Abstract
In the title salt, [Hg(NCS)(CH4N2S)3]Cl, the Hg2+ ion is coordinated in a severely distorted tetrahedral manner by three thiourea groups and one thiocyanate anion through their S atoms. The S—Hg—S angles vary widely from 87.39 (5) to 128.02 (4)°. Weak intramolecular N—H⋯S hydrogen bonds are observed, which form S(6) ring motifs. In the crystal, the ions are linked by N—H⋯N and weak N—H⋯Cl interactions, generating a three-dimensional network.
Related literature
For background to mercury(II) complexes with thiourea and thiocyanate ligands, see: Nawaz et al. (2010 ▶). For hard and soft acids and bases, see: Ozutsmi et al. (1989 ▶); Bell et al. (2001 ▶). For related structures, see: Safari et al. (2009 ▶); Nawaz et al. (2010 ▶); Ramesh et al. (2012 ▶). For graph-set notation, see: Bernstein et al. (1995 ▶).
Experimental
Crystal data
[Hg(NCS)(CH4N2S)3]Cl
M r = 522.49
Orthorhombic,
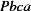
a = 8.2175 (3) Å
b = 16.3257 (8) Å
c = 22.6793 (10) Å
V = 3042.6 (2) Å3
Z = 8
Mo Kα radiation
μ = 10.83 mm−1
T = 293 K
0.30 × 0.25 × 0.20 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.140, T max = 0.221
38987 measured reflections
5125 independent reflections
3579 reflections with I > 2σ(I)
R int = 0.057
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.082
S = 1.05
5125 reflections
155 parameters
H-atom parameters constrained
Δρmax = 2.17 e Å−3
Δρmin = −1.21 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT and XPREP (Bruker, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: WinGX (Farrugia, 2012 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813011847/jj2165sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813011847/jj2165Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯Cl1i | 0.86 | 2.48 | 3.277 (4) | 155 |
| N1—H1B⋯S2 | 0.86 | 2.76 | 3.475 (5) | 142 |
| N2—H2A⋯Cl1i | 0.86 | 2.55 | 3.335 (4) | 152 |
| N3—H3B⋯Cl1 | 0.86 | 2.61 | 3.320 (5) | 141 |
| N4—H4B⋯Cl1ii | 0.86 | 2.51 | 3.370 (5) | 175 |
| N5—H5B⋯Cl1 | 0.86 | 2.54 | 3.363 (4) | 161 |
| N5—H5A⋯N7iii | 0.86 | 2.11 | 2.933 (7) | 160 |
Symmetry codes: (i) 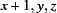 ; (ii)
; (ii) 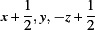 ; (iii)
; (iii) 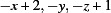 .
.
Acknowledgments
The authors thank Dr Babu Vargheese, SAIF, IIT, Madras, India, for his help in collecting the X-ray intensity data. KR thanks the University Grants Commission, Government of India, for financial support granted under a Major Research Project [F. No.41–1008/2012 (SR)].
supplementary crystallographic information
Comment
This work is part of a research project concerning the investigation of thiourea (N2H4CS) and thiocyanate (SCN) based metal organic crystalline materials and their derivatives (Ramesh et al., 2012). Transition metal thiourea and thiocyanate coordination complexes are candidate materials for device applications including their nonlinear optical properties. As ligands, both thiourea and thiocyanate are interesting due to their potential formation of metalcoordination complexes as they exhibit multifunctional coordination modes due to the presence of 'S' and 'N' donor atoms. With reference to the hard and soft acids and bases) concept (Ozutsmi et al., 1989; Bell et al., 2001), thesoft cations show a pronounced affinity for coordination with the softer ligands, while hard cations prefer coordination with harder ligands. Several crystallographic reports about mercury(II) complexes usually consist of discrete monomeric molecules with tetrahedral (somewhat distorted) coordination environments around mercury(II) (Nawaz et al., 2010). Here, we report the synthesis and structure of the title salt, [(SC(2NH2))3(SCN-)Hg(2+]+ . Cl-,(I).
