Abstract
RATIONALE
Episodic social defeat stress results in cross-sensitization to cocaine, characterized by augmentation of locomotor activation, dopamine (DA) levels in the nucleus accumbens (NAc), and cocaine self-administration during a 24-hour “binge” in male rats. However, females are more vulnerable than males at each phase of cocaine addiction, and while these sex differences have been replicated in rats, the role of social stress in females remains largely neglected.
OBJECTIVE
This study examined sex and estrous cycle differences in behavioral and dopaminergic cross-sensitization to cocaine, as well as cocaine taking in an unlimited access self-administration “binge.”
METHODS
Long-Evans rats underwent episodic social defeat and were assessed ten days later for either (1)behavioral sensitization, as determined by locomotor activity in response to acute cocaine (10 mg/kg, ip), (2)neural sensitization, as determined by in vivo microdialysis of DA in the NAc shell in response to acute cocaine, or (3)intravenous self-administration of cocaine (0.3 mg/kg/infusion) in an unlimited access “binge.”
RESULTS
Social defeat stress resulted in behavioral and dopaminergic cross-sensitization in both sexes, but the effect was larger and longer lasting in stressed females. Furthermore, while stress engendered a longer “binge” in both sexes, females had a significantly longer “binge” duration than males.
CONCLUSIONS
These data suggest that socially stressed females exhibit a larger and longer lasting behavioral and neural cross-sensitization, as well as more dysregulated cocaine taking, than males possibly due to different alterations in the dopaminergic response in the NAc. Furthermore, estrogens appear to play a facilitatory role in both behavioral and dopaminergic sensitization.
Keywords: social stress, sex differences, cocaine, dopamine, behavioral sensitization, neural sensitization, self-administration, microdialysis
INTRODUCTION
Stress is a major factor in vulnerability to drug addiction (Sinha 2001; 2008), with both clinical and preclinical work demonstrating that stress plays a key role in initiation, escalation, and relapse to drug abuse (Shaham et al. 2000; Sinha 2009; Sinha and Li 2007).
In rodents, episodic social defeat stress has proven to be a particularly powerful animal model to induce an increased cocaine response and instigate escalated cocaine-seeking behavior, resulting in long lasting physiological, behavioral, and neural changes (Covington et al. 2005). Episodic social defeat induces behavioral cross-sensitization to psychomotor stimulants in the form of augmented locomotor activity in male rats (Covington and Miczek 2001; Miczek et al. 1999; Miczek et al. 2011; Nikulina et al. 2004), as well as induces a neural cross-sensitization in the form of augmented extracellular dopamine (DA) in the nucleus accumbens (NAc), both at baseline and in response to acute d-amphetamine (Miczek et al. 1999) and cocaine (Miczek et al. 2011). Furthermore, episodic social defeat augments the reinforcement of cocaine as demonstrated by self-administration studies, accelerating acquisition (Tidey and Miczek 1997), increasing the breakpoint in a progressive ratio schedule of reinforcement (Covington et al. 2005; Covington and Miczek 2005) and significantly increasing the amount of cocaine taken during, as well as the duration of cocaine responding in, a 24 hour unlimited access “binge” protocol (Boyson et al. 2011; Covington et al. 2005; Covington and Miczek 2001; 2005; Miczek et al. 2011).
In addition to stress, gender plays a particularly influential role in risk to drug abuse. In 2010, 35.9% of cocaine users in the United States were female (SAMHSA, 2010), and women show a heightened vulnerability to cocaine addiction in every phase of the addiction cycle. For example, in comparison to men, women initiate cocaine use at a younger age (Chen and Kandel 2002), escalate from first use to dependence at a much faster rate (McCance-Katz et al. 1999), are more likely to engage in “binge”-like patterns of cocaine use (O'Brien and Anthony 2005), use cocaine more often (Chen and Kandel 2002) and are more prone to relapse (Ignjatova and Raleva 2009).
In parallel to clinical data, female rodents appear to be more sensitive to the reinforcing effects of cocaine than males, exhibiting a larger and longer lasting behavioral sensitization after repeated cocaine administration (van Haaren and Meyer 1991), and this effect is potentiated with estradiol replacement (Carroll et al. 2007; Hu and Becker 2003). Furthermore, ovariectomized females outperform males in every phase of cocaine self-administration, with faster acquisition (Lynch 2008; Lynch and Carroll 1999), higher breakpoints in progressive ratio schedules (Cummings et al. 2011; Roberts et al. 1989), and greater cocaine intake during extended access (Lynch and Taylor 2004; Roth and Carroll 2004), and all of these effects are potentiated with estradiol treatment. Neuronal sex differences, particularly in the mesocorticolimbic dopaminergic system, are thought to mediate these observed differences in behavior (see (Becker and Hu 2008) for review).
