Abstract
Aim
Despite predictions of increased clinical applications, little is known about primary care providers’ (PCPs’) readiness to apply genomics to patient care. The aim was to assess PCPs’ current experience with genetic testing, their assessment of the understandability and clinical utility of information in sample direct-to-consumer reports for genomic assessment of disease risk and warfarin dosing and attitudes toward genomic medicine.
Materials & methods
A web-based survey of PCPs who are members of Knowledge Networks’ Physician Consulting Network was conducted.
Results
Of the 502 respondents (23.3% response rate), most ordered genetic tests infrequently. When presented with the direct-to-consumer genomic testing reports, most believed the reports were understandable, and would be willing to review results with a patient, and many believed the results would be helpful in patient management.
Conclusion
Despite limited experience with genetic tests, PCPs are open to helping patients understand genomic information. However, additional physician education is needed.
Keywords: genetic testing, genetics, genomics, pharmacogenomics, physician experience, physician readiness
Primary care providers (PCPs) are increasingly expected to integrate genomic medicine into patient care [1,2]. However, this incorporation has been slow due to lack of knowledge and confidence, limited evidence of clinical utility and concerns about privacy and discrimination [2–7]. Currently, most genetic testing performed is done to look for mutations in single genes associated with Mendelian disorders. Increasingly, testing will focus on variation in a person’s entire genome as opposed to information about a single gene variant. This shift is likely to magnify the challenges of incorporating genomic medicine into healthcare [8].
Due in part to the slow diffusion of genomic medicine into patient care, commercial companies have begun selling tests to predict disease risk and drug response directly to consumers [9]. Although there are few data on utilization, approximately 40% of physicians report being aware of direct-to-consumer (DTC) genetic testing [10,11], and consumers’ interest is high [12,13]. Users of DTC tests are motivated by curiosity and an intention to improve health based on genetic predisposition [12,14]. Several studies document that users share or intend to share DTC results with their physicians [14–16]. This implies that PCPs increasingly will be asked to explain reports and make medical recommendations based on test results [12].
Of the available DTC tests, genomic risk profiles for complex diseases (e.g., diabetes and heart disease among others) and pharmacogenetic testing are most likely to demand the attention of PCPs. The medical relevance of the former is questionable as genetic variants identified to date account for only a fraction of the overall genetic contribution to common diseases, and the risk imparted by each variant is small with odds ratios (ORs) in the range of 1.1–1.4 [17]. Although both patients and providers may believe that genetic test results could motivate healthy behavioral changes [14,18], the extent to which results lead to behaviors to decrease risk, or to earlier diagnosis, is unclear [19]. Still, consumers receiving such results may seek, expect, or require changes in their medical management, follow-up care, or both.
Pharmacogenetic testing may improve patient care through tailored prescribing of medications [20]. Although over 70 US FDA-approved drugs contain pharmacogenetic information in their labeling, clear action-oriented information is often absent [21]. One exception is warfarin. The FDA updated the drug label in 2007 and 2010 with therapeutic dosing guidelines based on CYP2C9 and VKORC1 genetic test results. However, this recommendation was made despite the lack of prospective studies demonstrating superior outcomes, reduced toxicity or cost savings when using a pharmacogenetic approach to warfarin dosing [20]. This evidentiary gap, in addition to a lack of clinical practice guidelines and insurance reimbursement, may explain physicians’ reluctance to adopt pharmacogenetic testing to guide drug therapy [22].
Despite predictions of increased clinical applications of genomics and evidence that physicians are being asked about DTC testing, little is known about physicians’ readiness to apply genomics to patients’ care, nor about their opinions about DTC genomic testing. Accordingly, a survey of PCPs practicing in the USA was conducted to assess their responses to reports of DTC genomic testing presented by a hypothetical patient, and their attitudes toward personalized genomics.
Materials & methods
Survey design
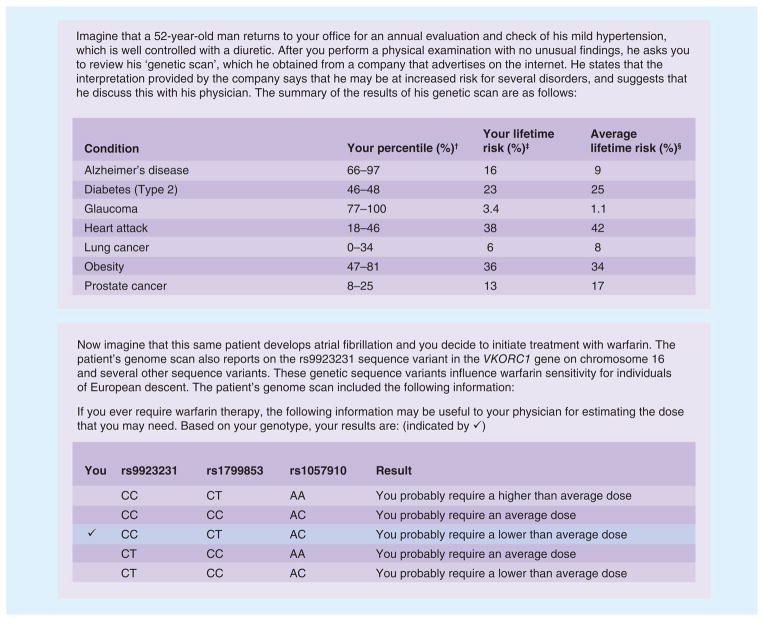
The survey was developed by the study team, which includes genetic counselors, physicians (including PCPs) and social science experts with experience in genomic medicine. Questions assessed providers’ current experience with ordering genetic tests and their perceived preparation for incorporating genomic medicine into practice. The survey included sample reports adapted from actual reports of two DTC companies offering genomic testing. The first vignette provided a brief description of a hypothetical patient and a report excerpt presenting the patient’s risk for several common complex disorders based on genotype, and the second vignette provided another report excerpt presenting information on the hypothetical patient’s sensitivity to warfarin based on VKORC1 genotype (Figure 1).
