS knockout downregulates but eNOS knockout upregulates CaSR expression. *P < 0.001 vs. other groups; one-way analysis of variance (ANOVA).
Normalization of Mounted Mesenteric Arteries.
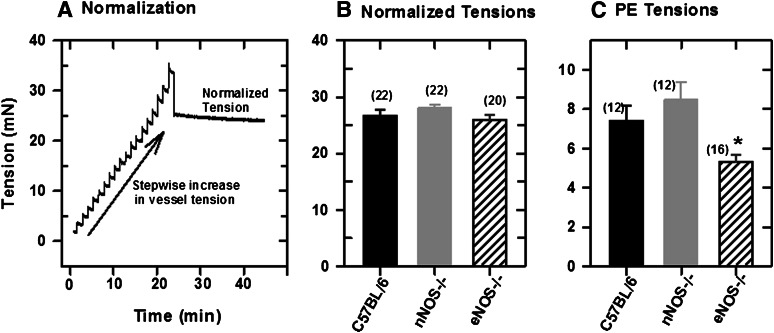
Figure 2A shows changes in vessel tension during “normalization” of a mounted mesenteric artery segment from a C57BL/6 mouse. The vessel was stretched stepwise following equilibration in PSS medium until a passive tension developed (Angus and Wright, 2000). Figure 2B shows the normalized tensions in isolated mesenteric arteries from control and NOS knockout mice, and Fig. 2C shows the developed tensions in these tissues following the application of 5 μM PE. Initial PE tensions were similar in tissues from control and nNOS−/− mice but lower in those from eNOS−/− mice. The PE tensions did not change significantly in the presence of NOS inhibitors.
Fig. 2.
Normalization of a mesenteric artery segment mounted in a wire myograph in PSS with 1 mM CaCl2. (A) Normalization: stepwise increase in vessel tension. (B) Normalized tensions in mesenteric artery segments mounted in the myograph chamber. (C) Tensions in normalized vessel segments following applications of 5 μM PE. Values plotted are means (± S.E.M.). Differences in normalized tensions are statistically significant (*P = 0.05; one-way analysis of variance).
Mediation of Ca2+-Induced Relaxation of Mesenteric Arteries by CaSR.
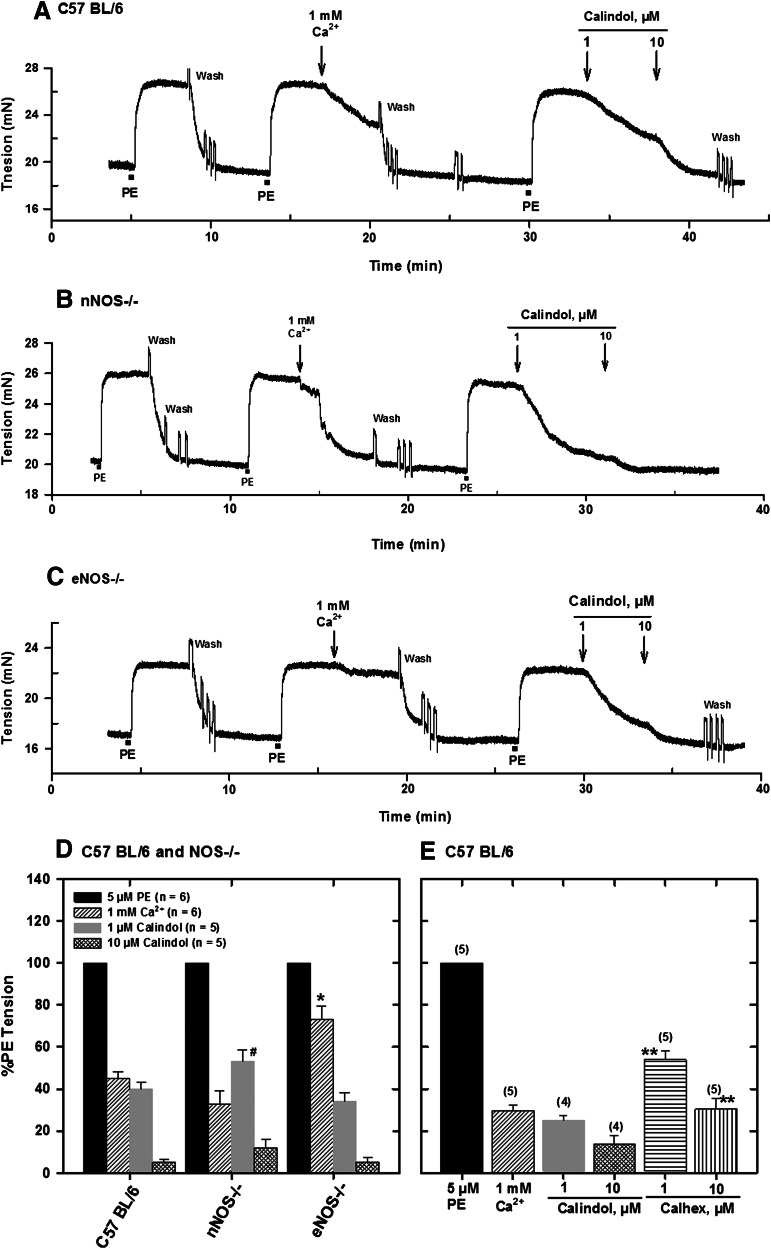
To confirm that Ca2+-induced relaxation of PE-contracted mesenteric arteries was mediated by the CaSR, we also determined responses to calindol (a CaSR agonist) and Calhex 231 (a CaSR antagonist), which act at the allosteric site of the receptor. Relaxations to both compounds were dependent on the concentration, and in all cases, Calindol (1 and 10 μM) relaxed the precontracted arteries by 75 and 90%, respectively (Fig. 3, A–D). On the other hand, 1 and 10 μM Calhex 231 caused only 45 and 70% relaxation, respectively, in tissues from control C57BL/6 mice (Fig. 3D). Ca2+-induced relaxation was similar in tissues from control and nNOS−/− mice and attenuated in those from eNOS−/− mice (Fig. 3E).