Interestingly, clinical data indicate there are gender differences in the effects of stress on drug dependence, with stress playing a larger role in vulnerability to drug abuse in women than in men (Fox and Sinha 2009). In comparison to men, women report greater feelings of cocaine craving and negative emotion following stressful stimuli (Back et al. 2005; Fox et al. 2008) and are more likely to relapse during periods of high psychosocial stress (McKay et al. 1996).
Sex differences in the role of social stress in cocaine cross-sensitization and self-administration in rodents have remained largely understudied. Haney et al. (1995) showed that females subjected to social defeat stress self-administer significantly more cocaine than similarly stressed males during the late phases of acquisition, but the role of circulating ovarian hormones was not assessed, nor were locomotor or dopaminergic responses to acute cocaine examined. The present study sought to systematically examine sex and estrous cycle differences in behavioral and dopaminergic cross-sensitization to cocaine, as well as cocaine taking behavior in an unlimited access cocaine self-administration “binge”, predicting a greater effect in intact females, with those in estrus or proestrus, when circulating ovarian hormones are higher, showing a larger effect than those in met/diestrus.
METHODS
General Methods
Subjects
Male and female Long-Evans rats (Charles River, Wilmington, MA) weighing 200–225g upon arrival were individually housed in custom-built clear acrylic chambers (30×30.5×24.5cm) with cellulose pellet bedding (CelluDri™, Shepherd Specialty Papers, Kalamazoo, MI) and adapted to the facilities for at least one week. Rats were then randomly assigned to stressed or non-stressed control groups. Males were housed in proximity to females to help ensure regular estrous cycles. Separate “resident” rats were housed in male-female pairs in large stainless steel cages (71×46×46 cm) as described previously (Miczek 1979). Rats were housed on a reverse 12-hour light cycle (lights on at 20:00) and provided food and water ad libitum. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee, following the guidelines of the Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Experimental Design
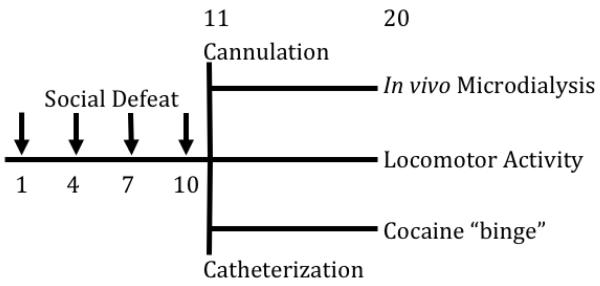
Animals were exposed to either episodic social defeat stress or handling for ten days, and ten days after the last defeat were evaluated for either (Experiment I) behavioral sensitization, assessed by locomotor activity in response to acute cocaine, (Experiment II) neural sensitization, assessed by in vivo microdialysis of DA in the NAc shell in response to acute cocaine, or (Experiment III) cocaine taking, assessed by an unlimited cocaine self-administration “binge” (see Figure 1).
Figure 1. Experimental Design.
Social defeats (indicated by arrows) occurred on days 1, 4, 7, and 10. One cohort of rats was implanted with intracerebral cannulae on day 11, and underwent in vivo microdialysis to determine neural sensitization on day 20. A separate cohort was assessed for behavioral sensitization by locomotor activity in response to acute cocaine on day 20. A final cohort was catheterized on day 11 and allowed to self-administer cocaine in an unlimited “binge” on day 20.
Estrous Cycle Examinations
Vaginal smears were taken daily and examined using the Giemsa staining method (Staples and Geils 1965).
Social Defeat Stress
A modification of a previously described resident-intruder paradigm (Tornatzky and Miczek 1993) was used. Briefly, rats were subjected to four defeats, each separated by 72 hours. Experimental rats were defeated by a same sex resident, either a larger male screened for reliable aggressive behavior or a lactating dam. First, the opposite sex resident rat was removed, and the experimental rat placed in a small protective cage (30×20×20 cm) inside the larger resident cage for 10 minutes. This allowed for olfactory and visual instigation, but prevented tactile contact. Next, the protective cage was removed and the experimental animal placed in the resident cage until defeated (defined as 10 bites and/or 6s held in supine position by resident) or until 5 minutes had elapsed following the first bite. After the defeat, experimental rats were again placed in the protective cage inside the resident cage for 10 minutes, then finally returned to their home cage.
