Summary
Background
Many women of childbearing potential take antiepileptic drugs, but the cognitive effects of fetal exposure are uncertain. We aimed to assess effects of commonly used antiepileptic drugs on cognitive outcomes in children up to 6 years of age.
Methods
In this prospective, observational, assessor-masked, multicentre study, we enrolled pregnant women with epilepsy on antiepileptic drug monotherapy (carbamazepine, lamotrigine, phenytoin, or valproate) between October, 1999, and February, 2004, at 25 epilepsy centres in the UK and the USA. Our primary outcome was intelligence quotient (IQ) at 6 years of age (age-6 IQ) in all children, assessed with linear regression adjusted for maternal IQ, antiepileptic drug type, standardised dose, gestational birth age, and use of periconceptional folate. We also assessed multiple cognitive domains and compared findings with outcomes at younger ages. This study is registered with ClinicalTrials.gov, number NCT00021866.
Findings
We included 305 mothers and 311 children (six twin pairs) in the primary analysis. 224 children completed 6 years of follow-up (6-year-completer sample). Multivariate analysis of all children showed that age-6 IQ was lower after exposure to valproate (mean 97, 95% CI 94–101) than to carbamazepine (105, 102–108; p=0·0015), lamotrigine (108, 105–110; p=0·0003), or phenytoin (108, 104–112; p=0·0006). Children exposed to valproate did poorly on measures of verbal and memory abilities compared with those exposed to the other antiepileptic drugs and on non-verbal and executive functions compared with lamotrigine (but not carbamazepine or phenytoin). High doses of valproate were negatively associated with IQ (r=−0·56, p<0·0001), verbal ability (r=−0·40, p=0·0045), non-verbal ability (r=−0·42, p=0·0028), memory (r=−0·30, p=0·0434), and executive function (r=−0·42, p=0·0004), but other antiepileptic drugs were not. Age-6 IQ correlated with IQs at younger ages, and IQ improved with age for infants exposed to any antiepileptic drug. Compared with a normative sample (173 [93%] of 187 children), right-handedness was less frequent in children in our study overall (185 [86%] of 215; p=0·0404) and in the lamotrigine (59 [83%] of 71; p=0·0287) and valproate (38 [79%] of 40; p=0·0089) groups. Verbal abilities were worse than non-verbal abilities in children in our study overall and in the lamotrigine and valproate groups. Mean IQs were higher in children exposed to periconceptional folate (108, 95% CI 106–111) than they were in unexposed children (101, 98–104; p=0·0009).
Interpretation
Fetal valproate exposure has dose-dependent associations with reduced cognitive abilities across a range of domains at 6 years of age. Reduced right-handedness and verbal (vs non-verbal) abilities might be attributable to changes in cerebral lateralisation induced by exposure to antiepileptic drugs. The positive association of periconceptional folate with IQ is consistent with other recent studies.
Funding
US National Institutes of Health, UK Epilepsy Research Foundation.
Introduction
Antiepileptic drugs are among the most common teratogens prescribed to women of childbearing potential.1 Knowledge of antiepileptic drug teratogenicity has increased in the past decade, including a concern that valproate is associated with impaired cognitive outcomes.2,3 Based largely on our previously published analysis3 of outcomes at 3 years of age, the US Food and Drug Administration issued a warning that fetal valproate exposure is associated with impaired cognitive outcomes.4 In this study, our primary aim was to determine how fetal exposure to different antiepileptic drugs affect intelligence quotient (IQ) at 6 years of age (age-6 IQ). Compared with measurements done at younger ages, age-6 IQ is a more stable measure, more strongly related to adult IQ, and more predictive of school performance.5 Thus, understanding whether differences at 3 years of age persist to 6 years is important. In addition, we present a more comprehensive assessment of other cognitive domains than can be assessed at younger ages, and test the hypothesis that antiepileptic drugs can change cerebral lateralisation.
Methods
Study design and participants
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was a prospective observational investigation with masked cognitive assessment. We enrolled pregnant women with epilepsy who were receiving antiepileptic drug monotherapy (ie, carbamazepine, lamotrigine, phenytoin, or valproate) between October, 1999, and February, 2004, from 25 epilepsy centres in the UK and the USA. No other antiepileptic drugs were used in adequate numbers to include in the assessments. We excluded women treated with polytherapy because of its association with worse outcomes compared with monotherapy2 A non-exposed control group was not included because the US National Institutes of Health review panel unanimously recommended its deletion from our original design.
Each site’s institutional review boards approved the study. Written informed consent was obtained according to the Declaration of Helsinki. A data safety monitoring board appointed by the US National Institutes of Health monitored study conduct. We excluded women with IQ scores of less than 70 to avoid floor effects because maternal IQ is the major predictor of child IQ.5 Other exclusions were positive syphilis or HIV serology, progressive cerebral disease, other major disease (eg, diabetes), exposure to known teratogenic drugs other than antiepileptic drugs, poor compliance to antiepileptic drugs, drug misuse in the previous year, or sequelae of drug misuse.
Procedures
We assessed potentially confounding variables (eg, maternal IQ, age, education, employment, ethnic group, types and frequency of seizures or epilepsy, antiepileptic drug dosages, compliance, medical history, socioeconomic status [appendix p 15], site [UK vs USA], periconception and pregnancy folate, concomitant medications, alcohol use, tobacco use, or other drug use during pregnancy, unwanted pregnancy, abnormalities or complications in present or previous pregnancies, enrolment and birth gestational ages, birthweight, breastfeeding, and childhood medical diseases). Apart from maternal IQ, we obtained information from the patients, their seizure or medication diary, or review of their medical records. We assessed compliance by interview, review of diaries, and assessment of clinically available antiepileptic drug levels. If antiepileptic drug levels were subtherapeutic or lower than expected by pharmacokinetic changes during pregnancy, mothers were asked again about compliance. If appropriate, doses were increased and blood levels rechecked. For almost all mothers in our study, compliance was excellent and blood levels of antiepileptic drugs remained therapeutic. Assessors, masked to treatment, evaluated cognitive outcomes with differential ability scales (DAS)6 at 3 years, 4·5 years, and 6 years of age, and Bayley scales of infant development (BSID)7 at 2 years of age. Testing at 6 years of age also included the children’s memory scale (CMS),8 the behavior rating inventory of executive function (BRIEF),9 the developmental neuropsychological assessment (NEPSY),10 the expressive one-word picture vocabulary test,11 and the developmental test of visual motor integration (DTVMI).12 See appendix p 15 for determination of handedness. Neuropsychological examiners were trained and monitored to ensure quality. We undertook annual workshops; assessors identified errors and corrections for videotaped test sessions containing administration or scoring errors. The Neuropsychology Core Director reviewed and approved videotapes of assessor practice test sessions and record forms. If assessors failed, they underwent additional video assessments.
