Abstract
Background
Development of pre-transplantation islet culture strategies that preserve or enhance β-cell viability would eliminate the requirement for the large numbers of islets needed to restore insulin independence in type 1 diabetes patients. We investigated whether glial cell line-derived neurotrophic factor (GDNF) could improve human islet survival and post-transplantation function in diabetic mice.
Methods
Human islets were cultured in medium supplemented with or without GDNF (100 ng/ml) and in vitro islet survival and function assessed by analyzing β-cell apoptosis and glucose stimulated insulin release. In vivo effects of GDNF were assessed in streptozotocin-induced diabetic nude mice transplanted under the kidney capsule with 2000 islet equivalents of human islets pre-cultured in medium supplemented with or without GDNF.
Results
In vitro, human islets cultured for 2–10 days in medium supplemented with GDNF showed lower β-cell death, increased Akt phosphorylation and higher glucose-induced insulin secretion than islets cultured in vehicle. Human islets pre-cultured in medium supplemented with GDNF restored more diabetic mice to normoglycemia and for a longer period after transplantation than islets cultured in vehicle.
Conclusions
Our study shows that GDNF has beneficial effects on human islet survival and could be used to improve islet post-transplantation survival.
Keywords: Islet transplantation, diabetes, GDNF, islet survival, normoglycemia
Type 1 diabetes (also known as juvenile diabetes) results from the autoimmune destruction of insulin-producing pancreatic β cells, and survival of individuals with the disease depends mainly on daily insulin injections or the use of insulin pumps. Transplantation of donor islets has recently proven effective at restoring insulin production and blood glucose control in type 1 diabetes mellitus patients (1–4). Its widespread application is, however, limited by availability of donor islets, the requirement for long-term use of immunosuppressive drugs to prevent graft rejection, and lower rates of long-term insulin independence (5). Moreover, the reduced islet viability and loss of insulin content that occurs during islet isolation and pre-transplantation culture necessitates the use of large numbers of islets, usually from multiple donors, to achieve insulin independence (6). Identification of factors that can enhance islet survival in vitro and improve islet function post-transplantation will thus help in extending the period of insulin independence in type 1 diabetes patients and reduce the number of donors required for successful islet transplantation.
Glial cell line-derived neurotrophic factor (GDNF) is a factor produced by glial cells that plays an important role in the development of the enteric nervous system. GDNF has previously been shown to promote the survival of β-cells in vitro, and to enhance β-cell mass and improve glucose control in mice when transgenically over-expressed under the control of the glial fibrillary acidic protein promoter (2, 7). In this study we investigated the ability of GDNF to improve human islet post-transplant survival and function. Using in vitro and in vivo methods we assessed the effects of culturing human islets in the presence of GDNF on β-cell survival, in vitro insulin secretion, and post transplantation glycemic control. We performed these studies to assess the potential for use of GDNF to enhance human islet viability before transplant in type 1 diabetes patients.
RESULTS
GFRα-1 and Ret receptors are expressed in human islets
GDNF signals through binding to a multi-unit receptor complex consisting of a GDNF-specific receptor, namely, GDNF family receptor α-1 (GFRα-1) and the ret co-receptor which it shares with other neurotrophic factors. To investigate the expression of these receptors in human islets, islets were lysed with Laemmli buffer and the presence of the receptors assessed by Western blotting. Fig. 1A shows that both GFRα-1 and ret receptors are expressed in human islets.
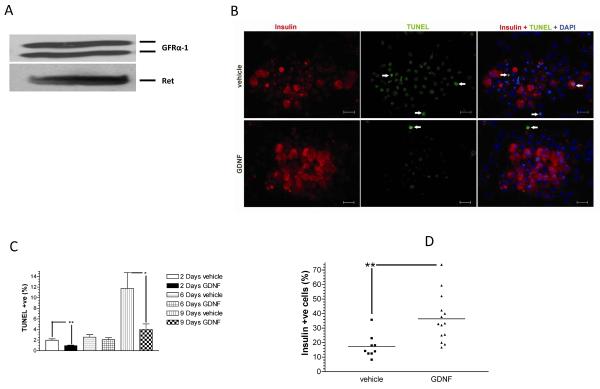
FIGURE 1. GDNF enhances human islet survival and function in vitro.