Figure 1. Scenarios and direct-to-consumer results used in the survey.
†Patient’s percentile: when compared to a sample population, your patient’s SNP-based risk for the condition falls within the given range of percentiles.
‡Patient’s lifetime risk: your patient’s risk of this condition over the course of their lifetime.
§Average lifetime risk: the average person’s risk of this condition over the course of their lifetime, depending on gender.
After reading each report, respondents were asked about factors related to readiness to incorporate the type of results presented in the report into patient care, including willingness and confidence to discuss results, understandability and clinical utility of the reports and previous exposure to such reports (using Likert scales). They were also asked attitudinal questions about various aspects of genomic medicine using Likert scale responses. Finally, the survey included questions about sociodemographics, experience with genetic testing and prior genetics education. For the sociodemographic questions, participants were given a ‘do not wish to respond’ option for the age, gender and race items.
The survey was piloted on five internists and modifications were made according to their recommendations. The physicians piloting the survey believed that most PCPs would be unfamiliar with the term ‘genomic testing’, and they recommended that we use the terms ‘genetic testing’, ‘genetic risk assessment’ or ‘genetic scan’. They further suggested that we explicitly state that respondents need not be familiar with genetic testing for disease risk or drug metabolism in order to complete the survey. Through piloting, we found that the survey took approximately 20 min to complete. A copy of the survey instrument is included in the Supplementary Material (see www.futuremedicine.com/doi/suppl/10.2217/pme.12.80).
Survey population
The web-based survey was delivered by the survey research firm Knowledge Networks (KN). KN sampled members of its Physician Consulting Network (PCN), an opt-in panel consisting of physicians invited to join from large national databases (e.g., American Medical Association [AMA] Masterfile). The PCN has been used in other published research assessing physician behavior [101], and data from KN comparing their PCN primary care sample with the AMA Masterfile indicates that their profile represents US PCPs in terms of region of practice and practice type, but has a slightly older sample and more male participants (80 compared with 70% male in the AMA Masterfile).
Recruitment
In August 2011, an email invitation including the survey URL was sent to a random sample of 2155 PCN panel members who listed family medicine or general internal medicine as their primary specialty. To reduce response bias, the email did not specify that the survey was about genetics. Participants who clicked the URL were taken to an online consent form, which then described the topic of the survey. Two email reminders were sent to nonresponders. Respondents were paid US$30 after completing the survey.
Analysis
First, frequencies were calculated to assess the distribution of responses to the main survey items. Next, bivariate linear or logistic regressions (depending on whether the variables were linear or dichotomous) were estimated to examine associations between key variables, such as frequency of ordering genetic tests and attitudinal responses to the testing scenarios. To examine differences between the two scenarios, tests of differences in proportions (z-tests) were conducted. These tests were one tailed given the expectation that providers would have greater familiarity with the pharmacogenetics scenario. Finally, bivariate linear and logistic regressions were estimated to explore differences in the main outcomes by physician specialty.
Open-ended responses were coded by two authors. To analyze management changes physicians would make based on the DTC report, responses were grouped into specific or general categories, and responses were coded thematically. Management changes were coded as ‘specific’ if respondents included a specific disease in their recommendation as opposed to general recommendations (e.g., modify diet and lifestyle) without mention of a specific disease. Changes were coded as ‘responding to an increased risk’ or ‘responding to a decreased risk’ respectively if the changes were in response to the risk for Alzheimer’s disease, glaucoma or obesity (disorders for which risk was increased according to the report) or diabetes, heart attack or lung or prostate cancer (disorders for which risk was decreased according to the report). Changes that included responses to both types of risk were coded as mixed.
The study was approved by the Institutional Review Boards of the University of Pennsylvania (PA, USA) and the Coriell Institute for Medical Research (NJ, USA).
Results
Participants
The survey was sent to 2155 physicians (777 family medicine and 1378 internal medicine physicians). Five hundred and twenty three physicians completed the survey, 502 of whom were qualified (21 respondents were excluded because they did not indicate their specialty or because they did not currently see patients). The survey was therefore completed by 23.3% of physicians contacted (24.1% of family practitioners and 22.9% of internists).