Statistical analysis
We calculated the sample size on the basis of an 80% power to detect a 0·5 SD difference in IQ, which is a clinically meaningful difference noted in previous studies.2 For the primary analysis, we examined group differences in IQ in the total-enrolled population (ie, all births). For secondary analyses, we assessed differences in IQ in children completing age-6 testing (age-6-completer sample), differences across outcomes at 2 years, 3 years, 4. 5 years, and 6 years, correlations of age-6 IQ to dose and maternal IQ by antiepileptic drug, and analyses of handedness, verbal or non-verbal abilities, memory, and executive functions. Analyses were done at the NEAD Data and Statistical Centre by NB with SAS and R statistical software.
For the primary analysis, we used linear regression models to examine group differences in IQ, adjusting for maternal IQ, antiepileptic drug type, standardised dose, gestational birth age, and use of periconceptional folate. Additional covariates were maternal age, ethnic group, maternal education, epilepsy or seizure types, seizure frequency during pregnancy, employment status, socioeconomic status, site, alcohol or tobacco use, concomitant drug use, birthweight, unwanted pregnancy, breastfeeding, birth defects and complications in present and past pregnancies, and antiepileptic drug compliance (appendix pp 3–4).
Because specific antiepileptic drug type and dose and maternal IQ are important covariates, we included these variables as predictors in a linear model with child IQ as the outcome. Other covariates were added individually to the model and included if significant (p<0·05) and not colinear with existing predictors. We inspected diagnostic plots to ensure that distributional assumptions of the models were met. For the total-enrolled analysis, we used Markov chain Monte Carlo methods13,14 to impute missing outcomes at 6 years of age from available outcomes at 2 years, 3 years, and 4·5 years of age and baseline variables related to outcome or likelihood of missing data. The imputation procedure generated 50 imputed datasets and fit regression models to each. We combined regression parameter estimates across imputations with standard errors that incorporated imputation uncertainty. The following variables were used in the imputation model: age 2 year, 3 year, and 4·5 year outcomes (BSID and DAS), maternal IQ, antiepileptic drug dose, folate, gestational age, unwanted pregnancy, maternal age, convulsions during pregnancy, employment status, US versus UK site, maternal education, and socioeconomic status. Mothers of children with missing outcomes at 6 years of age differed in terms of maternal age and site (the imputation model included these variables). Standard errors and CIs of parameter estimates incorporated imputation uncertainty. We did secondary analyses for age-6-completers without imputation for missing data.
To test the assumption that the proportion of children with lowest cognitive performance (ie, IQ <70 and <85) was unchanged across time, we used Cochran’s Q statistic with the sample of children who were tested at all four ages (2 years, 3 years, 4·5 years, and 6 years). We used Fisher’s exact test to assess differences between antiepileptic drugs in percentages of children with IQ scores of less than 70 and less than 85.
We did follow-up analyses to assess the importance of subgroups. We created forest plots to explore whether baseline differences explained the association of valproate with poorer cognitive outcomes. We defined subgroups by seizure type and propensity scores. Propensity scores are predicted probabilities of receiving a treatment in view of baseline covariates and are used to assess the effect of baseline group differences (appendix p 12 contains additional details on propensity score analyses).
This study is registered with ClinicalTrials.gov, number NCT00021866.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We included 305 mothers and 311 livebirths (six twin pairs) in the primary analysis. Table 1 shows baseline maternal characteristics for the total-enrolled sample and appendix p 5 shows maternal characteristics for the age-6-completer sample. We interviewed all mothers about compliance, reviewed all diaries, and assessed antiepileptic drug levels for 229 (75%) mothers. We noted significant associations between antiepileptic drug group and maternal IQ, maternal education, dose, epilepsy type, site, and ethnic group.
Table 1.
Characteristics of women enrolled in the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study
| Carbamazepine | Lamotrigine | Phenytoin | Valproate | Total* | p value† | |
|---|---|---|---|---|---|---|
| Maternal antiepileptic drug use | 93 (30%) | 99 (32%) | 52 (17%) | 61 (20%) | 305* | NA |
| Mean maternal IQ‡ | 99 (95–102) | 101 (98–104) | 93 (88–97) | 96 (92–100) | 98 (96–100) | 0·0138 |
| Mean maternal age | 30 (29–31) | 30 (29–30) | 30 (29–32) | 28 (27–30) | 30 (29–30) | 0·1096 |
| Mean dose, mg per day§ | 784 (697–870) | 457 (406–507) | 400 (364–435) | 1032–(877–1188) | NA | NA |
| Standardised dose¶ | 32 (29–36) | 35 (31–39) | 49 (44–54) | 26 (22–31) | 35 (32–37) | <0·0001 |
| Gestational age at enrolment, weeks∥ | 18 (17–20) | 18 (16–19) | 19 (16–21) | 17 (15–19) | 18 (17–19) | 0·7978 |
| Gestational age at birth, weeks | 39 (38–39) | 39 (39–40) | 39 (38–39) | 39 (39–40) | 39 (39–39) | 0·0703 |
| Years of maternal education | 14 (14–14) | 15 (14–15) | 13 (12–14) | 13 (13–14) | 14 (14–14) | 0·0001 |
| Enrolled at UK site | 37 (40%) | 29 (29%) | 7 (13%) | 32 (52%) | 105 (34%) | <0·0001 |
| Periconceptional folate | 55 (59%) | 59 (60%) | 21 (40%) | 39 (64%) | 174 (57%) | 0·0608 |
| Alcohol use** | 8 (9%) | 8 (8%) | 3 (6%) | 5 (8%) | 24 (8%) | 0·9632 |
| Epilepsy type†† | ||||||
| Localisation-related | 81 (87%) | 51 (52%) | 40 (77%) | 20 (20%) | 184 (60%) | <0·0001 |
| Idiopathic generalised | 7 (8%) | 39 (39%) | 8 (15%) | 43 (70%) | 97 (32%) | <0·0001 |
| GTCS | 5 (5%) | 9 (9%) | 4 (8%) | 6 (10%) | 24 (8%) | 0·7136 |
| Convulsions‡‡ | ||||||
| None | 71 (87%) | 68 (79%) | 40 (82%) | 46 (77%) | 225 (81%) | 0·3481 |
| >5 | 3 (3%) | 1 (1%) | 3 (6%) | 1 (2%) | 8 (3%) | 0·3483 |
| Ethnic origin | ||||||
| White | 75 (81%) | 87 (88%) | 30 (58%) | 53 (87%) | 245 (80%) | <0·0001 |
| Black | 7 (8%) | 1 (1%) | 5 (10%) | 1 (2%) | 14 (5%) | 0·0223 |
| Hispanic | 6 (6%) | 6 (6%) | 16 (31%) | 3 (5%) | 31 (10%) | <0·0001 |
| Other | 5 (5%) | 5 (5%) | 1 (2%) | 4 (7%) | 15 (5%) | 0·7355 |
Data are n (%) or n (95% CI). IQ=intelligence quotient. GTCS=generalise tonic-clonic seizures (unknown if generalised or secondary generalised).