Experiment I: Behavioral Sensitization
Ten days after the last defeat both stressed (n=16 male; n=12 female) and non-stressed (n=16 male, n=13 female) rats were given a challenge injection of a marginally effective dose of cocaine (10 mg/kg, ip) to evaluate locomotor sensitization (Covington and Miczek 2001). Under the conditions in our laboratory, using Long Evans rats and testing in the home cage, this dose does not alter walking behavior of unstressed, handling adapted rats, while increasing walking duration and frequency in stressed rats, indicative of cross-sensitization. We therefore choose to use this marginally effective dose in order to show a reliable cross-sensitization to cocaine due to stress. Rats were injected with saline (ip) once per day for three days prior to testing to ensure habituation to handling and injection. On the day of testing, rats were moved to an adjacent experimental room and given an injection of saline. Five minutes following the injection, behavior was recorded for a period of five minutes. Then, rats were injected with cocaine, and behavior recorded for a period of 5 minutes both 5 and 25 minutes later. For females, vaginal smears were taken immediately after recording to determine estrous cycle phase. Video recordings were analyzed by a reliable observer for walk duration, rearing, grooming and sniffing using a customized keyboard and computer software (The Observer Video-Pro© version 8.0, Noldus Information Technology, Wageningen, The Netherlands).
Experiment II: In vivo Microdialysis
A separate cohort of stressed (n=7 male; n=7 female with correct placement and intact sampling) and non-stressed (n=5 male; n=5 female with correct placement and intact sampling) rats was assessed for DA levels in the NAc shell in response to acute cocaine (10 mg/kg, ip) 10 days after the last defeat. Stereotaxic surgery, sample collection, and monoamine analysis have been described recently (Shimamoto et al. 2011). Briefly, rats were anesthetized with ketamine (100 mg/kg) and xylazine (6 mg/kg for males, 3 mg/kg for females) and implanted with a unilateral guide cannula (BASi, West Lafayette, IN) aimed at the NAc shell using the coordinates according to stereotaxic atlas (Paxinos and Watson 1997): AP, +1.2 mm from bregma; ML, +0.9mm (females) or +1.1mm (males) from midline; DV, −5.8mm from dura.
Rats were injected with saline (ip) once per day for three days prior to testing to ensure habituation to handling and injection. The day before sample collection, rats were anesthetized with isoflurane and the stylet in the cannula replaced with a 2-mm active membrane probe (BASi, West Lafayette, IN) connected to a syringe filled with artificial cerebrospinal fluid (CMA Microdialysis Inc., North Chelmsford, MA). The infusion rate was set to 0.5 μL/min overnight, and increased to 1.5 μL/min 30 minutes prior to sample collection the next day.
Samples were collected every 10 minutes using a refrigerated fraction collector (CMA 142, CMA Microdialysis Inc., North Chelmsford, MA) in vials containing 5μL antioxidant (20 mM phosphate buffer containing 25 mM EDTA-2NA and 0.5 mM ascorbic acid, pH 3.5). Tonic levels of DA were measured in the first five samples, followed by saline (ip, at 55 minutes) and cocaine (10 mg/kg, ip, at 75 minutes) injections. Sample collection continued for 115 minutes following the cocaine injection in order to assess the time course of cocaine-induced DA changes.
DA was analyzed by HPLC, consisting of an LC10-AD pump (Shimadzu, Columbia, MD), manual injector (model 7,125; Rheodyne, Cotati, CA) with a 100 μL sample loop, and an electrochemical detection (ECD) system (DECADE II, Antec Leyden BV, Zoeterwoude, Netherlands). A cation-exchange column (CAPCELL PAK, 1.5mm 250mm, 5μm ID, Shiseido, Tokyo, Japan) with temperature set at 30°C was used to separate monoamines. Mobile phase consisted of 150 mM ammonium acetate, 50 mM citric acid, 27μM EDTA, 10% methanol, and 1% acetonitrile, with pH adjusted to 4.6 and flow rate set at 0.2 mL/min. Concentrations of DA were calculated using a standard curve with known amounts of monoamines in a range of 1.875–18.75 pg. The limit of detection (LOD) for DA was 0.21 pg.