(A) Western blot analysis of Ret and GFRα-1 receptor expression in human islets. Data is representative of three separate experiments. (B) Representative images showing insulin (red) and TUNEL (green) staining with DAPI nuclear counter-staining (blue) in human islets cultured for 2 days in vehicle or medium containing GDNF. Scale 20 μm. (C) Score of TUNEL-positive cells in islets cultured for 2–9 days in CIT culture medium alone (vehicle) or CIT culture medium supplemented with GDNF (100 ng/ml). Plotted are means + SEM. ***, P<0.001, **, P<0.01, *, P<0.05, N≥5. (D) Score of insulin-positive cells in islets cultured for 2 days in vehicle or medium containing GDNF. Horizontal lines show the means for each group. **, P<0.01; GDNF, N=13 replicates; vehicle, N=11 replicates. (E) Western blot analysis of phospho-Akt and total Akt levels in human islets cultured for 6 and 10 days in vehicle or medium supplemented with GDNF. Histogram showing phospho-Akt band densities equalized to total Akt levels expressed as a percentage of vehicle. Plotted are means + SEM. ***, P<0.001.
GDNF enhances human islet survival in vitro
To investigate the effects of GDNF on human islet survival in vitro, islets were cultured for 2–9 days in CIT culture medium alone (vehicle) or CIT culture medium supplemented with GDNF (100 ng/ml) and cell survival assessed by TUNEL staining with insulin and DAPI counterstaining. Fig. 1B contains representative images showing TUNEL- and insulin-positive cells in human islets cultured for 2 days in vehicle or in medium supplemented with GDNF. Scoring of TUNEL-positive cells revealed significantly (P<0.01) lower numbers of apoptotic cells in islets cultured for 2 and 9 days in medium containing GDNF (respectively, 0.97 ± 0.098 %, N=5 and 4.03 ± 1.03 %, N=8) than in islets cultured for the same duration in vehicle (respectively, 1.99 ± 0.25 % and 11.76 ± 2.97 %, N=6) (Fig. 1C). In line with these observations, there were significantly more insulin-positive cells in islets cultured in medium containing GDNF than in islets cultured in vehicle (GDNF, 36.41 ± 4.4%, N=13 replicates, vs. vehicle, 17.41 ± 2.7%, N= 11 replicates; P<0.01) (Fig. 1D).
GDNF promotes human islet survival in vitro by enhancing Akt phosphorylation
To understand the mechanisms involved in GDNF-mediated improvement in human islet survival, islets were cultured in vehicle or medium supplemented with GDNF and Akt phosphorylation assessed by Western blotting. Islets cultured in medium supplemented with GDNF had significantly (P≤0.001) higher phospho-Akt levels than islets cultured in vehicle after 6 and 10 days of culture (Fig. 1E).
GDNF preserves in vitro islet function
Glucose stimulation indices of islets cultured for 10 days in vehicle and medium containing GDNF were compared with those of post-isolation islets cultured for 24 h in CIT medium to investigate the ability of GDNF to preserve islet function. No significant difference in glucose stimulation was observed between islets cultured for 10 days in medium supplemented with GDNF and post-isolation islets cultured for 24 h in CIT medium (GDNF, 3.80 ± 0.44 vs. post-isolation, 4.11 ± 0.17; P>0.05, N=6) (Fig. 2A). In contrast, islets cultured for 10 days in vehicle had significantly lower glucose stimulation indices than post-isolation islets cultured in CIT medium for 24 h (1.92 = 0.1, P<0.001; N=6). Comparison of insulin content between islets cultured in vehicle and medium containing GDNF after 2 and 9 days of culture revealed significantly (P<0.05) higher insulin content in islets cultured in medium containing GDNF, but no significant changes in insulin content over time for each condition (Fig. 2B).
FIGURE 2. GDNF preserves human islet function in vitro.
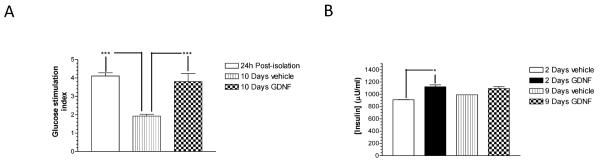
(A) Glucose stimulation indices of islets cultured in CIT culture medium for 24 h immediately post-isolation and islets cultured for 10 days in vehicle or medium supplemented with GDNF. Plotted are means + SEM. ***, P<0.001; N=6 replicates each from 2 isolations. (B) Insulin content of human islets cultured for 2 and 9 days in vehicle or GDNF. Plotted are means + SEM. *, P≤0.05. N=2.