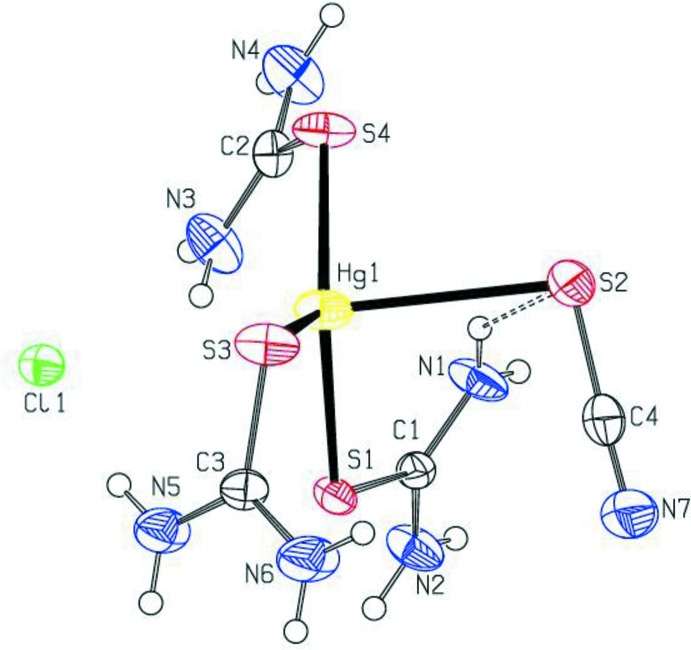
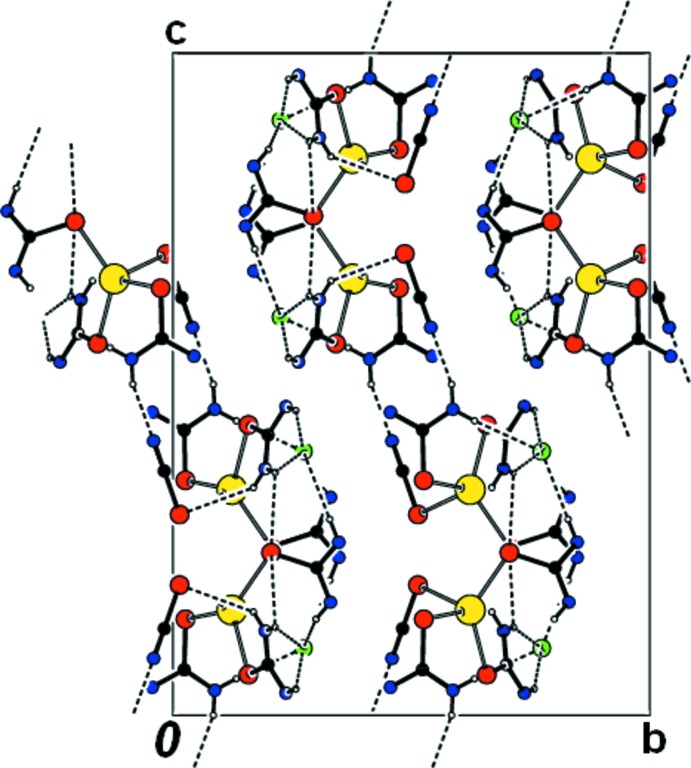
In (I), the Hg2+ ion is coordinated to three softer S atoms of thiourea and one softer S atom of a thiocyanate anion in addition to the isolated chlorine ion (Fig. 1). Intramolecular N—H···S hydrogen bonds are observed which form S(6) ring motifs (Bernstein et al., 1995). Bond distances and angles are in agreement with those reported for related compounds (Safari et al., 2009; Nawaz et al., 2010). The S—Hg—S angles vary widely from 87.39 (5)° to 128.02 (4)°, indicative of a distorted tetrahedral arrangement. The SCN- moiety is planar [to within 0.007 (1) Å] with the C—N and C—S bond lengths corresponding to the values intermediate between single and double bonds. The S2—C4—N7 unit is nearly linear with a bond angle of 177.9 (6)°. In the crystal, the ions are stabilized by weak N—H···Cl, and N—H···N intermolecular interactions (Table.1) which form a three-dimensional network (Fig. 2).
Experimental
A mixture of thiourea, ammonium thiocyanate and mercury (II) choloride were dissolved in aqueous solution in the molar ratio 3:1:1 and thoroughly mixed for an hour to obtain a homogenous mixture. The solution was allowed to evaporate slowly at ambient temperature. Colourless single crystals suitable for single-crystal XRD were obtained in 12 days.
Refinement
All H atoms were positioned geometrically with N—H = 0.86 Å and constrained to ride on their parent atoms with Uiso(H)=1.2Ueq.
Figures
Fig. 1.
View of the title compound showing the atom-numbering scheme. Displacement ellipsoids are drawn at the 40% probability level. H atoms are presented as a small spheres of arbitrary radius.
Fig. 2.
Packing diagram of (I) viewed along the a axis. Intramolecular N—H···S hydrogen bonds and weak N—H···Cl, and N—H···N intermolecular interactions are shown as dashed lines.