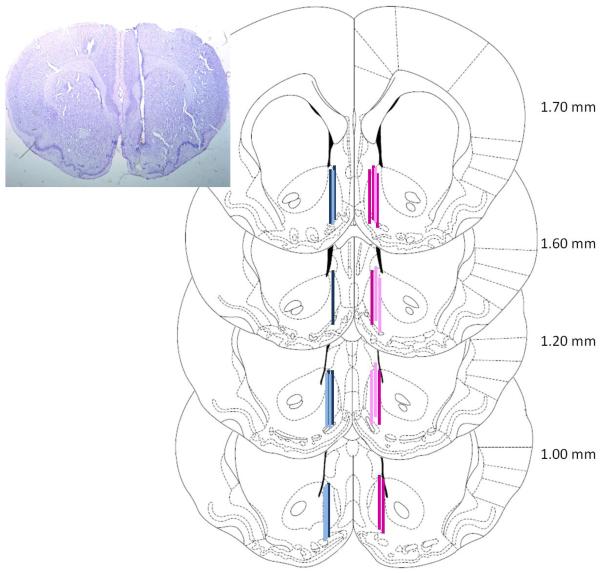
Following sample collection, rats were deeply anesthetized with pentobarbitol (100 mg/kg) and perfused with saline and 4% paraformaldehyde. Brains were removed for histological analysis of probe placement. Brains were sliced at 60 μm and mounted on slides, then stained with cresyl violet. Precise probe placement was determined using light microscopy. Correct placements are shown in Figure 3. Six male and six female rats were excluded due to missed placements.
Figure 3. Histological verification of probe placements.
(left) is a representative photograph of staining and probe placement. (right) shows placements for all male control (light blue) and stressed (dark blue) on the left and female control (light pink) and stressed (dark pink) on the right. Although all cannulae and probes were implanted on the right side, male placements are depicted on the opposite side for the purposes of this figure.
Experiment III: IV Cocaine Self-Administration
A third cohort of stressed (final n=14 male; n=18 female) and non-stressed (final n=15 male; n=18 female) rats was assigned to the self-administration experiment. After the last defeat, the animals were implanted with a catheter (SILASTIC silicon tubing; inner diameter, 0.63 mm; outer diameter, 1.17 mm) in the right jugular vein under ketamine (100 mg/kg) and xylazine (6 mg/kg for males, 3 mg/kg for females) anesthesia. The catheter was passed subcutaneously to the rat's back, where it exited through a small incision and was attached to a pedestal (Plastics1, Roanoke, VA) mounted to a harness system (Instech Laboratories, Plymouth Meeting, PA). Five days after surgery, rats were moved from their home cage to permanent housing in the IV self-administration chambers. Each morning catheters were flushed with 0.2 mL saline and 0.2 mL heparinized saline (20 IU/mL), and 0.17 mL pulses of saline were delivered every 30 minutes, except during the daily self-administration procedures.
Vaginal smears were taken daily prior to the self-administration session, and all females cycled for the duration of the self-administration protocol. Rats were reinforced for each press of the active lever by an infusion of cocaine (0.75 mg/kg/infusion) according to a fixed ratio 1 (FR1) schedule of reinforcement. Sessions were signaled by a stimulus light, and one wall of the home cage contained two retractable levers. Pressing the active lever resulted in an intravenous infusion, followed by a 30 second time out period, during which the stimulus light was turned off. Pressing the inactive lever was neither punished nor reinforced, but lever presses were recorded. Rats performed two daily sessions, which terminated after 15 infusions or 5 hours. Shaping began on the first session to ensure rapid acquisition. Seventeen of the initial 82 rats (n=6 non-stressed male, n=4 stressed male; n=4 non-stressed female, n=3 stressed female) were excluded for failing to meet acquisition criteria by the time of the “binge.”
After five days of self-administration (10 days after the last defeat), rats were given continuous access to cocaine (0.3 mg/kg/infusion) in an unlimited “binge” protocol. The “binge” terminated after 2 hours without any infusions, and “binge duration” was considered the number of minutes prior to the last infusion.
Statistical Analysis
Unless otherwise noted, statistical analyses were performed using Sigma Plot v11.0 (Systat Software, San Jose, CA). A Mann-Whitney Rank Sum Test was used to assess sex differences in quantitative aspects of the social defeat encounters. For behavioral sensitization, walk duration was analyzed using a split plot factor three-way repeated measures analysis of variance (ANOVA, SAS, SAS Institute, Cary, NC), followed by a priori hypothesis-driven two way ANOVAs with Holm-Sidak corrections for multiple comparisons. For neural sensitization, percent change from tonic DA concentrations during the time range where dopamine was elevated after cocaine were analyzed by three-way split plot, repeated measures ANOVA (SAS), followed by a priori hypothesis-driven two-way repeated measures ANOVAs with Holm-Sidak corrections for multiple comparisons. For cocaine “binge,” binge duration (time of last infusion) and total cocaine intake were analyzed with two-way ANOVAs with Holm-Sidak corrections for multiple comparisons.