AII mothers enrolled (311 children); data were available for seizure frequency in 277 mothers (91%), breastfeeding in 245 mothers (80%), antiepileptic drug compliance in 239 mothers (78%), and IQ in 304 mothers; data were available for infant birthweight in 308 children (99%); data for remaining covariates were complete; outcomes at age 6 years were available in 225 children (72%); cognitive outcomes were available for at least one test age (ie, 2 years, 3 years, 4·5 years, or 6 years) in 279 children (90% of total-enrolled sample).
We used an F-test from an ANOVA model for continuous variables and Fisher’s exact test for categorical variables.
See appendix for description of maternal IQ testing.
Average dose for whole pregnancy; median daily doses for the whole pregnancy were carbamazepine 700 mg, lamotrigine 433 mg, phenytoin 398 mg, and valproate 1000 mg; mean daily doses (ranges) were carbamazepine 784 mg (33–2350), lamotrigine 457 mg (50–1217), phenytoin 400 mg (67–750), and valproate 1032 mg (133–3583); notably, the dose range for phenytoin was smaller, which could limit sensitivity of analyses for dose effects.
Therapeutic doses (mg per day) vary between antiepileptic drugs, so doses were standardised to allow comparisons between drugs; ranges within each antiepileptic drug and total-enrolled group were used in the calculation 100 × (observed dose – minimum dose) ÷ range of doses (ie, maximum – minimum).3
Mean gestational age at enrolment was 18 weeks (range 1–38), which did not differ between antiepileptic drugs.
Any alcohol use during pregnancy (yes vs no).
Epilepsy types were localisation-related (includes cryptogenic and symptomatic), idiopathic generalised (includes absence, juvenile myoclonic, genetic, and other idiopathic generalised not otherwise classified), and GTCS.
Number (%) of mothers without convulsions or >5 convulsions during pregnancy; seizure frequency during pregnancy was not reported for 14 mothers.
Cognitive outcomes were available from at least one test age (ie, BSID at 2 years or DAS IQ at 3 years, 4·5 years, or 6 years) for 279 children (90% of total-enrolled sample). Age-6 IQ correlated with measures at each of the younger ages (appendix p 6). We included 221 mothers (72%) and 225 children (72%; four twin pairs) in the secondary age-6-completer analysis; however, an IQ score was missing for one mother so 224 children were included in the regression analysis. Mean testing at 6 years of age was at 74 months (range 70–87) and did not differ between antiepileptic drug type (p=0·99). Scores were standardised for age.
The appendix (p 3) shows the statistical details of total-enrolled and age-6-completer analyses, with additional analyses for paternal and child factors in the appendix table footnote. Independent predictors of child IQ in the total-enrolled sample were antiepileptic drug type (p<0·0001), maternal IQ (p<0·0001), use of periconceptional folate (p=0·0009), standardised dose (p=0·0060), and birth gestational age (p=0·0413). When added to the model, no other variables substantively changed inferences about antiepileptic drug group differences.