Islets cultured in GDNF restore normoglycemia longer in diabetic mice
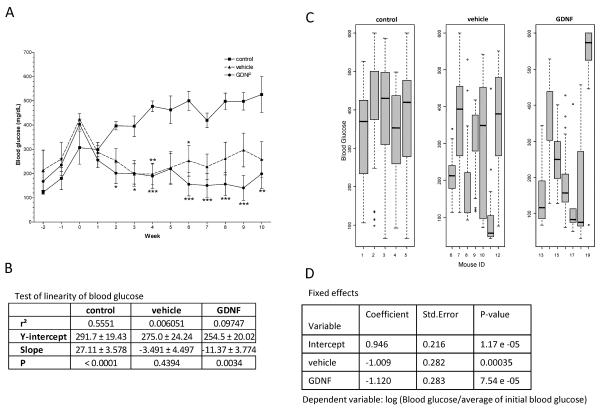
Diabetic nude mice were transplanted with human islets that had been pre-cultured for 14 days in CIT culture medium supplemented with or without GDNF to assess the effect of GDNF on the in vivo function of transplanted islets. This number of islets is recommended for in vivo islets function assessment using nude mice for CIT studies. Fig. 3A shows weekly changes in non-fasting blood glucose of control and transplant mice from the 3 studies. Blood glucose levels of the three treatment groups were statistically not significantly different from each other prior to islet transplantation (P> 0.05). Following transplantation, however, blood glucose levels of vehicle and GDNF transplant group mice fell 53% within a period of 4 weeks and continued falling until week 9 in GDNF group mice while rising gradually in vehicle group mice. In contrast, blood glucose levels of control mice rose throughout the duration of the study. Two-Way Anova with Bonferroni post tests comparisons of blood glucose levels between GDNF and control mice showed significant (P≤0.05) differences from week 4 forwards (Fig. 3A). Comparison of blood glucose levels between vehicle and control mice on the other hand showed significant differences (P≤0.05) only during weeks 4 and 6 post-transplantation. Linear regression fitting of the blood glucose data revealed significant (P<0.001) improvement in blood glucose levels for GDNF group mice following transplant, but no significant improvement for mice transplanted with islets cultured in vehicle (Fig. 3B). Analysis of daily blood glucose distribution showed non-fasting blood glucose higher than 200 mg/dL in only 3 out of 7 mice transplanted with islets cultured in medium containing GDNF (Fig. 3C). In contrast, all 5 control mice and 5 out of 7 mice transplanted with islets cultured in vehicle had non-fasting blood glucose higher than 200 mg/dL. Log linear mixed effects regression model tests further confirmed that blood glucose levels of mice transplanted with islets cultured in GDNF improved more compared to blood glucose levels of mice transplanted with islets cultured in vehicle only (Fig. 3D).
FIGURE 3. Diabetic mice transplanted with islets cultured in GDNF show better glycemic control than mice transplanted with islets cultured in vehicle.
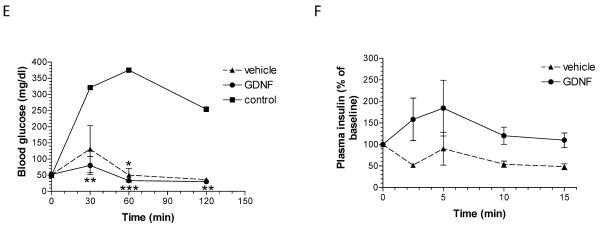
(A) Weekly non-fasting blood glucose levels for study 1–3 control, vehicle and GDNF transplant mice. Plotted are means ± SEM. Group means that are significantly different from control means at each time point are shown by asterisks either above (vehicle) or below (GDNF) the mean value. ***, P<0.001; **, P<0.01; *, P<0.05; control N=5, vehicle N=9, GDNF N=8. (B) Linear regression fit of blood glucose data. ***, P<0.001. (C) Non-fasting blood glucose distribution (quartiles and range) for control mice and mice transplanted with human islets pre-cultured for 14 days in vehicle or medium supplemented with GDNF. (D) Fixed effects and P-values of log linear mixed regression model fit tests of the significance of the difference in blood sugar levels between the treatment groups. (E) Blood glucose levels during intraperitoneal glucose (3 mg/kg) tolerance testing in control, vehicle and GDNF transplant mice performed 65 days post-transplantation. Plotted are means + SEM. N=3 mice. Group means that are significantly different from control mice means at each time point are shown by asterisks either above (vehicle) or below (GDNF) the mean value. ***, P<0.001; **, P<0.01; *, P<0.05. (F) Insulin release in response to intraperitoneal arginine (300 mg/kg) administration performed 63 days post-transplantation. Plotted are means + SEM of plasma insulin levels expressed as a percentage of baseline. N=3 mice in each group.