RESULTS
Social Defeat
No significant effect of sex in latency to the first bite, total number of bites received, or duration of defeat encounter was observed.
Experiment I: Behavioral Sensitization
Significant effects of cocaine (F2,98=33.6, p<0.001), sex/estrous cycle (F2,49=29.82, p<0.001), and episodic social defeat stress (F1,49=23.34, p<0.001) were observed for walk duration during the cocaine challenge.
In non-stressed rats there was no significant difference in walk duration prior to cocaine injection, and only females in estrus spent significantly more time walking at 5–10 (p<0.05) and 25–30 (p<0.05) minutes following cocaine injection. Additionally, walk duration for estrous females during each time period was significantly higher than that of males (p<0.001), but not non-estrous females (Figure 2).
Figure 2. Behavioral Sensitization.
Walk duration (seconds) 5–10 minutes after saline (SAL) and 5–10 (5) and 25–30 (25) minutes after cocaine (10 mg/kg, ip) in (a) males and (b) non-estrous and (c) estrous females. All values are expressed as means ± SEM; *=p<0.05, **=p<0.01, ***=p<0.001 vs. within group SAL; #=p<0.05, ##=p<0.01, ###=p<0.001 vs same sex/estrous phase control; ++=p<0.01, +++=p<0.001 vs same stress group male.
Within the stressed animals, at baseline females in estrus had a significantly longer walk duration than both males (p<0.05) and non-estrous females (p<0.01), but no difference was observed between males and non-estrous females. Walk duration 5–10 minutes after cocaine administration was significantly elevated compared to non-stressed rats for both sexes, regardless of estrous cycle (all p<0.001, Figure 2). However, a significantly larger increase in comparison to males for walk duration was observed in both groups of females (each p<0.001), with estrous females spending significantly more time walking than non-estrous females (p<0.001). Furthermore, both groups of stressed females exhibited a significantly elevated walk duration 25–30 minutes following cocaine injection (both p<0.001), whereas walk duration for stressed males did not differ from baseline.
Experiment II: In vivo Microdialysis
No difference in average tonic DA concentration (pmol) was observed (non-stressed male M=1.70, SEM=0.52; stressed male M=1.48, SEM=0.28; non-stressed female M=1.31, SEM=0.46; stressed female M=2.00, SEM=0.39). Percent change from baseline was used to reduce intergroup and intersex variability. Additionally, no statistical difference between all five baseline samples and the post-saline sample was observed across all groups, so baseline samples were excluded from subsequent analysis and all post-cocaine samples were compared to the post-saline sample. As a result, significant effects of sex (F1,19=4.51, p=0.0470), stress (F1,19=11.06, p=0.0036), and time (F8,152=17.57, p<0.0001), with significant sex × stress × time (F8,152=2.91, p=0.0047) but not sex × stress, sex × time, or stress × time interactions were observed.
Both stressed and non-stressed rats for both sexes had a significant change from baseline DA in response to cocaine. In males (Figure 4a), a significant overall effect of cocaine was observed in non-stressed (F11,44=5.571, p<0.001) and stressed (F11,66=17.964, p<0.001) rats, with significant increases over baseline from 15 to 35 minutes post-cocaine for non-stressed males (p<0.01) and 15-to 45 minutes post-cocaine for stressed males (p<0.01). Non-stressed females (Figure 4b) also showed a significant effect of cocaine (F11,44=3.423, p=0.002), with significant elevation in extracellular DA relative to baseline from 15 to 35 minutes after cocaine (p<0.05), and average standard error across the sampling was less than 15% of the mean. Stressed females showed an augmented DA percent increase from baseline (F11,55=2.862, p=0.005) for every sample after cocaine injection (Figure 4b).
Figure 4. Dopaminergic Sensitization.
DA levels in the NAc expressed as percent change from baseline in the same animals in (a) intermittently stressed (n=7, filled circles) or control (n=5, open circles) male rats and (b) intermittently stressed (n=7, filled triangles) or control (n=5, open triangles) female rats. All values are means ± SEM; *=p<0.05, **=p<0.01, ***=p<0.001 compared to within-group baseline and ##=p<0.01, ###=p<0.001 compared to same-sex control.