Crystal data
| [Hg(NCS)(CH4N2S)3]Cl | F(000) = 1968 |
| Mr = 522.49 | Dx = 2.281 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 5125 reflections |
| a = 8.2175 (3) Å | θ = 2.4–31.2° |
| b = 16.3257 (8) Å | µ = 10.83 mm−1 |
| c = 22.6793 (10) Å | T = 293 K |
| V = 3042.6 (2) Å3 | Block, colorless |
| Z = 8 | 0.30 × 0.25 × 0.20 mm |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 5125 independent reflections |
| Radiation source: fine-focus sealed tube | 3579 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.057 |
| ω and φ scans | θmax = 31.8°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −12→6 |
| Tmin = 0.140, Tmax = 0.221 | k = −23→24 |
| 38987 measured reflections | l = −32→33 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.082 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0291P)2 + 7.0058P] where P = (Fo2 + 2Fc2)/3 |
| 5125 reflections | (Δ/σ)max = 0.001 |
| 155 parameters | Δρmax = 2.17 e Å−3 |
| 0 restraints | Δρmin = −1.21 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 1.2410 (5) | 0.1993 (3) | 0.42350 (17) | 0.0280 (8) | |
| C2 | 1.0389 (5) | 0.3016 (3) | 0.2771 (2) | 0.0321 (9) | |
| C3 | 0.7067 (6) | 0.0234 (3) | 0.43089 (19) | 0.0333 (9) | |
| C4 | 1.2429 (7) | −0.0184 (3) | 0.3691 (2) | 0.0498 (13) | |
| N1 | 1.3113 (5) | 0.1934 (3) | 0.37245 (16) | 0.0488 (12) | |
| H1A | 1.4063 | 0.2142 | 0.3671 | 0.059* | |
| H1B | 1.2628 | 0.1687 | 0.3439 | 0.059* | |
| N2 | 1.3169 (5) | 0.2370 (3) | 0.46633 (17) | 0.0445 (10) | |
| H2A | 1.4119 | 0.2575 | 0.4604 | 0.053* | |
| H2B | 1.2718 | 0.2414 | 0.5004 | 0.053* | |
| N3 | 1.0072 (6) | 0.3302 (3) | 0.32922 (19) | 0.0511 (11) | |
| H3A | 1.0352 | 0.3794 | 0.3383 | 0.061* | |
| H3B | 0.9582 | 0.2999 | 0.3547 | 0.061* | |
| N4 | 1.1131 (6) | 0.3476 (3) | 0.2386 (2) | 0.0541 (12) | |
| H4A | 1.1409 | 0.3967 | 0.2477 | 0.065* | |
| H4B | 1.1342 | 0.3287 | 0.2040 | 0.065* | |
| N5 | 0.6681 (6) | 0.0872 (3) | 0.46201 (18) | 0.0496 (11) | |
| H5A | 0.6676 | 0.0844 | 0.4999 | 0.060* | |
| H5B | 0.6430 | 0.1324 | 0.4448 | 0.060* | |
| N6 | 0.7440 (8) | −0.0438 (3) | 0.4576 (2) | 0.0667 (15) | |
| H6A | 0.7430 | −0.0458 | 0.4955 | 0.080* | |
| H6B | 0.7698 | −0.0865 | 0.4375 | 0.080* | |
| N7 | 1.2853 (8) | −0.0407 (4) | 0.4142 (2) | 0.0779 (18) | |
| Hg1 | 0.92567 (2) | 0.123362 (12) | 0.340864 (7) | 0.03933 (7) | |
| S1 | 1.05319 (12) | 0.15817 (7) | 0.43857 (4) | 0.0313 (2) | |
| S2 | 1.18864 (19) | 0.01386 (9) | 0.30317 (6) | 0.0529 (4) | |
| S3 | 0.70350 (17) | 0.02592 (8) | 0.35487 (5) | 0.0430 (3) | |
| S4 | 0.98486 (18) | 0.20568 (7) | 0.25448 (5) | 0.0411 (3) | |