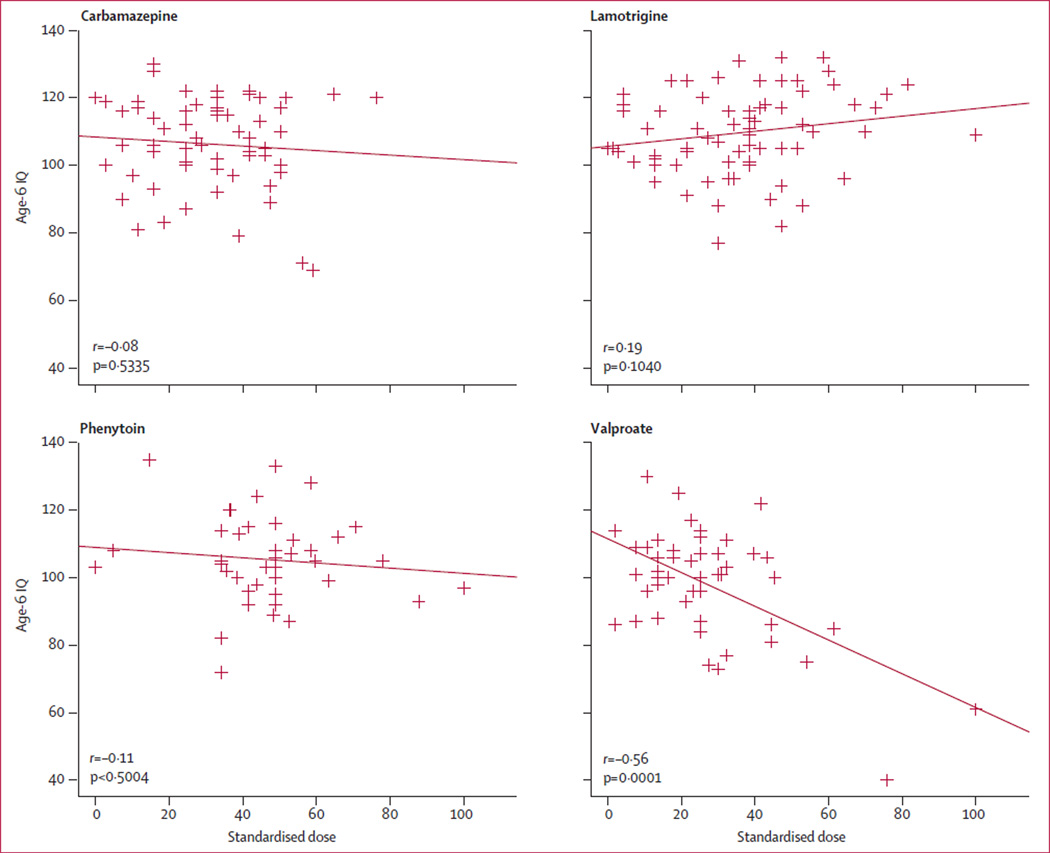
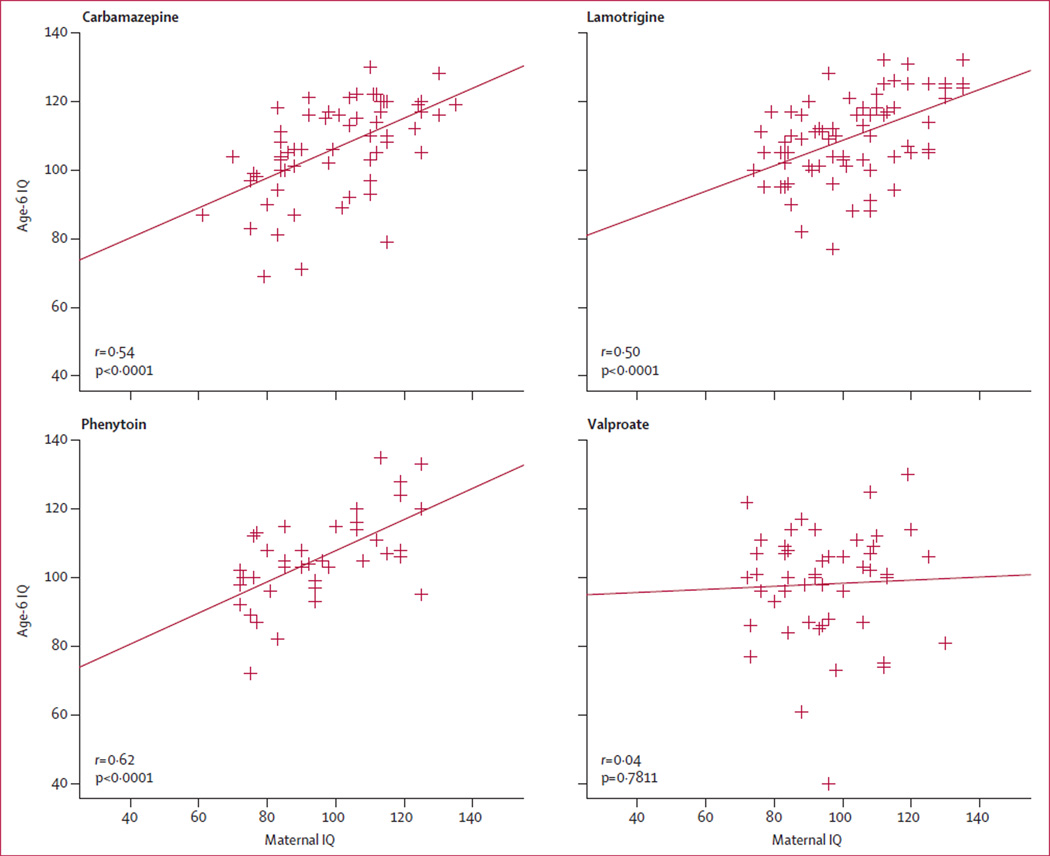
Table 2 lists adjusted mean age-6 IQ scores by antiepileptic drug group for total-enrolled and age-6-completer analyses. IQ scores were significantly lower for valproate than they were for the three other antiepileptic drugs. Dose-dependent effects were present for valproate but not for other antiepileptic drugs (figure 1). Linear regression results and correlations were similar with first or third trimester dosages. Child and maternal IQ scores were significantly correlated for all antiepileptic drugs apart from valproate (figure 2).
Table 2.
Differences from valproate in mean IQ scores in all children in the study (n=311) and in children at 6 years of age (n=224)
| Carbamazepine | Lamotrigine | Phenytoin | Valproate | |||
|---|---|---|---|---|---|---|
| Total-enrolled | ||||||
| Participants | 94 (30%) | 100 (32%) | 55 (18%) | 62 (20%) | ||
| Mean IQ* | 105 (102–108) | 108 (105–110) | 108 (104–112) | 97 (94–101) | ||
| Difference | 7 (3–12) | 10 (6–15) | 10 (5–16) | NA | ||
| p value† | 0·0015 | 0·0003 | 0·0006 | NA | ||
| Age-6-completers | ||||||
| Participants | 61 (27%) | 74 (33%) | 40 (18%) | 49 (22%) | ||
| Mean IQ* | 106 (103–109) | 108 (105–111) | 109 (105–113) | 98 (95–102) | ||
| Difference | 8 (3–13) | 10 (6–15) | 11 (5–16) | NA | ||
| p value† | 0·0010 | 0·0003 | 0·0004 | NA | ||
Data are n (%) or n (95% CI), unless otherwise stated. IQ=intelligence quotient. NA=not applicable.
Mean IQ scores at age 6 years were adjusted for maternal IQ, dose, periconceptional folate, and gestational age at delivery; total-en rolled analysis includes imputed IQ data; unadjusted means for the total-en rolled analysis were carbamazepine 105, lamotrigine 109, phenytoin 103, and valproate 98, and unadjusted means for age-6-completers were carbamazepine 106, lamotrigine 110, phenytoin 105, and valproate 98.
p values were adjusted for three pairwise comparisons to valproate with Hochberg’s correction.
Figure 1. Relation between age-6 IQ and standardised dose of every antiepileptic drug during pregnancy.
Therapeutic dosages (mg per day) vary between antiepileptic drugs, so doses were standardised to allow comparisons; ranges within each antiepileptic drug and total-enrolled group were used in following calculation: 100 × (observed dose-mini mum dose) ÷ range of doses (ie, maximum-mini mum).3 Note that this calculation is based on the total-enrolled sample, but scatter plots here depict only the subjects tested at 6 years of age. Correlations are parametric Pearson correlations; non-parametric rank correlations were much the same. Age-6 IQ=IQ score at 6 years of age. IQ=intelligence quotient.