The effects of stress on DA changes were significant in both sexes. Within males, a significant effect of stress was observed from 15 to 45 minutes following the cocaine injection (p<0.01), with stressed males significantly higher at each time point. Non-stressed and stressed females, on the other hand, significantly differed 5–15 minutes (p<0.001) following cocaine injection, and again from 45 to 105 minutes post cocaine, with no significant differences from 15–45 minutes following cocaine, as shown in Figure 4b.
Comparing non-stressed male and females, there was no significant effect of sex, although a significant difference between sexes was observed at 35–45 minutes following cocaine (p=.033), with females showing a higher elevation above baseline DA levels compared to males. A significant effect of sex was observed within the stressed animals (F1,11=4.81, p=0.05), and sex differences observed at 5 (p<0.05) and 45 to 105 (p<0.05) minutes after cocaine injection, with females again showing higher elevation above baseline compared to males.
Experiment III: IV Cocaine Self-Administration
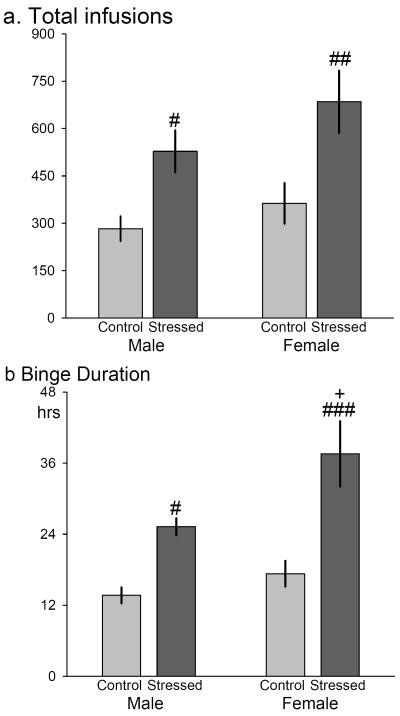
In the unlimited access “binge,” effects of both sex and stress were observed in overall duration. No effect of estrous cycle was found in total infusions or time of last infusion. Therefore data from females were collapsed across estrous cycle for all subsequent analyses.
In total number of infusions across the “binge,” a significant effect of stress (F1,34=13.792, p<0.001), but not sex or a sex × stress interaction (Figure 5a), was observed. Although there were no significant interactions, post-hoc analyses were performed based on the a priori hypothesis that sex and stress differences would exist in “binge” duration. There were significant stress effects within both males (p=0.042) and females (p=0.003), with stressed groups self-administering a significantly higher number of infusions across the “binge.”
Figure 5. Effects of stress and sex on the “binge”.
Effects were observed in (a) total infusions and (b) “binge” duration in an unlimited access cocaine self-administration (0.3 mg/kg/infusion, FR1) “binge” in male (control n=8, stressed n=8) and female (control n=12, stressed n=10) rats. “Binge” terminated after 120 minutes without a cocaine infusion. Values are expressed as mean ± SEM; #=p<0.05, ##=p<0.01, ###=p<0.001 vs same-sex control; +=p<0.05 vs stressed male
Effects of both stress (F1,34=21.131, p<0.001) and sex (F1,34=5.288, p=0.028) were observed in the time of last infusion, or “binge” duration, without a sex × stress interaction (Figure 5b). Non-stressed males stopped responding for cocaine significantly earlier than stressed males (p=0.034). Stressed females also “binged” significantly longer than non-stressed females (p<0.001). Furthermore, while sex differences were not observed in “binge” duration of non-stressed rats, a significant sex difference was observed in stressed rats such that stressed females “binged” significantly longer than stressed males (p=0.019).
DISCUSSION
This series of experiments has demonstrated that episodic social defeat engenders substantial behavioral and neurochemical changes in rats ten days after the last defeat experience, and that these changes occur to a significantly larger degree in females than males, regardless of circulating ovarian hormones. Furthermore, observed differences in social defeat were not likely due to differences in the nature of social defeat. The social defeats were kept as similar as possible, with identical fight duration and termination criteria. However, while no quantitative differences in the nature of the defeats were observed (i.e. equivalent attack latencies, fight durations, and number of attack bites), qualitative differences were observed. Whereas aggressive males primarily attack the back and necks, lactating dams are more prone to bite the face, forelimbs and flanks. While differences in the stressfulness of the encounters cannot be entirely ruled out, stressed males and females did not differ in the observable stress response, such as weight gain, corticosterone levels, and behavior. Sex differences in both the defeat experience and subsequent stress response may influence the dopamine response, locomotor cross-sensitization, and cocaine taking.