Fig. 3.
Effect of Ca2+e and the CaSR agonist calindol on relaxation of PE-contracted mesenteric arteries from C57BL/6 (A), nNOS−/− (Β), and eNOS−/− (C) mice mounted in PSS with 1 mM CaCl2. Responses of precontracted arteries to 1 mM Ca2+ and calindol (1 and 10 μM) are shown. (D) Relaxations of precontracted arteries to Ca2+ and calindol. (E) Relaxations of isolated, precontracted arteries from C57BL/6 mice to Ca2+, calindol, and the calcilytic Calhex 231. Values plotted are means (± S.E.) of 4–6 animals. *P < 0.05 vs. C57BL/6 and nNOS−/−; #P < 0.05 vs. C57BL/6 and eNOS−/−; **P < 0.05 vs. calindol responses (analysis of variance).
Effects of NOS and BK Inhibitors on Tension Changes in Mesenteric Arteries.
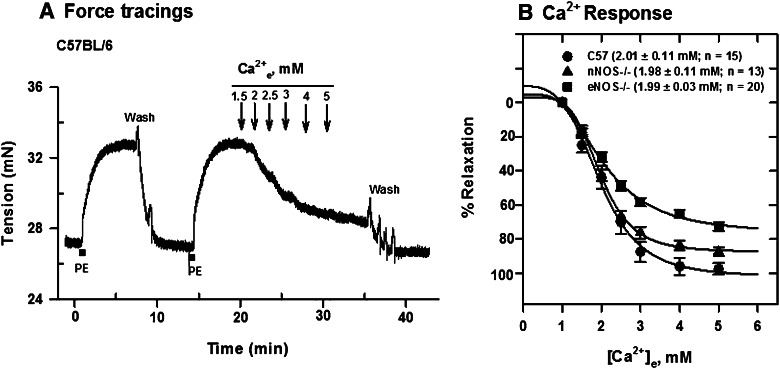
Cumulative applications of Ca2+e to PE-contracted mesenteric arteries from C57B/6 and NOS−/− mice induced relaxation; however, NOS knockout reduced maximum responses by about 25 and 8%, respectively, in tissues from eNOS−/− and nNOS−/− animals with no change in the EC50 values (Fig. 4). Deletion of eNOS reduced Ca2+e-induced relaxation maximum significantly compared with C57BL/6 (P < 0.05).
Fig. 4.
Ca2+e-induced relaxation of PE-contracted mesenteric arteries from C57BL/6, nNOS−/−, and eNOS−/− mice mounted in PSS containing 1 mM CaCl2. (A) Tension changes in an artery segment from C57BL/6 mouse following contraction with 5 μM PE and cumulative additions of Ca2+e. (B) [Ca2+]e-response curves generated from force tracing data obtained in tissues from C57BL/6, nNOS−/−, and eNOS−/− mice. Values plotted are means (± S.E.M.). NOS knockout reduced the maximum on the Ca2+-relaxation curve significantly (P < 0.05; one-way analysis of variance), with no change in EC50 values.
