5′-AMP induces pharmacological torpor in mice, where lymphopenia is governed by body temperature-independent suppression of lymphocyte egress from lymph nodes.
Keywords: hibernation, anesthesiology, inflammation, suspended animation, metabolism
Abstract
Natural hibernation consists of torpid phases with metabolic suppression alternating with euthermic periods. Induction of torpor holds substantial promise in various medical conditions, including trauma, major surgery, and transplantation. Torpor in mice can be induced pharmacologically by 5′-AMP. Previously, we showed that during natural torpor, the reduction in body temperature results in lymphopenia via a reduction in plasma S1P. Here, we show that during torpor induced by 5′-AMP, there is a similar reduction in the number of circulating lymphocytes that is a result of their retention in secondary lymphoid organs. This lymphopenia could be mimicked by engagement of A2BRs by a selective A2BR agonist (LUF6210) in the absence of changes in temperature and prevented by A2BR antagonists during 5′-AMP-induced torpor. In addition, forced cooling of mice led to peripheral blood lymphopenia, independent of A2BR signaling. The induction of torpor using 5′-AMP impacted the migration of lymphocytes within and between secondary lymphoid organs. During torpor, the homing into LNs was impaired, and two-photon intravital microscopy revealed that cell motility was decreased significantly and rapidly upon 5′-AMP administration. Furthermore, the S1P plasma concentration was reduced by 5′-AMP but not by LUF6210. S1P plasma levels restored upon arousal. Likely, the reduced migration in LNs combined with the reduced S1P plasma level substantially reduces lymphocyte egress after injection of 5′-AMP. In conclusion, 5′-AMP induces a state of pharmacological torpor in mice, during which, lymphopenia is governed primarily by body temperature-independent suppression of lymphocyte egress from LNs.
Introduction
Torpor is a widely conserved behavior in which metabolism and hence, energy demands are decreased, which is presumed to allow animals to cope with periods of harsh environmental conditions with low food supply [1]. During deep torpor, the body temperature might be as low as 0–4°C [2], lasting from a few days up to 35 days, depending on the species [3–7]. In species exhibiting daily torpor, the minimum body temperature typically remains above 18°C [5, 8, 9]. Both types of torpor alternate with euthermic arousal periods. Specific adaptations are thought to be crucial in allowing animals to safely undergo these states of physiological extremes without signs of organ injury [10–15]. For instance, the superior resistance to ischemia and hypothermia of hibernating animals is suggested to play an important role in maintaining homeostasis [16–18]. In addition, changes in the immune system occur during torpor. These lead to the induction of a reversible, immunodeficient state, which might not only conserve energy but also may prevent tissue injury by limiting inflammatory responses [19]. Upon entrance into deep torpor, the number of circulating leukocytes drop by ∼90% in all hibernating mammals studied so far [19]. Previously, we showed that lowering of the body temperature during torpor causes lymphopenia through retention of lymphocytes in secondary lymphoid organs subsequent to a reduction in the plasma level of S1P [20]. Lowering the metabolic rate while maintaining homeostasis, with depression of the immune system as occurs during torpor, may be of benefit in a number of medical conditions, where ischemia and/or inflammation are involved in the induction of organ injury, including trauma, cardiac arrest, organ transplantation, major cardiac, and brain surgery, or in patients in the intensive care unit [21]. Metabolic suppression in donor organs may improve preservation of transplant organs compared with the currently used cold and static preservation methods that are hampered by cell swelling, acidosis, and the production of ROS upon reperfusion [22].