Figure 2. Relation between age-6 IQ and maternal IQ for every antiepileptic drug during pregnancy.
Correlations are parametric Pearson correlations; non-parametric rank correlations were much the same. Age-6 IQ=IQ scores at 6 years of age. IQ=intelligence quotient.
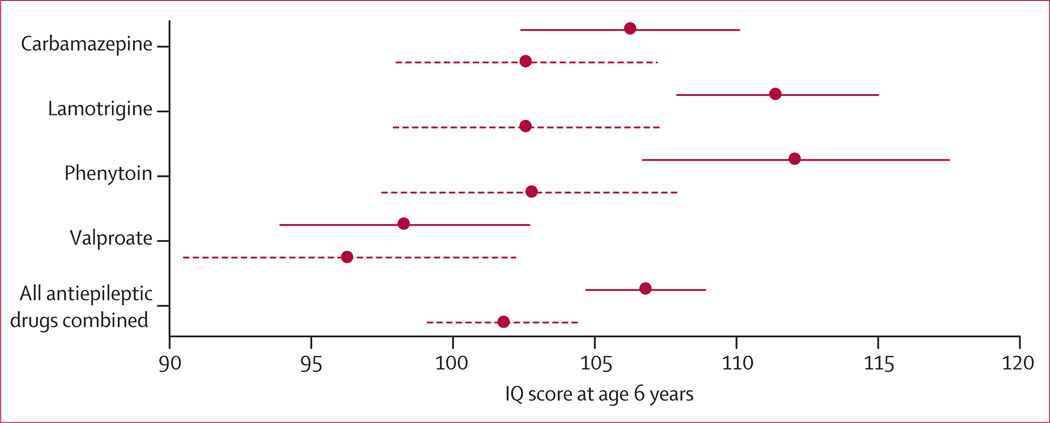
Higher child IQ scores were associated with higher maternal IQ (r=0·44; p<0·0001), older gestational age (r=0·14; p=0·0319), and periconceptional folate (p=0·0002 [t test]). Mean IQ for children whose mothers used periconceptional folate was 108 (95% CI 106–111) compared with 101 (98–104) for children of mothers who did not use periconceptional folate (figure 3); dose-dependent effects were present (appendix p 7).
Figure 3. Child IQ at 6 years, by exposure to maternal antiepileptic drug use and periconceptional folate.
Mean (95% CIs) are shown for folate (solid lines) and no folate (dashed lines). For carbamazepine, 56 children were exposed to periconceptional folate (mean IQ 106, 95% CI 102–110) and 38 children were not (103, 98–107); for lamotrigine 60 children were exposed to periconceptional folate (111, 108–115) and 40 children were not (103, 98–107); for phenytoin, 23 children were exposed to periconceptional folate (112, 107–118) and 32 children were not (103, 98–108); for valproate, 40 children were exposed to periconceptional folate (98, 94–103) and 22 children were not (96, 91–102); for all antiepileptic drugs combined, 179 children were exposed to periconceptional folate (107, 105–109) and 132 children were not (102, 99–105). Appendix p 7 shows means and analyses for dose effects. IQ=intelligence quotient.
Appendix pp 8–9 shows results for mixed-model analysis with repeated measures with child IQ at 2 years, 3 years, 4·5 years, and 6 years as dependent variables. Maternal IQ, age at testing, antiepileptic drug, dose, periconceptional folate, gestational age, and maternal age were significant. Appendix p 6 shows adjusted mean cognitive scores (BSID and DAS IQ) and percentages of children who were cognitively impaired (IQ<70 and IQ<80) for all antiepileptic drugs across ages. Cognitive scores improved as children aged (appendix p 13), and percentages of cognitively impaired children decreased (appendix p 6). This improvement did not differ by antiepileptic drug type. IQ was worse for valproate at ages 3 years, 4·5 years, and 6 years, but the BSID score did not differ statistically for the smaller sample at age 2 years.
Table 3 and appendix p 10 show verbal and non-verbal index score results at age 6 years. Children in the valproate group had significantly lower verbal functioning than did those in the other three antiepileptic drug groups, and did significantly worse on the non-verbal index than did those in the lamotrigine group.
Table 3.
Adjusted mean verbal and non-verbal index scores at age 6 years
| Verbal index*† |
Non-verbal index*‡ |
Non-verbal minus verbal* |
||||
|---|---|---|---|---|---|---|
| Score (95% CI) | p value (vs valproate) |
Score (95% CI) | p value (vs valproate) |
Difference (95% CI) | p value | |
| Carbamazepine | 104 (102–107) | 0·0005 | 104 (102–107) | 0·08 | 0·74 (−2·01 to 3·48) | 0·60 |
| Lamotrigine | 105 (102–107) | 0·0003 | 108 (105–110) | 0·0015 | 2·82 (0·31 to 5·33) | 0·0280 |
| Phenytoin | 106 (102–109) | 0·0005 | 106 (103–109) | 0·0514 | −0·89 (−4·48 to 2·70) | 0·63 |
| Valproate | 97 (94–100) | NA | 101 (98–104) | NA | 4·37 (1·24 to 7·49) | 0·0063 |
| All antiepileptic drugs combined | 103 (101–105) | NA | 105 (104–106) | NA | 1·93 (0·35 to 3·51) | 0·0168 |
IQ=intelligence quotient. NA=not applicable.
Means were adjusted for maternal IQ, maternal age, gestational age, and ethnic group.
Verbal index was created by averaging standard scores (mean 100 [SD 15]) from word definition and similarities subtests of the differential abilities scales,6 the expressive one-word picture vocabulary test,11 and phonological processing, comprehension of instructions and sentence repetition subtests of the developmental neuropsychological assessment.10
Non-verbal index was created by averaging the standard scores from the pattern construction, matrices, and recall of designs subtests of the differential abilities scales,6 the arrows subtest from the developmental neuropsychological assessment,10 and the developmental test of visual motor integration.12
Table 4 and appendix p 11 show the results for children aged 6 years of the general memory index of the children’s memory scale, the NEPSY executive index, and the BRIEF parent index. Valproate general memory index scores were significantly lower than those in the other three antiepileptic drugs, and executive index scores were worse for valproate than lamotrigine.
Table 4.