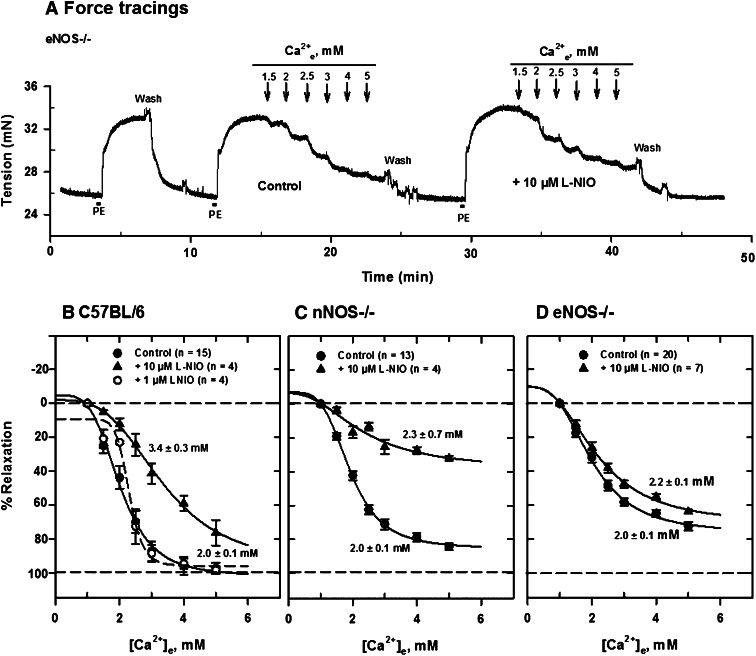
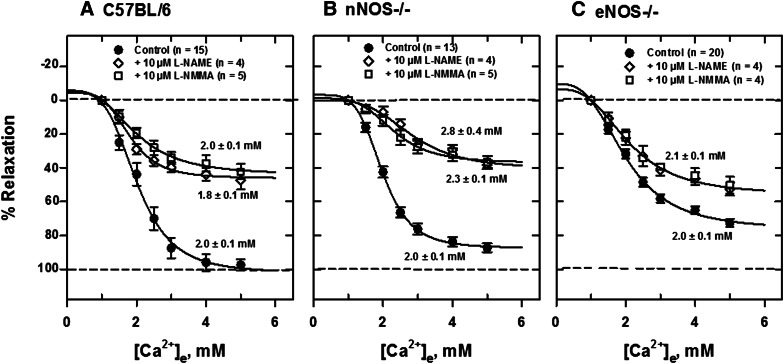
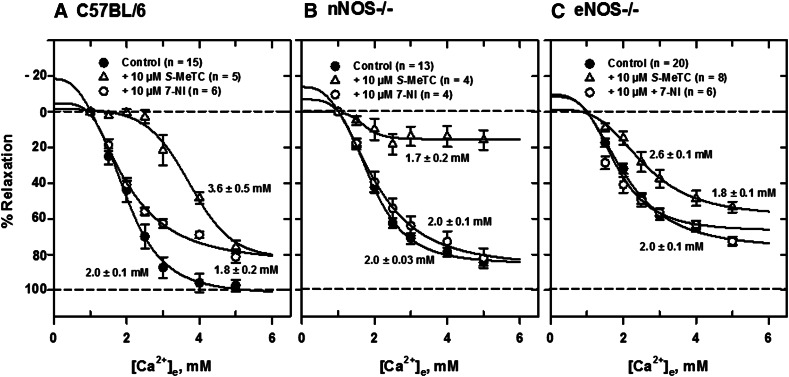
To determine the relative roles of neuronal and endothelial NO in Ca2+e-induced relaxation resulting from acute blockade, we examined the effect of several NOS inhibitors on relaxation of isolated, PE-contracted mesenteric arteries. Generally, the NOS inhibitors l-NIO, l-NAME, l-NMMA, and S-MeTC had less effect on Ca2+e-induced relaxations of arteries from eNOS−/− than nNOS−/− mice. Figure 5A shows a typical force tracing in an artery segment from eNOS−/− mice in the absence and presence of 10 μM l-NIO, a potent and nonselective NOS inhibitor (nNOS Ki, 1.7 μM in rat; eNOS Ki, 3.9 μM in bovine; 5 times more potent inhibitor of eNOS, IC50 = 500 nM, than l-NAME and l-NMMA). After equilibration of tissue for 30 minutes at 37°C with constant aeration, 5 μM PE was added to contract the vessel repeatedly until a constant tension developed. The tissue was then relaxed with cumulative additions of Ca2+e and Ca2+e concentration ([Ca2+]e)-response curves constructed as shown in Fig. 5, B–D. Ca2+e-induced relaxations were substantially reduced in arteries from C57BL/6 and nNOS−/− mice in the presence of the inhibitor compared with tissues from eNOS−/− mice. The [Ca2+]e-response curve was substantially shifted to the right in the presence of the inhibitor in tissues from control C57BL/6 mice. The EC50 for relaxation increased from 2.0 ± 0.1 mM to 3.4 ± 0.3 mM in the presence of the inhibitor in mesenteric arteries from C57BL/6 mice. l-NIO reduced the maximum response substantially (≈70%) in mesenteric arteries from nNOS−/− mice without changing the EC50. The inhibition pattern in control animals appears to be a composite of that observed in tissues from nNOS−/− and eNOS−/− mice. Similar procedures of contraction with 5 μM PE and relaxation with cumulative additions of Ca2+e were carried out with other NOS inhibitors and [Ca2+]e-response curves constructed. l-NAME and l-NMMA, competitive inhibitors of NOS, reduced Ca2+e-induced relaxation in similar fashion in C57BL/6 (Fig. 6A) and nNOS−/− (Fig. 6B) mice, leaving an insensitive component, which was much larger in tissues from eNOS−/− mice (Fig. 6C). Furthermore, the reversible and competitive NOS inhibitor 7-NI had no inhibitory effect in tissues from NOS−/− animals but apparently reduced the maximum response in tissues from C57BL/6 mice (Fig. 7A). S-MeTC, a NOS inhibitor with a greater selectivity for rat nNOS, shifted the [Ca2+]e-response curves to the right in tissues from C57BL/6 control and eNOS−/− mice (Fig. 7, A and C). This shift was more remarkable in C57BL/6 mice. Interestingly, S-MeTC almost abolished Ca2+e-induced relaxation of tissues from nNOS−/− mice (Fig. 7B). The EC50 for Ca2+ relaxation was 1.7 ± 0.2 mM and the maximum response was reduced by ≈80%. Table 1 shows the EC50 data in tissues from C57BL/6, nNOS−/−, and eNOS−/− mice.