To date, the exact mechanism(s) leading to the induction of natural torpor are not fully understood, although fasting (e.g., as a result of scarce food supply in winter) might play an important role [1]. Indeed, fasting of mice housed in constant darkness induces a state of torpor that is characterized by a drop in body temperature and numb behavior. Torpor in fasted mice is associated with an increased plasma level of 5′-AMP [23]. Interestingly, injection of 5′-AMP in mice induces a similar state of torpor [23, 24]. Mechanisms suggested to be involved in the induction of torpor by 5′-AMP include the activation of AMPK [1, 24, 25], a principal cytoplasmic molecular energy sensor [26], and the reduction in cardiac output as a result of stimulation of adenosine receptors by 5′-AMP-derived adenosine [27]. Addition of extracellular AMP might induce a rise in intracellular AMP levels through activation of adenosine receptors, leading to the formation of cAMP (from ATP) in the cell, which can, in turn, be converted into AMP. In addition, nucleoside transporters can modulate uptake of adenosine into cells; its phosphorylation leads to increased levels of AMP in the cell [28]. Thus, 5′-AMP may be considered a pharmacological tool to induce torpor. Lowering of body temperature after injection of 5′-AMP might induce lymphopenia. Alternatively, 5′-AMP-derived adenosine might influence lymphocyte migration through activation of adenosine receptors. Adenosine signaling is governed by four adenosine receptors: A1, A2A, A2B, and A3 [29]. In the immune system, A2AR is the predominant receptor expressed on lymphocytes, whereas A2AR and A2BR are expressed by APCs. Upon activation, expression of A2AR is up-regulated, and its activation selectively decreases the production of cytokines [30]. The best-characterized effect of the impact of adenosine on lymphocyte recirculation has been its impact on homing via its effect on HEVs. Activation of A2BR on HEVs reduces lymphocyte migration [31]. Here, we assessed (1) whether injection of 5′-AMP in mice affects lymphocyte recirculation and (2) whether these effects are mediated by activation of A2BR or lowered body temperature.
MATERIALS AND METHODS
Animals
C57BL/6 and CD45.1 congenic mice were housed under standard light:dark conditions (12:12) in the animal facilities of the University of Groningen (The Netherlands) and the NIH (Bethesda, MD, USA). C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA, or Harlan Netherlands B.V., The Netherlands), whereas CD45.1+ B6 mice were obtained from the National Institute of Allergy and Infectious Diseases contract colony at Taconic Farms (Hudson, NY, USA). Prior to experiments, animals were fed ad libitum using standard animal lab chow. All animal experiments were approved by the Animal Experimental Committees of the University of Groningen or the NIH.
Torpor induction by 5′-AMP
Torpor was induced pharmacologically by injecting 7.5 mmol/kg 5′-AMP (Sigma-Aldrich, St. Louis, MO, USA) in 0.9% saline (pH 7.5) i.p. To record body temperature during experiments, we surgically implanted miniaturized iButtons i.p. (Maxim Integrated, San Jose, CA, USA) and allowed mice to recover for a period of 3 weeks before torpor induction or measured the body temperature using a rectal probe (Physitemp Instruments, Clifton, NJ, USA). Mice were killed at different times after injection of 5′-AMP. The minimum body temperature during torpor was reached at 4–5 h following 5′-AMP injection, and full arousal with normalization of body temperature occurred by 10 h after 5′-AMP administration. Euthermic animals served as controls and were killed at the time that other animals were injected with 5′-AMP. At their death, animals were anesthetized using 3% isoflurane/oxygen, and ∼800 μl blood was drawn immediately by cardiac puncture into small EDTA-coated tubes. Automated hematological analysis was performed within 5 h using a Sysmex XE-2100 [32, 33] that was validated by manual counting of a Giemsa-stained blood smear. The remaining blood was collected for MS measurement of sphingosine/S1P in a polypropylene tube, mixed 1:10 v/v with a solution that minimizes platelet activation and contained PGE1 (94 nmol/l; Sigma-Aldrich), Na2CO3 (0.63 mmol/l; Sigma-Aldrich), EDTA (90 mM; Titriplex; Sigma-Aldrich), and theophylline (10 mM; Sigma-Aldrich). These samples were centrifuged (30 min, 17,000 g, 4°C), snap-frozen in liquid nitrogen, and stored at −80°C.