Diabetic mice transplanted with islets cultured in GDNF show better glucose tolerance and arginine-induced insulin release
Results of intravenous glucose tolerance testing performed 65 days post-transplantation on control, vehicle and GDNF transplant mice are shown in Fig. 3E. Baseline fasting blood glucose levels were not significantly different between the three groups. However, following an intravenous injection of 3 mg/kg glucose, blood glucose levels of GDNF transplant mice were significantly lower than blood glucose levels of control mice 30 min (P<0.01), 60 min (P<0.001) and 120 min (P<0.01) post injection while blood glucose levels of vehicle transplant mice were significantly lower than blood glucose levels of control mice only at 60 min (P<0.05) post injection (N=3 mice).
Comparison of the acute insulin release response to arginine administration performed 63 days post-transplantation showed a faster and stronger response in mice transplanted with islets cultured in medium containing GDNF than in mice transplanted with islets cultured in vehicle (P<0.01, N=3 mice in each group) (Fig. 3F).
Human islets cultured in GDNF show better post-transplantation survival
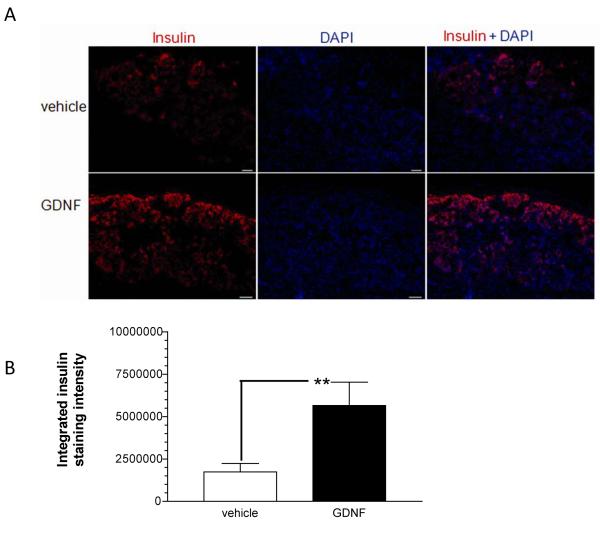
Sections from left kidneys collected 65 days post-transplantation from transplant group mice were stained for insulin to assess islets graft formation and post-transplantation survival. Islet grafts of nearly equal size were observed in both vehicle and GDNF transplant mice (Fig. 4A). Densitometric analysis of insulin staining intensity revealed 3.3 ± 0.79 fold (P<0.001) more intense insulin staining in grafts from GDNF transplant mice than in grafts from vehicle transplant mice (Fig. 4B).
FIGURE 4. GDNF improves islet post-transplantation survival.
(A) Representative images showing insulin (red) staining with DAPI (blue) nuclear counter-staining in kidney sections collected 65 days post-transplantation from vehicle (vehicle) and GDNF (GDNF) transplant mice. Scale, 20 μm. (B) Comparison of intensity of insulin staining in kidneys from vehicle (vehicle) and GDNF (GDNF) transplant mice. Plotted are means + SEM. (**, P<0.01; vehicle N=14 replicates, GDNF N=10 replicates from 4 mice each).
DISCUSSION
Results from our study demonstrate that human islets pre-cultured in GDNF are superior at restoring normal blood sugar levels in diabetic mice than islets pre-cultured in CIT medium alone. In our study, GDNF not only improved islet survival pre-transplantation, but also prolonged graft survival and the ability to restore normoglycemia after transplantation.