Fig. 5.
Inhibition of Ca2+e-induced relaxation of PE-contracted mesenteric arteries by l-NIO. (A) Tracings of tension changes in a mesenteric artery segment from an eNOS−/− mouse following contraction with 5 μM PE and cumulative additions of Ca2+e. (B–D) Inhibition of Ca2+e-induced relaxation by 10 μM l-NIO. [Ca2+]e-response curves were generated from tracing data obtained in tissues from C57BL/6 (B), nNOS−/− (C), and eNOS−/− (D) mice mounted in PSS with 1 mM CaCl2. l-NIO had a larger effect in tissues from C57BL/6 and nNOS−/− mice than those from eNOS−/− mice.
Fig. 6.
Inhibition of Ca2+e-induced relaxation of PE-contracted mesenteric arteries by l-NAME and l-NMMA. [Ca2+]e-response curves were generated from tracing data obtained in tissues from C57BL/6 (A), nNOS−/− (B), and eNOS−/− (C) mice mounted in PSS with 1 mM CaCl2. A larger component of the relaxation in tissues from eNOS−/− was insensitive to inhibition by both compounds.
Fig. 7.
Inhibition of Ca2+e-induced relaxation of PE-contracted mesenteric arteries by S-MeTC and 7-NI. [Ca2+]e-response curves were generated from tracing data obtained in tissues from C57BL/6 (A), nNOS−/− (B), and eNOS−/− (C) mice mounted in PSS with 1 mM CaCl2. The EC50 for Ca2+e-induced relaxation of tissues from C57BL/6 mice was substantially reduced by S-MeTC compared with control (P < 0.05). The EC50 in NOS−/− remained the same but the maximum responses were reduced by about 80% in nNOS−/− and 10% in eNOS−/−. 7-NI had no effect in tissues from NOS−/− mice.
TABLE 1.
EC50 values for Ca2+e-induced relaxation of PE-contracted mesenteric arteries in the absence (Control) and presence of IbTX (100 nM) or of NOS inhibitors (10 μM each)
Values are means (± S.E.M.) derived from data fitted to a standard four-parameter sigmoid curve in the pharmacology menu of the SigmaPlot 11.0 program. The number of animals for each group is indicated.
| Group | EC50 Values in Presence of: | ||||||
|---|---|---|---|---|---|---|---|
| Control | IbTX | l-NIO | l-NAME | l-NMMA | S-MeTC | 7-NI | |
| mM | |||||||
| C57BL/6 | 2.0 ± 0.1 (n = 15) | 1.9 ± 0.2 (n = 7) | 3.4 ± 0.3* (n = 4) | 2.0 ± 0.1 (n = 5) | 1.8 ± 0.1 (n = 5) | 3.6 ± 0.5* (n = 5) | 1.8 ± 0.2 (n = 6) |
| nNOS−/− | 2.0 ± 0.1 (n = 13) | 3.2 ± 0.3¶ (n = 5) | 2.3 ± 0.7¶ (n = 4) | 2.8 ± 0.4 (n = 4) | 2.3 ± 0.1 (n = 4) | 1.7 ± 0.2¶ (n = 4) | 2.0 ± 0.1 (n = 4) |
| eNOS−/− | 2.0 ± 0.1 (n = 20) | 4.4 ± 0.8¶ (n = 5) | 2.2 ± 0.1¶ (n = 7) | 2.1 ± 0.1 (n = 4) | 2.1 ± 0.1 (n = 4) | 2.6 ± 0.1¶ (n = 8) | 1.8 ± 0.1 (n = 6) |
P < 0.05 vs. Control; ¶P < 0.05 vs. C57BL/6.
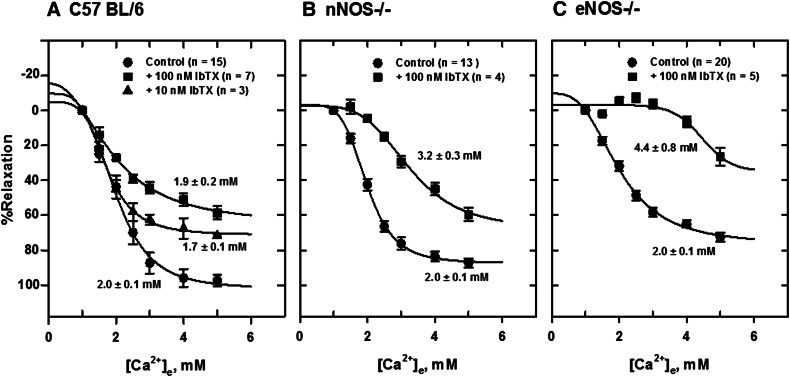
The BK channel blocker IbTX (100 nM) shifted the [Ca2+]e-response curves to the right in tissues from all animals (Fig. 8). A larger effect was seen in tissues from eNOS−/− mice, which show a higher level of CaSR expression. The order of IbTX potency in mesenteric arteries was eNOS−/− > nNOS−/− > C57BL/6. The EC50 values determined from nonlinear fitting of relaxation data to a four-parameter sigmoid curve were 1.9 ± 0.2 mM, 3.2 ± 0.3 mM, and 4.1 ± 0.6 mM for C57BL/6, nNOS−/−, and eNOS−/−, respectively (Fig. 9).