T cell LN homing assay
To investigate the impact of 5′-AMP on T cell homing to LNs, CD45.1+ T cells were purified by negative selection (MACS; Miltenyi Biotec, Auburn, CA, USA), and 1 × 107 cells were adoptively transferred i.v. into congenic C57BL/6 (CD45.2+) recipients. Simultaneously, 0.9% saline or 7.5 mmol/kg 5′-AMP was injected i.p. Two hours later, inguinal, axillary, and mesenteric LNs were collected from each recipient, cell suspensions made by homogenization through 70-μm cell strainers (BD Biosciences, San Jose, CA, USA), cell numbers enumerated, and cell fractions analyzed by flow cytometry. To assess whether blockade of T cell homing from blood to LNs prevented 5′-AMP-induced lymphopenia, LN entry was blocked in mice by i.p. administration of 100 μg anti-αL-integrin (clone M17/4) and 100 μg anti-α4-integrin (clone PS/2; both from Bio X Cell, West Lebanon, NH, USA) antibodies in PBS, 2 h prior to 5′-AMP injection [34]. Blood samples were drawn, as described above, 4 h after torpor induction.
Two-photon imaging data acquisition and analysis
Surgical preparation of the inguinal or popliteal LN was performed using a protocol modified from previous reports [35, 36], after having injected 5 × 106 purified T cells labeled with 1μM 5-chloromethylfluorescein diacetate (Invitrogen, Carlsbad, CA, USA), 18–24 h prior. Mice were anesthetized with 1–1.5% isoflurane (Baxter, Deerfield, IL, USA), vaporized in a 80:20 mixture of O2 and air during the surgery and microscopy. To inject 5′-AMP during imaging, a catheter was inserted i.p. via a 29.5GA insulin needle attached to plastic, 0.01-inch-wide microbore tubing (Tygon) and secured with Durapore tape (Fisher Scientific, Waltham, MA, USA) prior to placing the mouse under the microscope. Images were acquired on an LSM 710 NLO multiphoton imaging system (Carl Zeiss Microimaging, Thornwood, NY, USA), enclosed in a custom-built environmental chamber kept at 35°C through a 20× water-immersion lens (numerical aperture 1.0) and fluorescent excitation provided by a Chameleon Ultra II Ti Sapphire laser (Coherent, Santa Clara, CA, USA), tuned to 800-nm wavelength. Imaging planes were collected at 3-μm steps to form Z-stacks that were repeated every 30 s to yield 4-dimensional datasets that were processed with Imaris (Biplane, South Windsor, CT, USA) and analyzed in MatLab (MathWorks, Natick, MA, USA), as described previously [37] to obtain cell velocities. After Effects (Adobe Systems, San Jose, CA, USA) was used to produce video clips.
Flow cytometry
PBMCs were isolated from blood samples with erythrocyte lysis buffer (0.83% w/v ammonium chloride in H2O, incubated for 10 min on ice) or by spinning through a histopaque gradient, according to the manufacturer's protocol (Sigma-Aldrich). Cells were stained using anti-mouse antibodies CD44 (IM7), CD3ϵ (145-2C11), B220 (RA3-6B2), and CD19 (1D3), purchased from eBioscience (San Diego, CA, USA). All samples were acquired on a LSR II flow cytometer (BD Biosciences) and data analyzed using FlowJo (TreeStar, Ashland, OR, USA).
Measurement of calcium influx in lymphocytes
Mouse splenocytes were isolated, and erythrocytes were lysed using erythrocyte lysis buffer (0.83% w/v ammonium chloride in H2O, incubated for 10 min on ice). Next, cells were labeled with 5 μM Fluo-4 (Invitrogen) in 4 ml HBSS and incubated at 37°C for 20 min. Cells were washed in complete RPMI and then incubated in complete RPMI for another 20 min at 37°C. After washing cells 2× with PBS, they were divided into FACS tubes and labeled using anti-mouse antibodies CD3ϵ (145-2C11) and B220 (RA3-6B2), purchased from eBioscience. Cells were kept at 37°C until acquisition on a LSR II flow cytometer (BD Biosciences). Cells were acquired for 1 min before and 3 min after adding saline, 1 μM ionomycin, or 7.5 μmol 5′-AMP. Data were analyzed using FlowJo (TreeStar).