| Cl1 | 0.66976 (13) | 0.27345 (7) | 0.39858 (4) | 0.0323 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.025 (2) | 0.034 (2) | 0.0245 (18) | 0.0005 (16) | −0.0007 (15) | 0.0005 (16) |
| C2 | 0.029 (2) | 0.028 (2) | 0.040 (2) | −0.0008 (16) | 0.0019 (17) | −0.0016 (17) |
| C3 | 0.039 (3) | 0.029 (2) | 0.031 (2) | −0.0060 (18) | 0.0015 (18) | 0.0054 (17) |
| C4 | 0.060 (3) | 0.044 (3) | 0.046 (3) | 0.022 (3) | 0.006 (2) | −0.005 (2) |
| N1 | 0.032 (2) | 0.090 (3) | 0.0248 (18) | −0.021 (2) | 0.0068 (15) | −0.010 (2) |
| N2 | 0.036 (2) | 0.066 (3) | 0.0310 (19) | −0.019 (2) | 0.0034 (16) | −0.0137 (19) |
| N3 | 0.061 (3) | 0.037 (2) | 0.055 (3) | −0.013 (2) | 0.019 (2) | −0.0159 (19) |
| N4 | 0.076 (3) | 0.030 (2) | 0.057 (3) | −0.015 (2) | 0.024 (2) | −0.0046 (19) |
| N5 | 0.078 (3) | 0.034 (2) | 0.037 (2) | 0.007 (2) | 0.002 (2) | 0.0028 (18) |
| N6 | 0.123 (5) | 0.037 (3) | 0.040 (2) | 0.026 (3) | 0.002 (3) | 0.008 (2) |
| N7 | 0.110 (5) | 0.079 (4) | 0.044 (3) | 0.043 (4) | 0.005 (3) | 0.008 (3) |
| Hg1 | 0.04559 (12) | 0.04260 (11) | 0.02981 (9) | −0.01684 (8) | −0.00241 (7) | 0.00517 (7) |
| S1 | 0.0253 (5) | 0.0458 (6) | 0.0226 (4) | −0.0040 (4) | 0.0020 (4) | −0.0053 (4) |
| S2 | 0.0672 (10) | 0.0556 (8) | 0.0358 (6) | 0.0222 (7) | −0.0004 (6) | −0.0097 (6) |
| S3 | 0.0545 (8) | 0.0436 (7) | 0.0310 (5) | −0.0259 (6) | −0.0047 (5) | 0.0018 (5) |
| S4 | 0.0683 (8) | 0.0323 (6) | 0.0227 (5) | −0.0161 (6) | −0.0053 (5) | 0.0020 (4) |
| Cl1 | 0.0305 (5) | 0.0362 (5) | 0.0301 (5) | −0.0054 (4) | −0.0013 (4) | 0.0004 (4) |
Geometric parameters (Å, º)
| C1—N1 | 1.297 (5) | N2—H2B | 0.8600 |
| C1—N2 | 1.309 (5) | N3—H3A | 0.8600 |
| C1—S1 | 1.717 (4) | N3—H3B | 0.8600 |
| C2—N3 | 1.297 (6) | N4—H4A | 0.8600 |
| C2—N4 | 1.302 (6) | N4—H4B | 0.8600 |
| C2—S4 | 1.707 (4) | N5—H5A | 0.8600 |
| C3—N6 | 1.290 (6) | N5—H5B | 0.8600 |
| C3—N5 | 1.298 (6) | N6—H6A | 0.8600 |
| C3—S3 | 1.725 (4) | N6—H6B | 0.8600 |
| C4—N7 | 1.141 (7) | Hg1—S4 | 2.4250 (11) |
| C4—S2 | 1.647 (6) | Hg1—S3 | 2.4422 (12) |
| C4—S2 | 1.647 (6) | Hg1—S1 | 2.5162 (10) |
| N1—H1A | 0.8600 | Hg1—S2 | 2.9320 (14) |
| N1—H1B | 0.8600 | Hg1—S2 | 2.9320 (14) |
| N2—H2A | 0.8600 | ||
| N1—C1—N2 | 119.0 (4) | C2—N4—H4B | 120.0 |
| N1—C1—S1 | 123.3 (3) | H4A—N4—H4B | 120.0 |
| N2—C1—S1 | 117.7 (3) | C3—N5—H5A | 120.0 |
| N3—C2—N4 | 119.9 (4) | C3—N5—H5B | 120.0 |
| N3—C2—S4 | 123.4 (4) | H5A—N5—H5B | 120.0 |
| N4—C2—S4 | 116.7 (4) | C3—N6—H6A | 120.0 |
| N6—C3—N5 | 119.1 (4) | C3—N6—H6B | 120.0 |
| N6—C3—S3 | 119.6 (4) | H6A—N6—H6B | 120.0 |
| N5—C3—S3 | 121.4 (4) | S4—Hg1—S3 | 128.02 (4) |
| N7—C4—S2 | 177.9 (6) | S4—Hg1—S1 | 120.18 (4) |
| N7—C4—S2 | 177.9 (6) | S3—Hg1—S1 | 110.11 (4) |