Fig. 8.
Inhibition of Ca2+-induced relaxation of PE-contracted mesenteric arteries by 100 nM IbTX. [Ca2+]e-response curves were generated from tension data obtained in tissues from C57BL/6 (A), nNOS−/− (B), and eNOS−/− (C) mice mounted in PSS with 1 mM CaCl2. IbTX (100 nM) shifted the [Ca2+]e-response curves to the right. A larger effect of IbTX was observed in tissues from eNOS−/− mice. The EC50 values for Ca2+e-induced relaxation were significantly higher in tissues from nNOS−/− and eNOS−/− mice.
Fig. 9.
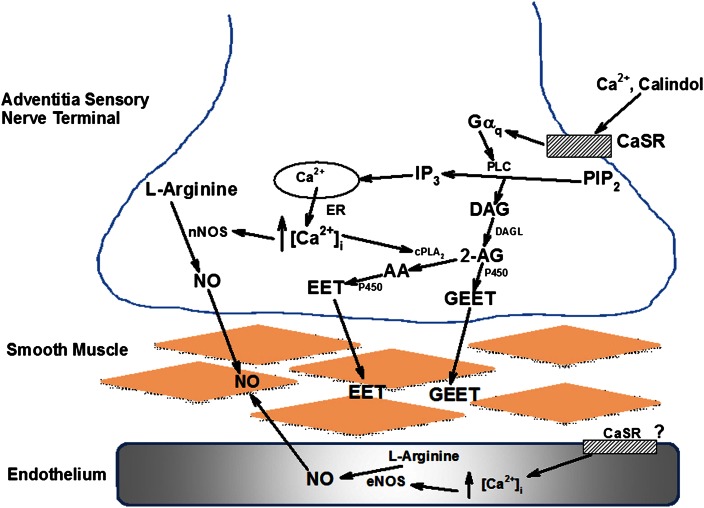
Proposed model for the PvN CaSR-mediated vasodilator release. Activation of the G-protein-coupled CaSR by agonist leads to hydrolysis of phosphatidylinositol 1,2-diphosphate (PIP2) by phospholipase C (PLC). to generate diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 releases Ca2+ from endoplasmic reticulum (ER) to activate Ca2+-sensitive nNOS to increase NO synthesis in the nerve terminal. Ca2+ release also activates cytosolic phospholipase A2 (cPLA2), which can metabolize the endocannabinoid 2-arachidonoylglycerol (2-AG) [from DAG following metabolism by diacylglycerol lipase (DAGL)] to arachidonic acid (AA). P450 can then metabolize 2-AG and AA to the vasodilators glycerated epoxyeicosatrienoic acid (GEET) and epoxyeicosatrienoic acid (EET), respectively. GEET and EET can then cross the vascular smooth muscle cell (VSMC) plasma membrane to activate BK channels and cause relaxation.
Discussion
Ca2+e-induced relaxation of isolated, PE-contracted rat mesenteric arteries is dependent on an intact sensory network (Bukoski et al., 1997; Mupanomunda et al., 1998; Wang and Bukoski, 1998, 1999; Mupanomunda et al., 1999; Bukoski, 2001). Endothelium denudation reduced relaxation, but a major component was due to endocannabinoids and their metabolites because cannabinoid receptor type 1 (CB1) antagonism blocked these responses (Awumey et al., 2008). However, endothelial factors, such as NO, may also play a role in this process. We carried out studies with isolated arteries from NOS−/− mice to determine the role of eNOS and nNOS in Ca2+e-induced relaxation of PE-constricted arteries.
CaSR Expression and Activation, NOS Knockout, and Pharmacological Inhibition of NOS on Ca2+-Induced Relaxation.