The brief in vitro islet culture period incorporated before transplantation in human islet transplantation protocols is a time during which islet quality is assessed and immunotherapy is initiated in diabetic patients (3, 8). Previous studies have compared the efficacies of several serum-free media commonly used to culture human islets before transplantation (9). More recently, studies have shown that supplementation of these culture media with factors such as nerve growth factor (NGF) or factors present in conditioned media from human mesenchymal stem cells can enhance the engraftment as well as survival and function of islets after transplantation (10, 11). In the study by Miao et al. (10), co-culture of murine islets with NGF for 96 h not only improved islet viability in vitro, but also restored normoglycemia longer in diabetic mice after transplantation showing that neurotrophic factors can play a beneficial role in islet transplantation. In another study conducted to investigate the potential for using pituitary adenylate cyclase-activating polypeptide (PACAP), an enhancer of glucose-induced insulin secretion, in human islet transplantation, PACAP enhanced β cell viability in culture and insulin secretion in vivo, but failed to improve blood glucose control (12). In our study GDNF improved human islet in vitro survival and function not only following short term culture, but also following prolonged culture. Moreover, while the insulin secretory capacity and the ability to restore normoglycemia in diabetic mice declined significantly following prolonged culture of human islets in vehicle, islets co-cultured with GDNF retained their insulin secretory capacity and their ability to restore normoglycemia in diabetic mice fairly well.
The mechanisms involved in GDNF influence on human islet post-transplantation survival and function were not explored fully in this study. Previously we have shown that in transgenic mice that over-express GDNF in glia GDNF enhances β cell mass and protects against streptozotocin-induced β cell damage (13). We have also shown that GDNF signaling through the PI3K/Akt signaling pathway promotes β cell survival (13). In line with these observations, in the present study GDNF enhanced Akt phosphorylation in cultured human islets and protected against β cell death both pre-and post-transplantation. In other studies GDNF has also been shown to provide a cue for migrating neural crest cells during embryonic development of the enteric nervous system (14–16). Acting through a similar mechanism, in our study GDNF could have enhanced islet engraftment into kidney tissue resulting in the increased insulin staining observed in the grafts in mice transplanted with islets pre-cultured in GDNF.
In conclusion, inclusion of GDNF in media used to pre-culture human islets before transplantation as used in this study may provide a tool for improving islet survival and function in patients with type 1 diabetes. Further studies involving primates and longer monitoring will be required to further evaluate the benefit of this therapy.
MATERIALS AND METHODS
Islet culture
Pancreata were obtained from brain-dead multi-organ donors ranging in age from 35 to 52 years and islets isolated as previously described (17). Details of the donors and isolations are shown in Table 1. Consent was obtained from the next of kin and the Emory University Institutional Review Board to use the pancreata for research. Two islet aliquots from each donor were cultured at 37°C in Clinical Islet Transplantation (CIT) Consortium (http://www.citisletstudy.org) culture medium consisting of CMRL 1066 medium (Mediatech Cellgro, Herindon, VA, U.S.A) supplemented with 25% human serum albumin, 1000 U/ml heparin, and 1 mg/ml insulin-like growth factor-1 (IGF-1). Recombinant rat GDNF produced as previously described (13) was added to one of the aliquot to a final concentration of 100 ng/ml at the start of culture and at each subsequent media change. The media were replaced every 3 days. Islet viability was assessed according to the CIT protocol for the estimation of islet viability using fluorescent dyes (SOP 3104, A02).
Table 1.
Transplant design and donor characteristics
| Study # | 1 | 2 | 3 | 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transplant group | vehicle | GDNF | control | vehicle | GDNF | control | vehicle | GDNF | control | vehicle | GDNF |
| Islet treatment | vehicle | GDNF | N/A | vehicle | GDNF | N/A | vehicle | GDNF | N/A | vehicle | GDNF |
| # of Recipients | 4 | 4 | 2 | 3 | 3 | 2 | 3 | 3 | 1 | In vitro studies | In vitro studies |
| Transplant duration (days) | 65 | 65 | 65 | 63 | 63 | 63 | 58 | 58 | 58 | N/A | N/A |
| Donor | 1 | None | 2 | None | 3 | None | 4 | ||||
| Age (years) | 52 | 35 | 48 | 48 | |||||||
| Sex (F or M) | F | M | F | F | |||||||
| Body weight (kg) | 98 | 108 | 68 | 78 | |||||||
| BMI | 36 | 35.3 | 24.8 | 28.5 | |||||||
| Cause of death | Cerebral hemorrhage | Cerebral hemorrhage | Cerebral hemorrhage | Cerebral hemorrhage | |||||||
| Total islet equivalent | 387,751 | 264,447 | 182,413 | 164,603 | |||||||
| Islet purity (%) | 80 | 90 | 90 | 97 | |||||||
| Islet viability (%) | 88 | not done | 96 | 95 | |||||||
| Stimulation index | 1.3 | 2.1 | 4.1 | 2.5 | |||||||
In vitro glucose stimulation test
Glucose stimulation tests were performed according to the NIH Consortium Method for Glucose Stimulation of Human Islet Cells (SOP 3104, A03) and insulin assayed using a human insulin ELISA kit (Mercodia AB, Uppsala, Sweden). The “stimulation index” was obtained by comparing the amount insulin produced in high (28.0 mM) glucose medium to that produced in low (2.8 mM) glucose medium.