Adjusted means for the general memory index, the executive index from neuropsychological assessment, and the parent index from the BRIEF at 6 years of age
| General memory index* | Executive index† | BRIEF‡ | ||||
|---|---|---|---|---|---|---|
| Score (95% CI) |
p value (vs valproate) |
Score (95% CI) |
p value (vs valproate) |
Score (95% CI) |
p value (vs valproate) |
|
| Carbamazepine | 104 (100–108) |
0·0010 | 105 (103–108) |
0·0546 | 101 (98–104) |
0·11 |
| Lamotrigine | 106 (102–110) |
0·0003 | 107 (104–109) |
0·0078 | 100 (97–103) |
0·11 |
| Phenytoin | 101 (96–107) |
0·0260 | 103 (100–106) |
0·28 | 100 (95–104) |
0·11 |
| Valproate | 92 (87–98) |
NA | 101 (98–104) |
NA | 105 (101–108) |
NA |
Statistical comparisons with valproate were adjusted with Hochberg’s method for multiple comparisons. IQ=intelligence quotient. BRIEF=behavioral rating inventory of executive function. NA=not applicable.
The general memory index standard score from the children’s memory scale8 shows general memory functioning and is generated by combining the immediate and delayed verbal and visual indexes; means were adjusted for maternal IQ, dose, maternal age, and alcohol use.
The executive index was created by averaging the standard scores from the tower, verbal fluency, and visual attention subtests from the developmental neuropsychological assessment;10 means were adjusted for maternal IQ, gestational age, and maternal age.
The parent index from the BRIEF9 measures global executive functions as rated by the child’s parent; thus, it incorporates all eight clinical scales (notably, by contrast with the other measures, a higher score on the BRIEF shows worse function); means are adjusted for dose.
Only valproate dose correlated with worse performance for the verbal index (r=−0·40, p=0·0045) and non-verbal index (r=−0·42, p=0·0028), general memory index (r=−0·30, p=0·0434), NEPSY executive index (r=−0·42, p=0·0004), and BRIEF parent index (r=0·35, p=0·0212 [higher score is worse for BRIEF]). No other antiepileptic drug had dose-dependent effects. Table 5 shows the means (CIs) and analyses for median dose split. High-dose valproate differed from all other groups.
Table 5.
IQ outcomes at age 6 years by median group dose for the age-6-completer sample (n=224)
| N | Mean age-6 IQ (95% CI) |
p value (vs below-median dose valproate) |
p value (vs above-median dose valproate) |
|
|---|---|---|---|---|
|
Carbamazepine (median dose 700 mg per day) | ||||
| Below group median | 28 | 107 (102–112) | 0·3994 | 0·0002 |
| Above group median | 33 | 106 (102–110) | 0·5990 | 0·0004 |
|
Lamotrigine
(median dose 433 mg per day) | ||||
| Below group median | 31 | 106 (102–111) | 0·4854 | 0·0003 |
| Above group median | 43 | 109 (105–113) | 0·1154 | <0·0001 |
|
Phenytoin (median dose 398 mg per day) | ||||
| Below group median | 20 | 108 (103–114) | 0·2551 | 0·0002 |
| Above group median | 20 | 106 (101–112) | 0·5501 | 0·0011 |
|
Valproate (median dose 1000 mg per day) | ||||
| Below group median | 23 | 104 (99–109) | NA | 0·0065 |
| Above group median | 26 | 94 (90–99) | 0·0065 | NA |
Means were adjusted for maternal IQ, gestational age at birth, and folate. IQ=intelligence quotient.
Verbal and non-verbal indices were equal in a normative sample, but verbal abilities were significantly lower than non-verbal abilities for lamotrigine (p=0·0280) and valproate (p=0·0063). In the normative sample of children from the Wechsler intelligence scale for children IV,15 173 (93%) of 187 children were right-handed, which is greater than was noted for children in this study (185 [86%] of 215 were right-handed; p=0·0404) with a logistic regression analysis. This difference was due to differences in the lamotrigine group (59 [83%] of 71 were right-handed; p=0·0287) and valproate group (38 [79%] of 48 were right-handed; p=0·0089). Right-handed frequency was lower for valproate than carbamazepine (54 [93%] of 58 were right-handed; p=0·0284), but not statistically lower than for phenytoin (34 [89%] of 38; p=0·0641). All antiepileptic drugs had higher mean doses for non-right versus right-handers, and a significant dose effect was present for all antiepileptic drugs combined (p=0·0329).
Our forest plot of propensity score subgroups (appendix p 12) suggests that results were not attributable to differences in baseline variables related to child IQ or chances of valproate treatment. In each propensity score subgroup, mean IQ for valproate was lower than mean IQs for other antiepileptic drugs. The forest plot displaying means by seizure type and antiepileptic drug group suggested that antiepileptic drug group differences cannot be explained by seizure type imbalances (appendix p 14). Patterns in these plots imply that baseline differences do not explain valproate’s association with poor IQ outcome. The total-enrolled versus age-6-completer analyses examining sensitivity of results to missing data suggest that the results cannot be explained by incomplete data.
Discussion
Similar to our findings in children aged 3 years and 4·5 years,3,16 children with fetal exposure to valproate had reduced IQ (7–10 points) at 6 years compared with other commonly used antiepileptic drugs (ie, carbamazepine, lamotrigine, and phenytoin). Valproate exposure was also associated with worse verbal and memory abilities compared with the other antiepileptic drugs, and worsened non-verbal and executive functions compared with lamotrigine. Teratogens act in a dose-dependent manner and according to genetic susceptibility. An increased valproate dose was associated with reduced IQ, verbal, non-verbal, memory, and executive function, but other antiepileptic drugs had no dose effects. Thus, fetal exposure to valproate is associated with a range of cognitive deficits.
Outcomes in children exposed to low-dose valproate (<1000 mg per day) did not differ from those in children exposed to the other low or high dose antiepileptic drugs; however, sample sizes might have restricted delineation of differences. For example, low-dose valproate (<700 mg per day) had an increased risk for major malformation compared with low-dose lamotrigine (<300 mg per day).17 Studies in animals suggest that apoptotic effects of valproate on the immature brain begin below typical therapeutic doses.18 Thus, a safe dose of valproate is unknown.
One limitation of this study was the number of participants lost to follow-up. However, cognitive testing was available for at least one age in 279 (90%) of 311 children. Because age-6 IQ is correlated strongly to earlier cognitive outcomes, these data help compensate for children lost to follow-up. The relation of earlier outcomes to age-6 IQ might allow for early detection and intervention.