Our results show that eNOS−/− upregulates but nNOS−/− downregulates CaSR expression in mesenteric arteries. The expression of the receptor doublet indicates the presence of mature (complex carbohydrate) and immature (high mannose) glycosylated forms of the receptor (Bai et al., 1996, 1998; Wang et al., 2003). Generally, three different protein bands between 100 and 160 kDa, representing different monomeric forms of the receptor, are observed in Western blot analysis. In this study, the 130- to 140-kDa and 150- to 160-kDa proteins correspond to the immature and mature forms, respectively. The former is expressed in the endoplasmic reticulum (Huang and Miller, 2007) and the latter on the cell surface (Bai et al., 1998; Brown and MacLeod, 2001). Basal tensions in tissues from control and NOS−/− mice were similar, but PE tensions were reduced in tissues from eNOS−/− mice, probably as a result of high levels of endocannabinoid-derived vasodilators produced in the vessels via the CaSR signaling pathway in the presence of low NO release. Endothelial NO is important in the regulation of vascular tone (Pilette et al., 1996; Sogni et al., 1996); therefore, the reduced PE tension in eNOS−/− arteries may be attributable to stimulation of the CaSR-cannabinoid pathway to generate P450-derived vasodilators in addition to NO from residual eNOS activity. Hyporesponsiveness to vasoconstrictors in mesenteric resistance arteries is due to high NO production, which counteracts the effects of vasoconstrictive agents, and targeted disruption of the eNOS gene has been shown to produce higher myogenic tone (Scotland et al., 2001, 2005). Ca2+e-induced relaxation of isolated, PE-contracted rat mesenteric arteries is mediated by endocannabinoids (Awumey et al., 2008). Other studies have shown that CB1 receptor antagonism inhibits Ca2+e-induced relaxation in CB1 receptor–deficient mice, indicating that direct activation of this receptor is not involved (Bukoski et al., 2002). Thus, in tissues from eNOS−/− mice, the reduction in PE tension in the presence of reduced NO synthesis may be due to activation of the CaSR-endocannabinoid metabolic pathway and generation of vasodilators that act directly on smooth muscle cells to cause hyperpolarization and relaxation. The present data also confirm the CaSR as the mediator of the Ca2+e relaxation responses, as calindol, a CaSR agonist, enhanced while Calhex 231, a calcilytic at the receptor, reduced relaxation of PE-contracted arteries in a concentration-dependent manner. Both compounds bind to the allosteric site of the CaSR to increase or reduce Ca2+ sensitivity (Kessler et al., 2004; Ray et al., 2005; Weston et al., 2005; Ciceri et al., 2012).
A comparison of Ca2+ responses of arteries from nNOS−/− and eNOS−/− mice with that from C57BL/6 mice indicates that NOS knockout reduced Ca2+e-induced relaxation maxima, with eNOS gene deletion having a larger effect than nNOS gene deletion, but the EC50 for Ca2+ relaxation remained the same. NOS−/− had no effect on the potency of Ca2+ but reduced its efficacy, indicating a functional change in the CaSR-mediated response system and suggesting a contributory role for eNOS-derived NO. Furthermore, nonselective, acute inhibition of NOS reduced Ca2+ relaxation maxima in control and nNOS−/− mice but not in eNOS−/− mice, indicating that endothelium-derived NO modulates the response. The effects of the NOS inhibitors l-NIO, l-NAME, l-NMMA, and S-MeTC on Ca2+e-induced relaxation of PE-contracted arteries were similar. The data support our earlier findings that the overall Ca2+e-induced relaxation was largely due to nonendothelial factors (Awumey et al., 2008), but eNOS-derived NO may have a regulatory role. The inhibitory effects of l-NAME and l-NMMA in tissues from C57BL/6 and nNOS−/− mice are consistent with a primary action of Ca2+e on eNOS either as a result of activation of the CaSR or following influx through Ca2+ entry pathways to increase [Ca2+]i and NO synthesis. This conclusion is consistent with our earlier finding using rats in which endocannabinoids were reported to mediate a large component of the response to CaSR activation (Awumey et al., 2008). Endothelial and smooth muscle cells have also been reported to express the CaSR (Weston et al., 2005; Berra Romani et al., 2009); therefore, it is possible that activation of the receptor by Ca2+e stimulates both NO and endocannabinoid synthesis. Such a mechanism will be unique because it is manifested only after PE contraction of vessels. Thus, under conditions of increased tone, small changes in interstitial fluid Ca2+ concentration can activate the CaSR signaling pathway that leads to vasodilator synthesis and release from the nerve network (Mupanomunda et al., 1999; Palmer et al., 2003). Based on the current data and earlier findings (Awumey et al., 2008), it is reasonable to conclude that vasodilator release occurs in the nerve network of mesenteric arteries and acts to hyperpolarize the adjacent smooth muscle cells to cause relaxation. It therefore appears that NO from eNOS modulates the relaxation response but does not account for the majority of CaSR dilator activity.
Cellular Mechanisms Involved in NO Modulation of CaSR Expression and Signaling.
The current findings suggest that NO from eNOS exerts a negative-feedback effect on the CaSR signaling. Such a mechanism will be physiologically important in regulating vasodilation and blood pressure. This mechanism is consistent with the finding that 7-NI, a primary inhibitor of nNOS, has no inhibitory effect on Ca2+e-induced relaxation of PE-contracted arteries from nNOS−/− and eNOS−/− mice. 7-NI significantly reduced Ca2+e relaxation maxima in tissues from C57BL/6 mice and had no effect in tissues from nNOS−/− mice and a small effect in eNOS−/− mice. The major relaxing factors in the vasculature are endocannabinoids, NO, prostacyclin, and endothelium-derived hyperpolarizing factors such as epoxyeicosatrienoic acid (Feletou and Vanhoutte, 2006). eNOS, nNOS, and iNOS compensate for each other; therefore, one has to be cautious in interpreting the inhibition data. The effect of residual NO production can complicate the interpretation of the effects on Ca2+e-induced relaxation. However, iNOS is not involved, as we did not detect its expression in the mesentery. In nNOS−/− mice, the main source of NO is from the endothelium; therefore, the reduced relaxation maxima without a change in the EC50 indicates a negative effect of endothelial NO on Ca2+e-induced relaxation, suggesting eNOS activation. This is further supported by the data obtained with S-MeTC, which indicate a much larger effect of the drug on eNOS compared with nNOS. S-MeTC is considered to be an nNOS-selective inhibitor (Seddon et al., 2009).