Assessment of insulin content
Aliquot containing 1000–1300 cultured islets were homogenized using acid-alcohol (1.5% HCL in 70% ethanol) as previously described (13) and insulin content assessed using a human insulin ELISA kit (Millipore). DNA content in the homogenates was estimated by fluorometry using bisBenzimide H 33258 (Hoechst 33258, Sigma) and used to normalize insulin values.
Islet Transplantation
Six weeks-old athymic nude-foxn1<nu> nude mice (Harlan Laboratories, Indianapolis, IN, U.S.A) were used as islet recipients following guidelines established by the Emory University Institutional Animal Use and Care Committee. Diabetes was induced by two intraperitoneal injections of 75 mg streptozotocin (Sigma)/kg body weight given on two consecutive days. Glycemia was assessed by drawing a small drop of blood from the tail vein and measuring blood glucose levels using an ACCU-CHEK Aviva automatic glucometer (Roche Diagnostics, Indianapolis, IN, U.S.A.). Mice consistently showing non-fasting blood glucose readings above 300 mg/dL were considered diabetic. The mice were then assigned to either of 3 treatment groups (Table 1). GDNF transplant group mice (N=10) and vehicle transplant group mice (N=10) were each transplanted under the left kidney capsule under anesthesia with 2000 IEQ of human islets pre-cultured for 14 days in CIT culture medium supplemented with 100 ng/ml GDNF (GDNF) or in CIT culture medium alone (vehicle) according to recommended procedure (CIT protocol for in vivo islets function assessment using nude mice, SOP 3104, A04). Control diabetic mice (N=5) did not receive any islet transplantation. Random non-fasting blood glucose levels were subsequently monitored at least once a week.
Glucose tolerance testing and assessment of insulin secretion
Glucose tolerance testing was performed 60 days post transplantation. Following an overnight fast, the mice were injected intraperitoneally with glucose (3 mg/kg body weight) in sterile PBS and blood glucose levels measured 30, 60 and 120 min after injection. For in vivo insulin secretion assessment, 6-h fasted mice were injected with 300 mg arginine /kg body weight in sterile PBS and tail vein blood collected 0, 2.5, 5, 10 and 15 min post-injection into heparinized tubes. Plasma was collected by centrifugation at 3000 × g for 15 min at 4°C and stored at −20°C until used. Insulin levels were measured using a human insulin ELISA kit (Millipore).
Western blotting
Western blotting was performed as previously described (7) using rabbit polyclonal antibodies to human GFRα-1 (H-70; Santa Cruz, Santa Cruz, CA, U.S.A.), human c-Ret (R787; Immuno-Biological Laboratories, Takasaki-shi, Gunma, Japan), phospho-Akt (Ser473) and total Akt (Cell Signaling Technologies, Danvers, MA, U.S.A.) at 1:1000 dilution. Horseradish peroxidase conjugated anti-rabbit IgG (Cell Signaling Technologies) secondary antibody was used at 1:2000 dilution. A semi quantitative measurement of band density was performed using Scion Image for Windows software (Scion Corp, MD, U.S.A.).
Immunofluorescence
The left kidney was removed from each transplant mouse and frozen in Tissue-Tek O. C. T. compound (Sakura Finetek, Torrance, CA, U.S.A.) using standard techniques. Insulin and DAPI nuclear staining were performed as previously described (18).
Apoptosis Scoring
TUNEL staining of cytospin preparations of cultured human islets was performed (7) followed by insulin and DAPI nuclear counter-staining (18). Scoring of TUNEL-positive cells was achieved by taking images from randomly selected fields from each slide. The number of TUNEL-positive β cells was determined and expressed as a percentage of the total number of β cells in each field. At least 2000 β cells per condition were scored.