IQ scores improved with age. Practice effects might have contributed to this improvement, and improvements might also be related to intervention programmes. Families were provided results at each test age and assisted in referral to intervention programmes if indicated. Another limitation was that data for intervention programme enrolment were not collected systematically. The effects of practice and intervention programmes might have reduced the apparent effects of fetal exposure to antiepileptic drugs. The absence of a healthy control group was a further limitation. However, the 7–10 IQ point reduction for valproate compared with other antiepileptic drugs is clinically significant even if the mean valproate IQ was in the normal range.
Verbal and non-verbal indexes were reported as standard scores, so they should be equivalent in the absence of selective effects. However, verbal scores were lower than non-verbal scores for lamotrigine and valproate. Practice effects might differentially affect verbal versus non-verbal scores, but this explanation seems unlikely in view of the small practice effects for DAS verbal and non-verbal cluster scores.6 Furthermore, the difference was present on the first exposure to DAS at 3 years of age, and the difference decreased from 3–6 years of age with repeated testing. This finding is also consistent with other studies that have reported verbal impairments associated with fetal valproate exposure19–21 and impaired language in children with fetal antiepileptic drug exposure.22
Typical functional brain asymmetries include left cerebral dominance for language and handedness. The development of these asymmetries can be changed by environmental factors during early neural development.23 Atypical lateralisation is increased in various developmental disorders including fetal alcohol syndrome.23 The relatively lower verbal versus non-verbal performances and the reduced frequency of right-handedness in the lamotrigine and valproate groups suggest that fetal exposure to some antiepileptic drugs might affect normal cerebral lateralisation. However, our study was limited because we did not assess family handedness or directly measure brain lateralisation.
Other factors related to child IQ in our study include higher maternal IQ and older gestational age. However, maternal IQ was not related to child IQ for the valproate group, although it was for each of the other antiepileptic drug groups, suggesting valproate exposure disrupts this otherwise strong association.
Maternal periconceptional folate was associated with higher age-6 IQ. This finding should be interpreted with caution because periconceptional folate was one of several confounding variables, and was established by retrospective maternal interview. The US Public Health Service and Center for Disease Control and Prevention recommend that all women of childbearing age consume 0·4 mg folate daily to prevent birth defects (ie, spina bifida).24 Folate is important for normal fetal development, and severe deficiency or inborn errors of folate metabolism can result in mental retardation.25 Folate deficiency can lead to fetal neuronal apoptosis and reduced neuronal progenitor cells.26 A study of maternal folate status later in pregnancy and one older periconceptional folate study found no relation with cognitive outcome, but seven recent studies have reported positive associations of periconceptional folate with neurodevelopmental outcomes (appendix pp 15–16).27,28 Thus, our finding adds to the evidence that periconceptional folate might have beneficial effects on cognitive development.
Strengths of this study are our prospective design, use of masked cognitive assessments with standardised measures, and detailed monitoring of multiple potential confounding factors. Limitations are its relatively small non-population-based sample, loss to follow-up, non-randomisation, inadequate pharmacokinetics, and no unexposed controls. Detection of dose effects for non-valproate antiepileptic drugs might require larger sample sizes as noted for malformations associated with carbamazepine, lamotrigine, and phenobarbital.17 Antiepileptic drug clearance is variable during pregnancy;29 future studies should measure antiepileptic drug levels to better assess intrauterine exposure. Randomised pregnancy trials of antiepileptic drugs are not possible, and observational studies might be confounded by differences in baseline characteristics (eg, maternal IQ, dose, ethnic group, or epilepsy type). Our analyses suggest that these baseline group differences do not explain our findings, but some residual confounding effects are possible. Thus, observational studies require replication.
Most women with epilepsy cannot avoid use of antiepileptic drugs during pregnancy because of the risks from seizures to both mother and child. Based on anatomical and cognitive risks,2,3 we propose that valproate is a poor first-choice antiepileptic drug for most women of childbearing potential. However, a few women with generalised epilepsy can only be controlled by valproate.
Many gaps exist in our knowledge about epilepsy care during preconception and pregnancy.2 The mechanisms underlying observed deficits are uncertain, but antiepileptic drugs might affect fetal neurodevelopment via neuronal apoptosis induced by antiepileptic drugs, reduced neurotrophin expression, decreased neuronal survival-promoting proteins, reduced neurogenesis, or impaired physiology in remaining neurons.18 Many antiepileptic drugs are untested in animal models and have not been assessed in clinical cohorts. Additional research is needed to confirm our findings and to improve our knowledge for care in this population. In conclusion, we noted that fetal valproate exposure has dose-dependent associations with reduced cognitive abilities across a range of domains at 6 years of age. Women requiring an antiepileptic drug and their clinicians should be aware of these findings in choosing their treatment (panel).
Supplementary Material
Panel: Research in context.
Systematic review
We examined the American Academy of Neurology evidence-based review,2 a systematic review from 2012,30 and pertinent articles listed in these sources. We also searched PubMed to identify articles related to effects of periconceptional folate on cognition and antiepileptic drug effects on cognition and cerebral lateralisation. Additional studies are listed in the appendix.
Interpretation
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study is the largest prospective investigation of cognitive outcomes after fetal exposure for carbamazepine, lamotrigine, phenytoin, and valproate monotherapies. Neuropsychological assessments in almost all previous studies were restricted to intelligence quotient (IQ) or a developmental measure. Most did not control for maternal IQ, which is the major predictor of child IQ,5 and many did not prospectively collect other important potential confounding factors. The NEAD study provides the most detailed neuropsychological assessment, prospectively collecting a large array of factors, including maternal IQ. To our knowledge, NEAD is the first study to show that fetal valproate exposure has dose-dependent associations with reduced cognitive abilities across many domains, show longitudinal changes in intelligence across ages 2–6 years, and show reduced right-handedness. The verbal and non-verbal asymmetries match the findings of atypical handedness providing support for our hypothesis that fetal exposure to some antiepileptic drugs might alter cerebral lateralisation. The positive association of periconceptional folate with IQ is the first reported in children of mothers with epilepsy and is consistent with other recent studies, but requires replication.