Sociodemographic characteristics of respondents is included in Table 1. Approximately two out of three of respondents specialized in internal medicine and one out of three in family medicine. Nearly all respondents had been in practice for at least 10 years, reported seeing patients 4 or more days per week, seeing at least ten patients each day and maintained private office-based practices. The majority of respondents were male, white and 50 years of age or older. There were no significant differences between respondents and nonrespondents with regard to gender and age, the only variables available from KN about nonrespondents.
Table 1.
Respondent demographics†.
| Characteristic | n (%) |
|---|---|
| Specialty | |
| Internal medicine | 315 (62.8) |
| Family medicine | 187 (37.2) |
| Years in practice | |
| 0–9 | 22 (4.4) |
| 10–19 | 213 (42.4) |
| 20+ | 267 (53.2) |
| Age (years) | |
| <40 | 29 (5.8) |
| 40–49 | 160 (32.1) |
| 50–59 | 221 (44.3) |
| ≥60 | 89 (17.8) |
| Gender | |
| Male | 401 (80.4) |
| Female | 98 (19.6) |
| Race‡ | |
| American–Indian | 3 (0.6) |
| Asian | 90 (18.6) |
| Black | 15 (3.1) |
| Native Hawaiian | 4 (0.8) |
| White | 368 (76.0) |
| Other | 11 (2.3) |
n = 502.
Respondents could choose more than one racial identity.
Experience with genetics
Only 50% of respondents ordered a genetic test more than once a year, and only 16% ordered tests once a week or more. There was no statistically significant association between either age or years in practice and frequency of genetic test ordering (all p-values > 0.05). Of the 361 respondents who indicated which test(s) they most frequently ordered, slightly under half indicated that BRCA testing was most frequently ordered, followed by DNA testing for hemochromatosis (Table 2).
Table 2.
Experience with genetics†.
| Variable | n (%) |
|---|---|
| Frequency of genetic test ordering | |
| Never | 88 (17.5) |
| Once a year | 162 (32.3) |
| Once a month | 173 (34.5) |
| Once a week | 52 (10.3) |
| More than once a week | 27 (5.4) |
| Tests most frequently ordered‡ | |
| BRCA | 165 |
| Hemochromatosis | 65 |
| Carrier screening | 43 |
| HLA | 40 |
| Factor V Leiden/other tests for clotting disorders | 39 |
| Paternity | 14 |
| α-1-antitrypsin | 13 |
| Other | 38 |
| Confidence in interpreting results of genetic tests | |
| Not at all confident | 41 (8.2) |
| Somewhat unconfident | 58 (11.6) |
| Neutral | 112 (22.3) |
| Somewhat confident | 209 (41.6) |
| Very confident | 82 (16.3) |
| Type of genetic education§ | |
| CME/CEU course in genetics | 97 (19.3) |
| Self-directed education (journal reading and so on) | 160 (31.9) |
| Genetics course in medical school | 283 (56.4) |
| Grand rounds in genetics | 94 (18.7) |
| No genetics education | 99 (19.7) |
| Sufficiency of training in genetics | |
| Not at all sufficient | 90 (17.9) |
| Somewhat insufficient | 150 (29.9) |
| Neutral | 150 (29.9) |
| Sufficient | 101 (20.0) |
| More than sufficient | 11 (2.2) |
n = 502.
451 tests were named as most commonly ordered by 361 respondents.
Respondents could choose more than one.
CEU: Continuing education units; CME: Continuing medical education.
A total of 58% of respondents reported feeling confident in interpreting genetic test results (Table 2). A total of 20% had no genetics education, while 56% had a genetics course in medical school; only 22% felt their training in genetics was sufficient to work with their patients who have had genetic testing. Physicians ordering genetic tests at least once a month were more likely to feel confident interpreting test results (p < 0.001), and to report that their training in genetics was ‘sufficient’ (p < 0.001).
DTC genetic testing scenarios
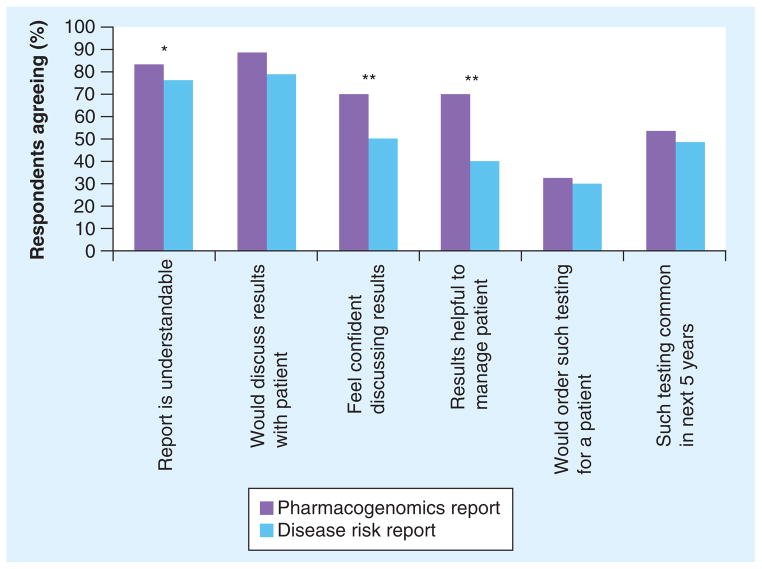
Most respondents believed the complex disease report was understandable and would be willing to discuss it with the patient (Figure 2). As an assessment of clinical utility, 40% agreed that such results would be helpful in patient management. A total of 49% of respondents agreed that this kind of testing will be commonplace in the next 5 years. Respondents who ordered genetic tests at least once a month were significantly more likely to agree that: the report is understandable (p = 0.007); they would feel confident discussing results (p = 0.024); such test results are helpful in patient management (p = 0.003); and they would order such testing for their patients (p = 0.005).