In smooth muscle cells, the BK channel contributes to vascular tone by facilitating feedback regulation against an increase in [Ca2+]i, membrane depolarization, and vasoconstriction, thus promoting a K+ outward current leading to membrane hyperpolarization (Zhao et al., 2007). The current findings suggest that NO from eNOS may interact with PvN CaSR signaling that leads to BK channel activation to reduce hyperpolarization of smooth muscle cells and cause relaxation. The effects of IbTX on relaxation in tissues from eNOS−/− mice indicate that NO from nNOS had no effect on BK channel activity, allowing full manifestation of the effect of the inhibitor. Previous studies from our group showed that tetraethylammonium and IbTX (BK inhibitors) blocked Ca2+e relaxation of PE-contracted rat mesenteric arteries but apamin and charybdotoxin (small KCa inhibitors), glinbenclamide (KATP inhibitor), and 4-aminopyridine (voltage-dependent K+ channel inhibitor) had no effect (Ishioka and Bukoski, 1999). NO regulates the activity of various K+ channels, but the hyperpolarizing effects of NO on vascular smooth muscle cells substantially contribute to their relaxation (Vanhoutte et al., 2005; Toda and Okamura, 2011). The larger reduction in the Ca2+ response maxima in tissues from eNOS−/− mice was probably due to inefficiency in the CaSR signaling mechanism, as a larger level of receptor expression was observed. It appears that higher CaSR expression in eNOS−/− mice is necessary to compensate for the reduction in NO from the endothelium.
Our studies suggest that in the absence of eNOS, CaSR activation provides a mechanism for relaxation that reduces the increased tone associated with reduced NO production from eNOS. Thus, CaSR in the mesentery may provide a new pathway for vasodilator generation to protect vascular integrity. The findings are novel and the proposed mechanism (Fig. 9) may be important in endothelial dysfunction. Because CaSR activation leads to release of P450-derived metabolites of 2-arachidonoylglycerol that act at the BK channel (Awumey et al., 2008), the interaction of NO and the receptor may be at the BK channel in smooth muscle cells.
In conclusion, we have identified a novel CaSR signaling mechanism in mesenteric arteries that may serve a protective cardiovascular function and can be a target for the development of new vasodilator compounds.
Abbreviations
- BK
Ca2+-activated big potassium (channel)
- Ca2+e
extracellular calcium
- [Ca2+]e
extracellular Ca2+ concentration
- [Ca2+]i
intracellular Ca2+ concentration
- CaSR
Ca2+-sensing receptor
- CB1
cannabinoid receptor type 1
- eNOS
endothelial nitric-oxide synthase
- IbTX
iberiotoxin
- iNOS
inducible nitric oxide synthase
- l-NAME
NG-nitro-l-arginine methylester
- l-NMMA
NG-monomethyl-l-arginine
- 7-NI
7-nitroindazole
- l-NIO
l-N5-(1-iminoethyl)-ornithine
- nNOS
neuronal nitric-oxide synthase
- NO
nitric oxide
- NOS
nitric-oxide synthase
- P450
cytochrome P450
- PE
phenylephrine
- PSS
physiological salt solution
- PvN
perivascular nerve
- S-MeTC
S-methyl-l-thiocitrulline
Authorship Contributions
Participated in research design: Awumey, Diz.
Conducted experiments: Bridges, Williams.
Performed data analysis: Awumey, Bridges.
Wrote or contributed to the writing of the manuscript: Awumey, Diz.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL064761, HL059868, HL099139, and HL051952]; and National Institutes of Health National Institute on Minority Health and Health Disparities [Grant MD000175].