Behavioral Sensitization
In agreement with extensive work in males, episodic social defeat stress induces long lasting behavioral changes in females. In particular, episodically stressed females in this experiment displayed an enhanced locomotor response to cocaine compared to controls, and this observed behavioral sensitization was greater in magnitude than that of similarly stressed males. Moreover, the increased locomotion in response to cocaine was detected 25–30 minutes after the injection in stressed females, while locomotor activity returned to baseline levels in males by this time period. This coincides with previous studies on sex differences in drug-induced behavioral sensitization, which also show a larger and longer lasting behavioral sensitization in females compared to males (Becker et al. 2001; Hu and Becker 2003).
Although episodically socially defeated females of all estrous phases in this experiment displayed behavioral sensitization to a greater degree than males, effects of the estrous cycle were still observed, such that females in estrus showed an augmented response compared to non-estrous rats in metestrus or diestrus. This is consistent with extensive studies in females showing a facilitatory effect of estradiol on behavioral sensitization. Despite substantial differences in sensitization protocols, strains and ages of rats used, and means of measuring locomotor activity, most studies agree that estradiol augments cocaine-induced behavioral sensitization. For example, although some studies show no behavioral sensitization in OVX females (Puig-Ramos et al. 2008; Sircar and Kim 1999) while others do (Becker et al. 2001; Hu and Becker 2003; Sell et al. 2000; Sell et al. 2002), behavioral sensitization is higher in OVX females treated with estradiol in each of these reports, regardless of dose or route of administration.
Neural Sensitization
No group difference in baseline NAc DA concentration was observed between groups, at variance with studies showing a higher baseline DAergic tone in males compared to females (Castner et al. 1993) and in stressed compared to non-stressed males (Miczek et al. 2011). However, a trend towards similar sex and stress effects were observed, and it is possible that they would become statistically significant with a larger number of subjects. Sex differences in tonic dopamine levels have been observed even in the absence of circulating gonadal hormones, with extracellular dopamine in the striatum of castrated males twice as high as ovariectomized females, as determined by no net flux microdialysis (Castner et al. 1993). This indicates a partial neural source for lower dopaminergic tone in females, which could be a result of differences in spontaneous firing, or conversely as a result of the often reported sex differences in D1, D2 and DAT distributions. Within females, estradiol can affect DA tone, such that females in estrus have significantly higher DA tone in the striatum than diestrous and ovariectomized females (Xiao and Becker 1994). However, no differences in DA tone were observed between intact and castrated males (Xiao and Becker 1994), and estradiol did not have any effect on castrated males (Castner et al. 1993). Thus, it appears that while there are sex differences in the dopamine system unrelated to circulating hormones, estradiol modulates DA in female but not male rats.
Overall, as expected, significant effects of sex, stress, and cocaine were observed, as well as a three-way interaction. All males showed an increase in DA after cocaine, but males previously exposed to episodic social defeat stress showed a significantly higher percent increase from baseline in extracellular DA than non-stressed males, indicative of neural cross-sensitization to cocaine due to episodic stress (Miczek et al. 2011; Tidey and Miczek 1996; 1997).
Prior experiences with stress showed clear sex differences in accumbal DA in response to acute cocaine. Particularly, in contrast to the significantly greater change from baseline DA levels observed in stressed compared to non-stressed males in response to cocaine, there was no statistical difference in the initial percent change from baseline after cocaine between stressed and non-stressed females. However, the increase of extracellular DA in the NAc occurred earlier in stressed than non-stressed females, and the DA remained elevated for a longer period of time. This persistent elevation in NAc DA could be a result of either increased firing rate by DA neurons in the VTA, increased amount of DA released from DA neurons in the VTA, or a reduced ability to clear DA from the synapse in the NAc. While it is possible that episodic stress could engender long lasting neuroadaptations in the firing rate of mesocorticolimbic DA neurons, it is unlikely that these neuroadaptations would lead to a prolonged firing in response to cocaine 105 minutes after the injection. As such, it seems much more likely that episodic stress may modify the reuptake mechanisms for DA. Future work is directed towards evaluating the DA transporter and D2 receptor protein expression on NAc DAergic terminals.
It seems likely that an interaction exists between stress exposure and circulating estradiol on DAergic function in female rats. As the number of females in the microdialysis experiment was low due low variability, it was not possible to statistically compare the effects of estrous cycle phase, although it was tracked. Accordingly, more causal effects of estradiol need to be evaluated in further studies using OVX rats with and without estradiol and/or progesterone treatment.