Figure 2. Responses to genetic testing scenarios.
From a 5-point Likert scale, ‘agree’ and ‘strongly agree’ responses were combined (n = 502).
*p < 0.01; **p < 0.001.
When presented with the DTC test report containing results for warfarin sensitivity, 83% of respondents agreed the report is understandable, and 88% would be willing to review the results with the patient (Figure 2). Although 70% believed the results would be helpful in managing the patient and therefore clinically useful, only 32% would order such testing before initiating therapy.
Nearly every physician (97%) reported starting a patient on warfarin in the past 2 years; however, most (89%) had not ordered a pharmacogenetic test to guide warfarin dosing. When asked in an open-ended question why they had not ordered pharmacogenetic testing before initiating warfarin therapy, 34% reported testing was not indicated, 27% were unaware of testing, 15% indicated testing was unavailable, 14% cited cost or lack of insurance coverage, and 7% said it was not standard of care.
Comparing genetic testing for susceptibility to common complex diseases with pharmocogenetic testing, providers were significantly more likely to agree that: the pharmacogenetic test report was understandable (p = 0.003); they would feel confident discussing pharmacogenetic results (p < 0.001); and pharmacogenetic results would be helpful in managing the patient (p < 0.0001).
Use of genomic test results in management decisions
After viewing the complex disease report, 43% of respondents indicated they would be likely or very likely to change the management of the hypothetical patient. Of the 213 respondents who would change patient management, approximately one-third did not mention the disorders they would address (in an open-ended question), and gave nonspecific responses (Table 3). Approximately one-third of respondents who would use the genomic results in management indicated they would address Alzheimer’s disease and/or glaucoma risk (for which increased risk was indicated in the report). For Alzheimer’s disease risk, respondents reported that they would conduct more frequent screening for early dementia, provide recommendations for risk reduction, or educate the patient about increased Alzheimer’s disease risk; several respondents would recommend ordering imaging (e.g., CT, MRI or PET scans). Respondents also indicated they would address the increased glaucoma risk through more frequent eye exams. The other one-third would address the risk of cardiovascular disease or Type 2 diabetes (for which decreased risk was indicated) by recommending lifestyle changes including increased exercise, weight loss and smoking cessation. Some would also recommend checking blood glucose levels regularly, lowering lipid levels or performing stress tests. No respondent would reduce screening in response to the reported reduced disease risk. Respondents were more likely to state they would change patient management when they indicated the report was understandable (OR: 2.26; p < 0.001) or that they felt confident discussing results (OR: 3.54; p < 0.001).
Table 3.
Reported modifications to management based on genomic testing for disease risk†.
| Modifications reported | n (%) | Example |
|---|---|---|
| None | 289 (57.6) | NA |
| Nonspecific | 59 (11.8) | “Emphasize regular exercise, proper nutrition, weight management and recommend medications if necessary” |
| Specific only to Alzheimer’s disease and/or glaucoma risk (increased risk stated in report) | 68 (13.6) | “Baseline and periodic rescreening using an Alzheimer’s screening tool; place reminder in chart to prompt me to remind him regarding annual eye exams including intraocular pressure testing” |
| Specific only to diabetes, heart disease and/or cancer risk (decreased risk stated in report) | 72 (14.3) | “I would look strongly on the patient’s risk for heart disease and diabetes and may start treatment for these disorders earlier. I would also counsel more for weight loss and be more vigilant in ordering screening and monitoring of blood sugar and lipids” |
| Specific to disorders with both increased and decreased risk | 14 (2.8) | “Do mini mental exam to check for early sign of dementia, monitor blood sugar and cholesterol on a regular basis” |
Modifications were reported on open-field responses and categorized by the authors (n = 502).
NA: Not applicable.
Experience with DTC genetic testing & attitudes towards genomic testing in general
Thirty-five respondents (7%) indicated that they had previously seen a patient’s DTC genetic risk assessment report, and only two reported that they personally had been tested.
Although 40% of respondents believed that genetic testing for common disease risk currently offers clinically useful information, 57% believed that genomic medicine will improve clinical outcomes within 5 years (Table 4). A minority of respondents (45%) felt knowledgeable about the genetic basis of common diseases or about pharmacogenetics (35%), and only 37% reported being ready to take care of patients who have had genetic testing for complex diseases. Although 72% of respondents believed that patients will be interested in having genetic testing, only 46% agreed that it will motivate patients to adopt healthy behaviors. Sixty-eight percent reported being concerned that genetic testing will lead to insurance discrimination.