References
- Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, Vizard TN, Sage AP, Martin D, Ward DT, et al. (2009) Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res 81:260–268 [DOI] [PubMed] [Google Scholar]
- Angus JA, Wright CE. (2000) Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods 44:395–407 [DOI] [PubMed] [Google Scholar]
- Awumey EM, Hill SK, Diz DI, Bukoski RD. (2008) Cytochrome P-450 metabolites of 2-arachidonoylglycerol play a role in Ca2+-induced relaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 294:H2363–H2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awumey EM, Howlett AC, Putney JW, Jr, Diz DI, Bukoski RD. (2007) Ca(2+) mobilization through dorsal root ganglion Ca(2+)-sensing receptor stably expressed in HEK293 cells. Am J Physiol Cell Physiol 292:C1895–C1905 [DOI] [PubMed] [Google Scholar]
- Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM. (1996) Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem 271:19537–19545 [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Brown EM. (1998) Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem 273:23605–23610 [DOI] [PubMed] [Google Scholar]
- Berra Romani R, Raqeeb A, Laforenza U, Scaffino MF, Moccia F, Avelino-Cruz JE, Oldani A, Coltrini D, Milesi V, Taglietti V, et al. (2009) Cardiac microvascular endothelial cells express a functional Ca+ -sensing receptor. J Vasc Res 46:73–82 [DOI] [PubMed] [Google Scholar]
- Bridges LE, Williams CL, Pointer MA, Awumey EM. (2011) Mesenteric artery contraction and relaxation studies using automated wire myography. J Vis Exp (55):e3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–297 [DOI] [PubMed] [Google Scholar]
- Bukoski RD. (2001) Dietary Ca(2+) and blood pressure: evidence that Ca(2+)-sensing receptor activated, sensory nerve dilator activity couples changes in interstitial Ca(2+) with vascular tone. Nephrol Dial Transplant 16:218–221 [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Bátkai S, Járai Z, Wang Y, Offertaler L, Jackson WF, Kunos G. (2002) CB(1) receptor antagonist SR141716A inhibits Ca(2+)-induced relaxation in CB(1) receptor-deficient mice. Hypertension 39:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukoski RD, Bian K, Wang Y, Mupanomunda M. (1997) Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension 30:1431–1439 [DOI] [PubMed] [Google Scholar]
- Ciceri P, Volpi E, Brenna I, Elli F, Borghi E, Brancaccio D, Cozzolino M. (2012) The combination of lanthanum chloride and the calcimimetic calindol delays the progression of vascular smooth muscle cells calcification. Biochem Biophys Res Commun 418:770–773 [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. (2006) Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26:1215–1225 [DOI] [PubMed] [Google Scholar]
- Huang C, Miller RT. (2007) The calcium-sensing receptor and its interacting proteins. J Cell Mol Med 11:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka N, Bukoski RD. (1999) A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther 289:245–250 [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Ruat M, Dauban P, Dodd RH. (2004) N2-benzyl-N1-(1-(1-naphthyl)ethyl)-3-phenylpropane-1,2-diamines and conformationally restrained indole analogues: development of calindol as a new calcimimetic acting at the calcium sensing receptor. Bioorg Med Chem Lett 14:3345–3349 [DOI] [PubMed] [Google Scholar]
- McCarron RM, Chen Y, Tomori T, Strasser A, Mechoulam R, Shohami E, Spatz M. (2006) Endothelial-mediated regulation of cerebral microcirculation. J Physiol Pharmacol 57 (Suppl 11):133–144 [PubMed] [Google Scholar]
- Molostvov G, Fletcher S, Bland R, Zehnder D. (2008) Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell Physiol Biochem 22:413–422 [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Ishioka N, Bukoski RD. (1999) Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am J Physiol 276:H1035–H1042 [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Wang Y, Bukoski RD. (1998) Effect of chronic sensory denervation on Ca(2+)-induced relaxation of isolated mesenteric resistance arteries. Am J Physiol 274:H1655–H1661 [DOI] [PubMed] [Google Scholar]
- Palmer CE, Rudd MA, Bukoski RD. (2003) Renal interstitial Ca2+ during sodium loading of normotensive and Dahl-salt hypertensive rats. Am J Hypertens 16:771–776 [DOI] [PubMed] [Google Scholar]
- Pilette C, Moreau R, Sogni P, Kirstetter P, Cailmail S, Pussard E, Lebrec D. (1996) Haemodynamic and hormonal responses to long-term inhibition of nitric oxide synthesis in rats with portal hypertension. Eur J Pharmacol 312:63–68 [DOI] [PubMed] [Google Scholar]
- Ray K, Tisdale J, Dodd RH, Dauban P, Ruat M, Northup JK. (2005) Calindol, a positive allosteric modulator of the human Ca(2+) receptor, activates an extracellular ligand-binding domain-deleted rhodopsin-like seven-transmembrane structure in the absence of Ca(2+). J Biol Chem 280:37013–37020 [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. (2001) An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension 38:833–839 [DOI] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. (2005) Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111:796–803 [DOI] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. (2009) Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119:2656–2662 [DOI] [PubMed] [Google Scholar]
- Sogni P, Sabry S, Moreau R, Gadano A, Lebrec D, Dinh-Xuan AT. (1996) Hyporeactivity of mesenteric resistance arteries in portal hypertensive rats. J Hepatol 24:487–490 [DOI] [PubMed] [Google Scholar]
- Toda N, Okamura T. (2011) Modulation of renal blood flow and vascular tone by neuronal nitric oxide synthase-derived nitric oxide. J Vasc Res 48:1–10 [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M, Taddei S. (2005) Endothelium-dependent contractions in hypertension. Br J Pharmacol 144:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, Wu L. (2003) Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur J Biochem 270:2680–2688 [DOI] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD. (1998) Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br J Pharmacol 125:1397–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD. (1999) Use of acute phenolic denervation to show the neuronal dependence of Ca2+-induced relaxation of isolated arteries. Life Sci 64:887–894 [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G. (2005) Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res 97:391–398 [DOI] [PubMed] [Google Scholar]
- Wonneberger K, Scofield MA, Wangemann P. (2000) Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol 175:203–212 [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, et al. (2007) Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res 101:493–502 [DOI] [PubMed] [Google Scholar]