Statistical Analysis
Two-way ANOVA with Bonferroni post test, t test and linear regression were performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego California USA). Log linear mixed regression model analysis was done using R-version 2.9.2.
ACKNOWLEDGEMENTS
Special thanks go to Wenxin Pang and Johanna Moreno from the Emory Transplant Center for their help with mice surgery and human islet viability and functional assays. We would also like to thank Dr. Michael Hart and Dr. Mauricio Rocca from the Emory University Division of Pulmonary, Allergy, and Critical Care Medicine for allowing us use of their cryostats and histological tissue processing equipment.
S.M. participated in research design, performance of the research, data analysis and the writing of the paper; I.J., J.A., J.C., N.S., and Y.U. participated in the performance of the research; V.K.C. participated in data analysis; C.P.L. provided reagents and participated in research design; and S.V.S. and S.S. mentored the study and participated in research design.
This work was supported by the following grants: NIH-RO1 DK080684 (S. Srinivasan), Juvenile Diabetes Foundation (26-2008-878, S. Srinivasan), VA MERIT award (S. Srinivasan) and DDRDC DK064399 (S. Srinivasan).
Abbreviations
- CIT
Clinical Islet Transplantation
- DAPI
4',6-diamidino-2-phenylindole
- GDNF
glial cell line-derived neurotrophic factor
- GFRα-1
Glial cell line-derived neurotrophic factor family receptor alpha-1
- IEQ
islet equivalent
- PBS
phosphate buffered saline
- STZ
streptozotocin
Footnotes
The authors of this manuscript have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-Donor, Marginal-Dose Islet Transplantation in Patients With Type 1 Diabetes. JAMA. 2005;293(7):830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, et al. Islet Transplantation in Type 1 Diabetes Mellitus Using Cultured Islets and Steroid-Free Immunosuppression: Miami Experience. American Journal of Transplantation. 2005;5(8):2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell PJ, Hawthorne WJ, Holmes-Walker DJ, et al. Clinical islet transplantation in type 1 diabetes mellitus: results of Australia's first trial. Med J Aust. 2006;184(5):221. doi: 10.5694/j.1326-5377.2006.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanson MS, Park EE, Sears ML, et al. A Simplified Approach to Human Islet Quality Assessment. Transplantation. doi: 10.1097/TP.0b013e3181d54bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39(4):515. doi: 10.2337/diab.39.4.515. [DOI] [PubMed] [Google Scholar]
- 9.Carter J, Karmiol S, Nagy M, et al. Pretransplant islet culture: a comparison of four serum-free media using a murine model of islet transplantation. Transplant Proc. 2005;37(8):3446. doi: 10.1016/j.transproceed.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 10.Miao G, Mace J, Kirby M, et al. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation. 2006;81(4):519. doi: 10.1097/01.tp.0000200320.16723.b3. [DOI] [PubMed] [Google Scholar]
- 11.Park KS, Kim YS, Kim JH, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 89(5):509. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 12.Sakuma Y, Ricordi C, Miki A, et al. Effect of pituitary adenylate cyclase-activating polypeptide in islet transplantation. Transplant Proc. 2009;41(1):343. doi: 10.1016/j.transproceed.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mwangi S, Anitha M, Mallikarjun C, et al. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology. 2008;134(3):727. doi: 10.1053/j.gastro.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229(2):503. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 15.von Boyen GB, Reinshagen M, Steinkamp M, Adler G, Kirsch J. Enteric nervous plasticity and development: dependence on neurotrophic factors. J Gastroenterol. 2002;37(8):583. doi: 10.1007/s005350200093. [DOI] [PubMed] [Google Scholar]
- 16.Worley DS, Pisano JM, Choi ED, et al. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development. 2000;127(20):4383. doi: 10.1242/dev.127.20.4383. [DOI] [PubMed] [Google Scholar]
- 17.Turgeon NA, Avila JG, Cano JA, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10(9):2082. doi: 10.1111/j.1600-6143.2010.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mwangi SM, Usta Y, Raja SM, et al. Glial cell line-derived neurotrophic factor enhances neurogenin3 gene expression and beta-cell proliferation in the developing mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G283. doi: 10.1152/ajpgi.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]