Table 4.
Attitudes towards genomic testing†.
| Statement | Strongly disagree (%) | Disagree (%) | Neither agree nor disagree (%) | Agree (%) | Strongly agree (%) |
|---|---|---|---|---|---|
| At this time, genetic testing for risk for common diseases offers information that is clinically useful | 3.0 | 17.3 | 39.4 | 35.1 | 5.2 |
| Within the next 5 years, genetic medicine will improve clinical outcomes | 1.2 | 6.6 | 35.4 | 46.4 | 10.4 |
| I am knowledgeable about the genetic basis of common disease | 2.2 | 21.3 | 31.1 | 42.0 | 3.4 |
| I am knowledgeable about pharmacogenetics | 5.8 | 23.7 | 35.4 | 30.9 | 4.2 |
| I feel ready to take care of patients who have had genetic testing for complex diseases | 3.8 | 24.1 | 34.9 | 31.6 | 5.6 |
| Patients will be interested in having genetic testing | 0.4 | 3.2 | 24.7 | 57.3 | 14.4 |
| Within the next 5 years, medical insurance will cover the cost of genetic testing for disease risk | 9.6 | 29.1 | 35.4 | 22.1 | 3.8 |
| Genetic testing will motivate my patients to adopt healthy behaviors | 2.2 | 16.5 | 34.9 | 40.6 | 5.8 |
| I am concerned that genetic testing will lead to insurance discrimination | 2.0 | 5.6 | 24.1 | 46.0 | 22.3 |
n = 502.
There were no significant differences in responses to the scenarios or attitudes about genomic testing by respondents’ specialty.
Discussion
We found that PCPs share the excitement expressed in the popular media and by members of the general public that genomic advances will eventually improve clinical outcomes [23,24]. They also share some of the public’s concerns about genomic medicine, especially with regard to potential insurance discrimination [25]. Although most respondents are willing to try to help patients understand genomic information, many remain skeptical both about their ability to interpret genomic information and the clinical utility of genomic information.
Consistent with the findings of other research [26,27], the majority of PCPs we surveyed do order genetic tests, albeit infrequently. Tests for mutations in BRCA1 and BRCA2 are most commonly ordered, potentially attributed in part to Myriad Genetics’ (UT, USA) marketing efforts to both healthcare consumers and providers [28,29]. Although physicians who order genetic tests infrequently are less confident in their ability to interpret the results of those tests, we cannot ascertain if lack of confidence leads to ordering fewer tests, or if less exposure to genetic testing reports results in feeling less confident.
Despite limited previous experience with genetic testing, most respondents would be willing to discuss a hypothetical patient’s DTC test results, agreed that the test reports (especially the pharmacogenetic report) are understandable and would be confident discussing the reported results. This finding is counter to other research showing that physicians generally do not feel prepared to discuss DTC testing with patients [11]. In the study by Powell et al., participants were not presented with a sample test report [11]. Therefore, perceived lack of preparation could relate to lack of familiarity with the types of reports DTC companies provide. By contrast, our study included a sample report which most participating providers believed to be understandable, likely contributing to providers’ confidence. In addition, providers may have felt more confident interpreting results for the disorders included in the report as they are likely addressed in routine risk assessment and counseling, albeit not using genetic testing.
As reported by Stanek et al., most respondents held positive views towards pharmacogenetic testing, believing that such testing, at least in theory, would be helpful in managing patients [30]. This is surprising considering that few respondents had ever ordered genetic testing before starting a patient on warfarin, and most would be reluctant to use such testing in hypothetical patients. Stanek et al. have suggested that although physicians are receptive to future use of pharmocogenomic testing, their failure to order testing relates to more practical barriers such as lack of awareness of how and when to order tests [30]. In our study, respondents cited those barriers, and also frequently believed that such testing was not indicated, possibly reflecting clinicians’ confidence in traditional approaches to warfarin management. Findings from previous surveys have documented providers’ concerns about the uncertainty and ambiguity of genomic findings in general [18] and skepticism about the clinical benefits of warfarin pharmacogenetic testing in particular [31].
Compared with the pharmacogenetic scenario results, respondents felt less prepared to deal with common disease risk information. Despite this, 43% of respondents cited modifications they would make to their management of the hypothetical patient based only on small relative risks of disease. Few other studies are available reporting on what actions PCPs actually take or might take in response to patients’ DTC testing for risk of common diseases. One study reported that only one of five PCP who had patients bring in reports of DTC testing modified management in response to test results [11]. Another study found that the majority of medical students would recommend increased screening in response to DTC test results on a hypothetical patient presenting with test results indicating a modestly increased risk of either breast cancer or macular degeneration [32].
Of concern, some respondents would increase screening in response to a reported decreased disease risk, implying confusion about the risk data supplied. This finding supports previously reported concerns about provider misunderstanding of probabilistic risk information [33,34], and provider response to DTC testing by ordering unnecessary follow-up procedures [33]. Although most recommendations the respondents made followed general public health guidelines (lose weight, stop smoking and so on), a few providers would order tests that were not indicated clinically based on the sample report supplied.