| C1—N1—H1A | 120.0 | S4—Hg1—S2 | 87.39 (5) |
| C1—N1—H1B | 120.0 | S3—Hg1—S2 | 101.05 (5) |
| H1A—N1—H1B | 120.0 | S1—Hg1—S2 | 95.02 (4) |
| C1—N2—H2A | 120.0 | S4—Hg1—S2 | 87.39 (5) |
| C1—N2—H2B | 120.0 | S3—Hg1—S2 | 101.05 (5) |
| H2A—N2—H2B | 120.0 | S1—Hg1—S2 | 95.02 (4) |
| C2—N3—H3A | 120.0 | C1—S1—Hg1 | 106.69 (14) |
| C2—N3—H3B | 120.0 | C4—S2—Hg1 | 97.45 (18) |
| H3A—N3—H3B | 120.0 | C3—S3—Hg1 | 97.71 (15) |
| C2—N4—H4A | 120.0 | C2—S4—Hg1 | 108.55 (16) |
| N1—C1—S1—Hg1 | −14.6 (4) | S2—Hg1—S2—C4 | 0 (9) |
| N2—C1—S1—Hg1 | 166.6 (3) | N6—C3—S3—Hg1 | −115.5 (4) |
| S4—Hg1—S1—C1 | −32.03 (16) | N5—C3—S3—Hg1 | 66.4 (4) |
| S3—Hg1—S1—C1 | 161.59 (16) | S4—Hg1—S3—C3 | −160.34 (16) |
| S2—Hg1—S1—C1 | 57.87 (16) | S1—Hg1—S3—C3 | 4.69 (17) |
| S2—Hg1—S1—C1 | 57.87 (16) | S2—Hg1—S3—C3 | 104.27 (17) |
| S2—C4—S2—Hg1 | 0 (100) | S2—Hg1—S3—C3 | 104.27 (17) |
| S4—Hg1—S2—S2 | 0.00 (8) | N3—C2—S4—Hg1 | −17.9 (5) |
| S3—Hg1—S2—S2 | 0.00 (8) | N4—C2—S4—Hg1 | 163.4 (4) |
| S1—Hg1—S2—S2 | 0.00 (8) | S3—Hg1—S4—C2 | 138.25 (16) |
| S4—Hg1—S2—C4 | 150.5 (2) | S1—Hg1—S4—C2 | −25.45 (17) |
| S3—Hg1—S2—C4 | −81.2 (2) | S2—Hg1—S4—C2 | −119.74 (17) |
| S1—Hg1—S2—C4 | 30.5 (2) | S2—Hg1—S4—C2 | −119.74 (17) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···Cl1i | 0.86 | 2.48 | 3.277 (4) | 155 |
| N1—H1B···S2 | 0.86 | 2.76 | 3.475 (5) | 142 |
| N2—H2A···Cl1i | 0.86 | 2.55 | 3.335 (4) | 152 |
| N3—H3B···Cl1 | 0.86 | 2.61 | 3.320 (5) | 141 |
| N4—H4B···Cl1ii | 0.86 | 2.51 | 3.370 (5) | 175 |
| N5—H5B···Cl1 | 0.86 | 2.54 | 3.363 (4) | 161 |
| N5—H5A···N7iii | 0.86 | 2.11 | 2.933 (7) | 160 |
Symmetry codes: (i) x+1, y, z; (ii) x+1/2, y, −z+1/2; (iii) −x+2, −y, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JJ2165).
References
- Bell, N. A., Branston, T. N., Clegg, W., Parker, L., Raper, E. S., Sammon, C. & Constable, C. P. (2001). Inorg. Chim. Acta, 319, 130–136.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2004). APEX2, SAINT and XPREP Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Nawaz, S., Sadaf, H., Fettouhi, M., Fazal, A. & Ahmad, S. (2010). Acta Cryst. E66, m952. [DOI] [PMC free article] [PubMed]
- Ozutsmi, K., Takamuku, T., Ishiguro, S. & Ohraki, H. (1989). Bull. Chem. Soc. Jpn, 62, 1875–1879.
- Ramesh, V., Rajarajan, K., Kumar, K. S., Subashini, A. & NizamMohideen, M. (2012). Acta Cryst. E68, m335–m336. [DOI] [PMC free article] [PubMed]
- Safari, N., Amani, V., Abedi, A., Notash, B. & Ng, S. W. (2009). Acta Cryst. E65, m372. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813011847/jj2165sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813011847/jj2165Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report