Acknowledgments
Conflicts of interest
KJM has received research support from the GlaxoSmithKline, Eisai Medical Research, Myriad Pharmaceuticals, Marinus Pharmaceuticals, NeuroPace, Pfizer, SAM Technology, Schwartz Biosciences, and UCB Pharma, the Epilepsy Foundation, and the US National Institutes of Health (NIH); received salary support to Emory University from the Epilepsy Consortium for research consultant work related for NeuroPace, Novartis, Upsher-Smith, and Vivus; served as a consultant for Eisai, Glaxo SmithKline, Johnson and Johnson (Ortho McNeil), Medtronics Spherics, and UCB Pharma, but the monies went to a charity of the company’s choice; received travel support from Sanofi-Aventis; and also serves on the Professional Advisory Board for the Epilepsy Foundation and the editorial boards for Cognitive and Behavioral Neurology, Epilepsy and Behavior, Neurology, and Journal of Clinical Neurophysiology. GAB has served on paid advisory boards, receiving lecture fees from Pfizer, UCB, and Janssen, receiving grant support from Sanofi-Aventis, Pfizer, UCB and the NIH, and has served as an expert witness in litigation related to neurodevelopment effects of antiepileptic drugs. NB has received research support from the NIH. MJC has received research support from the NIH and is author of the children’s memory scale. RLB has received honorarium from Sanofi-Aventis and an educational grant from UCB Pharma, and has served as an expert witness in litigation related to neurodevelopment effects of antiepileptic drugs. JC-S is journal editor for Clinical Dysmorphology and has served as an expert witness in litigation related to diagnosis of fetal anticonvulsant syndrome. LAK has received lecture fees from Glaxo SmithKline. AK has received grant support from Pfizer JDL has received lecture fees from UCB Pharma, and also is a member of the Professional Board of Epilepsy Foundation of Eastern PA. PBP has received grant support from the NIH, Milken Family Foundation, and the Epilepsy Therapy Project; and serves on the Professional Advisory Board for the Epilepsy Foundation and the Board of Directors for the American Epilepsy Society. MP has received research support from UCB and Eisai, and receives reimbursement for services on data safety monitoring boards for clinical trials for Lilly and Upsher Smith. DWL has received consulting fees from NeuroPace and grant support from UCB and Pfizer.
This work was supported by the US National Institutes of Health [NS038455 to KJM and NS050659 to NB] and the UK Epilepsy Research Foundation [RB219738 to GAB]. We thank the children and families who gave their time to participate in the Neurodevelopmental Effects of Antiepileptic Drugs Study
Footnotes
Members listed in appendix
Contributors
KJM drafted the manuscript and supervised the study. NB did the statistical analyses and wrote the analysis and results section. KJM, NB, MJC, and DWL contributed to study design and interpreted the data. KJM and GAB obtained funding. All authors were involved in acquisition of data and reviewed and revised the manuscript.
References
- 1.Schwarz EB, Maselli J, Norton M, Gonzales R. Prescription of teratogenic medications in United States ambulatory practices. Am J Med. 2005;118:1240–1249. doi: 10.1016/j.amjmed.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis, perinatal outcomes Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US FDA. [accessed June 23, 2012];FDA drug safety communication: children born to mothers who took Valproate products while pregnant may have impaired cognitive development. http://www.fda.gov/Drugs/DrugSafety/ucm261543.htm.
- 5.Sattler JM. Assessment of children: cognitive foundations. 5th edn. San Diego, CA: Jerome M Sattler; 2008. [Google Scholar]
- 6.Elliott CD. Differential abilities scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 7.Bayley N. Bayley scales of infant development. 2nd edn. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 8.Cohen MJ. The children’s memory scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 9.Gioa GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 10.Korkman M, Kirk U, Kemp S. NEPSY: a developmental neuropsychological assessment. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 11.Brownell R. Expressive one-word picture vocabulary test. Novato, CA: Academic Therapy Publications; 2000. [Google Scholar]
- 12.Beery KE. Developmental test of visual motor integration. Cleveland, OH: Modern Curriculum; 1997. [Google Scholar]
- 13.Li KH. Imputation using Markov chains. J Stat Comput Simul. 1988;30:57–79. [Google Scholar]
- 14.Little RJA, Rubin DB. tStatistical analysis with missing data. 2nd edn. New York: John Wiley and Sons; 2002. [Google Scholar]
- 15.Wechsler D. The Wechsler intelligence scale for children—. 4th edn. London: Pearson Assessment; 2004. [Google Scholar]
- 16.Meador KJ, Baker GA, Browning N, et al. Effects of fetal antiepileptic drug exposure: outcomes at age 4·5 years. Neurology. 2012;78:1207–1214. doi: 10.1212/WNL.0b013e318250d824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 18.Meador KJ. Cognitive effects of epilepsy and of antiepileptic medications. In: Wyllie E, Cascino GD, Gidal BE, Goodkin HP, editors. The treatment of epilepsy: principles and practice. 5th edn. Philadelphia: Lippincott Williams and Wilkins; 2011. pp. 1028–1036. [Google Scholar]
- 19.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 21.Nadebaum C, Anderson VA, Vajda F, Reutens DC, Barton S, Wood AG. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology. 2011;76:719–726. doi: 10.1212/WNL.0b013e31820d62c7. [DOI] [PubMed] [Google Scholar]
- 22.Thomas SV, Sukumaran S, Lukose N, George A, Sarma PS. Intellectual and language functions in children of mothers with epilepsy. Epilepsia. 2007;48:2234–2240. doi: 10.1111/j.1528-1167.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- 23.Domellof E, Ro L, Titran M, Esseily R, Fagard J. Atypical functional lateralization in children with fetal alcohol syndrome. Dev Psychohiol. 2009;51:696–705. doi: 10.1002/dev.20404. [DOI] [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention. [accessed June 23, 2012];Folic Acid: Recommendations. http://www.cdc.gov/ncbddd/folicacid/recommendations.html.
- 25.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- 26.Craciunescu CN, Brown EC, Mar M-H, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 27.Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzi L, Papadopoulou E, Koutra K, et al. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutr. 2012;8:1–9. doi: 10.1017/S1368980012000067. [DOI] [PubMed] [Google Scholar]
- 29.Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol. 2008;83:227–240. doi: 10.1016/S0074-7742(08)00013-5. [DOI] [PubMed] [Google Scholar]
- 30.Nadebaum C, Anderson V, Vajda F, Reutens D, Wood A. Neuro behavioral consequences of prenatal antiepileptic drug exposure. Dev Neuropsychol. 2012;37:1–29. doi: 10.1080/87565641.2011.589483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.