The need for additional physician education about the clinical applications of new genomic technologies and knowledge is unquestioned [2,35–37]. To date, genetic education has focused on diseases with Mendelian inheritance, ignoring the contribution of genetic variation throughout the genome to complex disease risk and drug response [36]. In addition to traditional educational approaches through didactic courses in medical school or CME courses, several studies have explored more innovative ways to expand PCPs knowledge of genomics. One somewhat controversial educational approach to increase provider knowledge of and understanding of probabilistic results from common disease testing is offering physicians their own genotyping [27,32,34]. Despite these innovative approaches to education, neither personal experience nor clinician education alone is likely to foster widespread adoption of genomic medicine. Clinical practice guidelines, evidence-based outcomes data, and evidence of the efficacy of protections against breaches of confidentiality and discrimination, such as those addressed by the Genetic Information Nondiscrimination Act of 2008, will be needed [11,38].
Our study has several limitations. First, the sample was composed of members of Knowledge Network’s PCN, who are slightly older and more male compared with US PCPs in general. Second, our participation rate was low, but typical of the response rate to other web-based surveys of physicians [39], and far exceeding the response rate of other physician surveys on similar topics [11,30,40]. Third, with any survey, issues of response bias should be considered, where respondents are different in important ways from nonrespondents; in this study, participation was unlikely to be biased based on interest in or knowledge about genetics since providers were not informed of the topic during recruitment and respondents were representative of the PCN panel. Fourth, we used hypothetical scenarios to understand how providers might respond to an actual patient. Responses to hypothetical vignettes may not mirror actual behavior [41,42]. In addition, the scenarios and report excerpts were short and relatively uncomplicated. Physicians may respond differently to reports that are many pages long, that include actual genotypes or SNP numbers, or that include results for hundreds of conditions and traits. Finally, we did not provide a definition of genetic testing, so providers may have interpreted this term in diverse ways. However, an open-ended question about genetic tests ordered suggests respondents were using similar and accepted definitions of genetic testing.
Conclusion
Despite these limitations, our results provide insight on how prepared PCPs are for incorporating personalized genomic information into patient care. Although most of the PCPs surveyed claimed to be fairly confident discussing results and found the reports understandable, some would make recommendations that are not justified by test results, particularly the very small increase in disease risk or the dearth of data supporting tests’ clinical utility. More research is needed on how providers can effectively provide recommendations that incorporate both genetic and nongenetic risk factors [43]. Additional research is also needed on how physicians react to actual patient genomic testing information, and on clinical and behavioral outcomes of testing.
Future perspective
For over a decade, there have been predictions of a genetics revolution leading to a new era of personalized medicine in which knowledge of a patient’s personal genomics will reveal predispositions or guide personalized treatments, leading patients and physicians to appropriate actions to reduce morbidity and mortality [44]. Unfortunately, outside of oncology, most patients have not been able to benefit from genetically targeted treatments and prevention. Ultimately, the extent to which genomic medicine diffuses into patient care is likely to depend on sufficient evidence of improved outcomes compared with current practices. As data on the comparative effectiveness of genomically informed interventions are gathered, physicians will need to be educated so they can interpret genomic data, including data obtained from DTC companies and educate patients about their genetic risk [45].
Supplementary Material
Executive summary.
Background
Genomic testing to predict disease risk and medication response is being obtained by healthcare consumers through direct-to-consumer (DTC) genetic testing companies.
People obtaining such testing are likely to seek guidance from their primary care physicians about their DTC test results.
Practicing physicians providing primary care to adults only infrequently order genetic tests and thus lack experience with interpreting genomic testing results.
Materials & methods
We conducted a web-based survey of primary care providers (general internists and family practitioners who are members of Knowledge Networks’ Physician Consulting Network).
The survey assessed physicians’ current experience with genetic testing, their assessment of the understandability and clinical utility of information in sample DTC reports for genomic assessment of disease risk and warfarin dosing, and attitudes toward genomic medicine.
Results
If presented by a hypothetical patient with a report about disease risk and medication response obtained from a DTC genetic testing company, most responding physicians would agree to review the report and believed the report to be understandable.
Although the majority of responding physicians believe that pharmocogenetic testing results would be helpful in patient management and would feel confident discussing results with patients, few had ever ordered a pharmacogenetic test for warfarin dosing.
Physicians feel less well prepared to deal with genomic information relating to the risk of common disease and are skeptical about the clinical utility of genomic information to predict disease risk.
The majority of primary care providers are concerned that genomic medicine will lead to insurance discrimination.
Discussion
Additional research is needed on how physicians react to actual patient genomic testing information, and on clinical and behavioral outcomes of testing.
Additional physician education about the clinical applications of new genomic technologies is needed.