Cocaine “binge”
In line with previous research at later time points after stress, stressed males self-administered significantly more cocaine for a significantly longer duration during the unlimited access “binge” (Boyson et al. 2011; Covington and Miczek 2001; 2005; Miczek et al. 2011). Cumulative records (not shown) demonstrate that both groups of males initially self-administer cocaine at similar rates, but non-stressed males ceased responding approximately 12–13 hours into the “binge,” whereas stressed males continued responding for an average of 25 hours. There was only overlap of two animals in regards to “binge” duration, indicating two discrete groups.
In females, however, an effect of stress did not emerge until after the first 24 hours of the “binge.” There was a much wider range in binge durations of stressed females than any other group, and this effect was not as a result of levels of circulating gonadal hormones. As females self-administer more cocaine than males in extended access protocols (Lynch and Taylor 2004; Roth and Carroll 2004), the longer “binge” duration in non-stressed females was not surprising. Furthermore, given the enhanced acquisition and cocaine intake in females following cocaine-induced sensitization (Zhao and Becker 2010), as well as the enhanced intake in male rats following stress induced cross-sensitization (Boyson et al. 2011; Covington and Miczek 2001; Miczek et al. 2011; Tidey and Miczek 1997), it was also expected that stressed females would have a longer “binge” duration than all other groups.
Unlike the behavioral sensitization data, and previous reports of the effects of estradiol on cocaine self-administration (see Anker and Carroll 2011 for recent review), no effect of estrous cycle at initiation of “binge” was observed. This lack of effect was likely due to different fluctuations of ovarian hormones across the several day “binge” within these intact females, such that the estrous cycle phase at the beginning of the “binge” did not remain constant. Therefore, ovariectomized females with estradiol and/or progesterone replacement should be assessed in this protocol to more adequately assess the role of gonadal hormones “binge” duration and cocaine intake.
Conclusions and Implications
These three experiments complement and inform one another. Dopamine plays a key role in both locomotor sensitization (Kalivas and Duffy 1993) and cocaine self-administration (De Wit and Wise 1977). Considerable research also indicates social defeat stress in males results in neuroadaptations in the mesocorticolimbic DA system, (see Cabib and Puglisi-Allegra 2012 for recent review). While this has been well studied in males, DAergic neuroadaptations and their subsequent effects on behavior have remained understudied in females. This set of experiments provides a substantial first examination of the interaction between sex and episodic social defeat stress on the behavioral and dopaminergic response to acute cocaine, as well as cocaine taking and satiation in an unlimited “binge” protocol, with a particular focus on the role of circulating ovarian hormones in intact, freely-cycling females. Estradiol plays a key facilitatory role in the expression of episodic social defeat induced behavioral cross-sensitization to cocaine, possibly through modulation of mesocorticolimbic DAergic function. As the role of estrogens and progestins is only correlational in intact females, more work needs to be directed at adequately assessing a more causal role of estradiol and progesterone both alone and in combination in all aspects of these experiments. Furthermore, the mechanism underlying dopaminergic sensitization as a result of episodic social defeat stress appears to differ between males and females, and this difference requires further study.
Thus far, preclinical and clinical data on the role of ovarian hormones on the cocaine response and cocaine taking/seeking behaviors seem to converge, but preclinical examinations to compare with clinical reports of heightened, estrogen-dependent effects of stress on the response to cocaine in females are lacking. This crucial missing translational evidence needs to be explored further, so that new treatments for cocaine dependence and addiction tailored to females can be pursued.
Acknowledgements
This research was supported by NIH Grant DA031734. We thank Rachel Doyle, Melanie Monroe, and Andrew Terrano for their great help.
REFERENCES
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–69. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2007;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr., Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–21. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–98. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Canadian journal of psychology. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–9. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatova L, Raleva M. Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy. 2009;110:285–9. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya. J Neurosci. 1993;13:276–84. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–46. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–51. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8:300–11. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–22. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. d-amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 1999;147:190–9. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–57. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. Eighth Edition. The National Academies Press; 2011. [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr., Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–18. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3 edn. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Puig-Ramos A, Santiago GS, Segarra AC. U-69593, a kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behavioral neuroscience. 2008;122:151–60. doi: 10.1037/0735-7044.122.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2010 National Survey on Drug Use and Health: National Findings. In: Studies OoA., editor. NSDUH Series H-36. SAMHSA; Rockville, MD: 2010. [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–86. [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–90. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 2011;218:271–9. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Staples RE, Geils HD. An Observation on the Vaginal Smear of the Rat. J Endocrinol. 1965;32:263–4. doi: 10.1677/joe.0.0320263. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–93. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–7. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav. 2010;58:8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]