Acknowledgments
The authors would like to thank K Armstrong, L Fishbein and A Owens for their assistance with developing the survey instrument.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by funding from the National Human Genome Research Institute, NIH (grant numbers 1RC1HG005369 and 1-50HG004487). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Greendale K, Pyeritz RE. Empowering primary care health professionals in medical genetics: how soon? How fast? How far? Am J Med Genet Semin Med Genet. 2001;106(3):223–232. doi: 10.1002/ajmg.10010. [DOI] [PubMed] [Google Scholar]
- 2▪.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299(11):1320–1334. doi: 10.1001/jama.299.11.1320. Reviews studies that have addressed the outcomes, consumer information needs, delivery and challenges in integrating genomic risk information into healthcare. [DOI] [PubMed] [Google Scholar]
- 3.Suther SG, Goodson P. Texas physicians’ perceptions of genomic medicine as an innovation. Clin Genet. 2004;65(5):368–377. doi: 10.1111/j.0009-9163.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- 4.Billings PR, Carlson RJ, Carlson J, et al. Ready for genomic medicine? Perspectives of health care decision makers. Arch Intern Med. 2005;165(16):1917–1919. doi: 10.1001/archinte.165.16.1917. [DOI] [PubMed] [Google Scholar]
- 5.Klitzman R, Chung W, Marder K, et al. Attitudes and practices among internists concerning genetic testing. J Genet Couns. 2012 doi: 10.1007/s10897-012-9504-z. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nippert I, Harris HJ, Julian-Reynier C, et al. Confidence of primary care physicians in their ability to carry out basic medical genetic tasks – a European survey in five countries – part 1. J Community Genet. 2011;2(1):1–11. doi: 10.1007/s12687-010-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman LC, Cooper HP, Webb JA, Weinberg AD, Plon SE. Primary care physicians’ attitudes and practices regarding cancer genetics: a comparison of 2001 with 1996 survey results. J Cancer Educ. 2003;18(2):91–94. doi: 10.1207/S15430154JCE1802_11. [DOI] [PubMed] [Google Scholar]
- 8.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 9.Foster MW, Sharp RR. The contractual genome: how direct-to-consumer genomic services may help patients take ownership of their DNA. Per Med. 2008;5(4):399–404. doi: 10.2217/17410541.5.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolor K, Liu T, St Pierre J, Khoury MJ. Health care provider and consumer awareness, perceptions, and use of direct-to-consumer personal genomic tests, United States, 2008. Genet Med. 2009;11(8):595. doi: 10.1097/GIM.0b013e3181b1cc2c. [DOI] [PubMed] [Google Scholar]
- 11.Powell KP, Cogswell WA, Christianson CA, et al. Primary care physicians’ awareness, experience and opinions of direct-to-consumer genetic testing. J Genet Couns. 2012;21(1):113–126. doi: 10.1007/s10897-011-9390-9. [DOI] [PubMed] [Google Scholar]
- 12.McGuire AL, Diaz CM, Wang T, Hilsenbeck SG. Social networkers’ attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9(6–7):3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leighton JW, Valverde K, Bernhardt BA. The general public’s understanding and perception of direct-to-consumer genetic test results. Public Health Genomics. 2012;15(1):11–21. doi: 10.1159/000327159. [DOI] [PubMed] [Google Scholar]
- 14.Gollust SE, Gordon ES, Zayac C, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15(1):22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. ‘It’s not like judgment day’: public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2012;21(3):423–432. doi: 10.1007/s10897-011-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman D, Bollinger JM, Dvoskin R, Scott J. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21(3):413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle – will we get our wish? N Engl J Med. 2008;358(2):105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 18▪.Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485–494. doi: 10.2217/pme.10.47. Qualitative study highlighting the opportunities and challenges related to the delivery of genomic medicine services. [DOI] [PubMed] [Google Scholar]
- 19.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;10:CD007275. doi: 10.1002/14651858.CD007275.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damani SB, Topol EJ. Emerging clinical applications in cardiovascular pharmacogenomics. Wiley Interdiscip Rev Syst Biol Med. 2011;3(2):206–215. doi: 10.1002/wsbm.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson KL. Genomics, health care, and society. N Engl J Med. 2011;365(11):1033–1041. doi: 10.1056/NEJMra1010517. [DOI] [PubMed] [Google Scholar]
- 22.Manolopoulos VG, Dechairo B, Huriez A, et al. Pharmacogenomics and personalized medicine in clinical practice. Pharmacogenomics. 2011;12(5):597–610. doi: 10.2217/pgs.11.14. [DOI] [PubMed] [Google Scholar]
- 23.Tambor ES, Bernhardt BA, Rodgers J, Holtzman NA, Geller G. Mapping the human genome: an assessment of media coverage and public reaction. Genet Med. 2002;4(1):31–36. doi: 10.1097/00125817-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Etchegary H, Miller F, deLaat S, Wilson B, Carroll J, Cappelli M. Decision-making about inherited cancer risk: exploring dimensions of genetic responsibility. J Genet Couns. 2009;18(3):252–264. doi: 10.1007/s10897-009-9218-z. [DOI] [PubMed] [Google Scholar]
- 25.Hahn S, Letvak S, Powell K, et al. A community’s awareness and perceptions of genomic medicine. Public Health Genomics. 2010;13(2):63–71. doi: 10.1159/000218712. [DOI] [PubMed] [Google Scholar]
- 26.Shields AE, Burke W, Levy DE. Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genet Med. 2008;10(6):404–414. doi: 10.1097/GIM.0b013e3181770184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haga SB, Carrig MM, O’Daniel JM, et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J Gen Intern Med. 2011;26(8):834–840. doi: 10.1007/s11606-011-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers MF, Chang MH, Jorgensen C, et al. Genetic testing for susceptibility to breast and ovarian cancer: evaluating the impact of a direct-to-consumer marketing campaign on physicians’ knowledge and practices. Genet Med. 2006;8(6):361–370. doi: 10.1097/01.gim.0000223544.68475.6c. [DOI] [PubMed] [Google Scholar]
- 29.Mouchawar J, Hensley-Alford S, Laurion S, et al. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: a naturally-occurring experiment. Genet Med. 2005;7(3):191–197. doi: 10.1097/01.gim.0000156526.16967.7a. [DOI] [PubMed] [Google Scholar]
- 30▪.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharm Ther. 2012;91(3):450–458. doi: 10.1038/clpt.2011.306. National survey highlighting the need for more effective physician education on the clinical value, availability and interpretation of pharmacogenomic tests. [DOI] [PubMed] [Google Scholar]
- 31.Kadafour M, Haugh R, Posin M, Kayser SR, Shin J. Survey on warfarin pharmacogenetic testing among anticoagulation providers. Pharmacogenomics. 2009;10(11):1853–1860. doi: 10.2217/pgs.09.117. [DOI] [PubMed] [Google Scholar]
- 32.Ormond KE, Hudgins L, Ladd JM, Magnus DM, Greely HT, Cho MK. Medical and graduate students’ attitudes toward personal genomics. Genet Med. 2011;13(5):400–408. doi: 10.1097/GIM.0b013e31820562f6. [DOI] [PubMed] [Google Scholar]
- 33▪▪.McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA. 2008;300(22):2669–2671. doi: 10.1001/jama.2008.803. Outlines how clinicians might appropriately prepare for the medical integration of clinically meaningful genomic test results obtained by direct-to-consumer testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke W, Evans JP. Teaching with single nucleotide polymorphisms: learning the right lessons. Genet Med. 2011;13(1):17–18. doi: 10.1097/GIM.0b013e3182049618. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Feero WG, Green ED. Genomics education for health care professionals in the 21st century. JAMA. 2011;306(9):989–990. doi: 10.1001/jama.2011.1245. Brief review of how clinicians will need to be educated about clinical applications of genomic discoveries so that patients will benefit from the genetic revolution. [DOI] [PubMed] [Google Scholar]
- 36.McInerney JD. Education in a genomic world. J Med Philos. 2002;27(3):369–390. doi: 10.1076/jmep.27.3.369.2977. [DOI] [PubMed] [Google Scholar]
- 37.Powell KP, Christianson CA, Cogswell WA, et al. Educational needs of primary care physicians regarding direct-to-consumer genetic testing. J Genet Couns. 2012;21:469–478. doi: 10.1007/s10897-011-9471-9. [DOI] [PubMed] [Google Scholar]
- 38.Laedtke AL, O’Neill SM, Rubinstein WS, Vogel KJ. Family physicians’ awareness and knowledge of the Genetic Information Non-discrimination Act (GINA) J Genet Couns. 2012;21(2):345–352. doi: 10.1007/s10897-011-9405-6. [DOI] [PubMed] [Google Scholar]
- 39.Braithwaite D, Emery J, de Lusignan S, Sutton S. Using the internet to conduct surveys of health professionals: a valid alternative? Fam Pract. 2003;20(5):545–551. doi: 10.1093/fampra/cmg509. [DOI] [PubMed] [Google Scholar]
- 40.Dodson C, Van Riper M. Analysis of clinicians’ attitudes towards pharmacogenomics. Per Med. 2011;8(5):533–540. doi: 10.2217/pme.11.43. [DOI] [PubMed] [Google Scholar]
- 41.Eccles MP, Hrisos S, Francis J, et al. Do self-reported intentions predict clinicians’ behaviour: a systematic review. Implement Sci. 2006;1:28. doi: 10.1186/1748-5908-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persky S, Kaphingst KA, Condit CM, McBride CM. Assessing hypothetical scenario methodology in genetic susceptibility testing analog studies: a quantitative review. Genet Med. 2007;9(11):727–738. doi: 10.1097/gim.0b013e318159a344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyeritz RE. The family history: the first genetic test, and still useful after all those years? Genet Med. 2012;14(1):3–9. doi: 10.1038/gim.0b013e3182310bcf. [DOI] [PubMed] [Google Scholar]
- 44▪.Offit K. Personalized medicine: new genomics, old lessons. Hum Genet. 2011;130:3–14. doi: 10.1007/s00439-011-1028-3. Suggests how the diffusion of new genetic technologies can be informed by the previous practice of genetic medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. doi: 10.1038/nature09764. Provides the current view of the National Human Genome Research Institute, NIH, for the future of genomic research and describes the path towards an era of genomic medicine. [DOI] [PubMed] [Google Scholar]
Website
- 101.Interim results: influenza A (H1N1) 2009 monovalent and seasonal influenza vaccination coverage among health-care personnel United States, August 2009–January 2010. 2010 www.cdc.gov/mmwr/pdf/wk/mm5912.pdf. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.