LC-ESI-MS/MS
Sphingolipids were extracted and analyzed by LC-ESI-MS/MS on a PE-Sciex API 3000 triple-quadrupole mass spectrometer, equipped with a turbo ionspray source, as described previously [38, 39]. HPLC separation was performed as described previously [40], with the following changes: an Alltima C18 column (2.1×150 mm, 5 μ; Grace Davison Discovery Sciences, Deerfield, IL, USA) was used at a flow rate of 200 μl/min. N2 was used as the nebulizing gas and drying gas for the turbo ionspray source. The ion spray needle was held at 5500 V; the orifice temperature was set to 500°C. N2 was used to induce dissociations collisionally in Q2. MRM scans were acquired by setting Q1 and Q3 to pass the precursor and product ions of the most abundant sphingolipid molecular species. MRM transitions were optimized for each individual component (C-17SoP: 366.2/250.4; C-17SaP: 368.2/270.4; C-18SoP: 380.2/264.4; C-18SaP: 382.2/284.4; C-17So: 286.2/238.1; C-17Sa: 288.2/240.1; C-18So: 300.2/252.3; C18Sa: 302.2/254.2). Quantitation was achieved by spiking the samples before extraction with sphingosine (d17:1), sphinganine (d17:0), S1P (d17:1), and sphinganine-1-phosphate (d17:0; Avanti Polar Lipids, Alabaster, AL, USA).
Statistical analysis
To calculate statistical differences, SPSS 20.0 for Windows workstations was used. Data were analyzed using a one-way ANOVA, and after testing for homogeneity of variances, post hoc testing was performed using least significant difference or Games-Howell. In the case where fewer than three groups were to be compared, an independent-samples Student's t-test or Mann-Whitney test was used. To estimate the effect of different dosages, an ANOVA linear trend analysis was applied. In all situations, P < 0.05 was considered significantly different. Data are presented as means ± sem.
RESULTS
Administration of 5′-AMP reduces the body temperature and induces peripheral blood lymphopenia
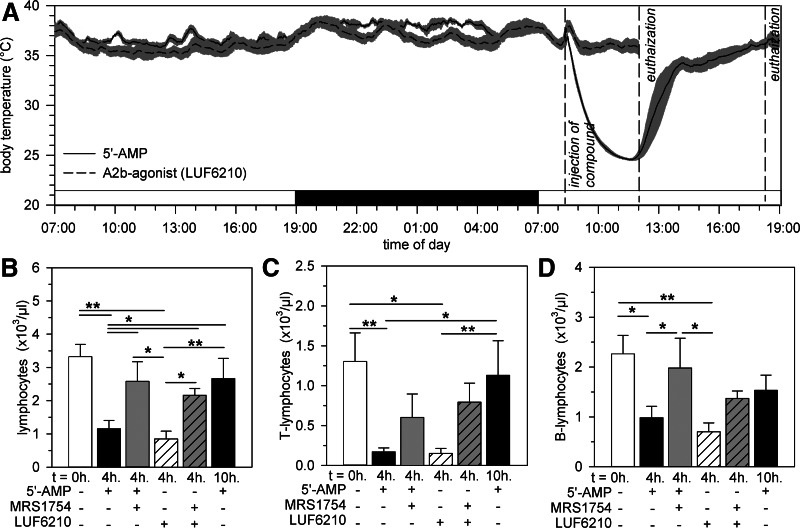
As described previously [23], injection of 5′-AMP into mice induces a torpor-like state, characterized by numb behavior and a profound reduction of body temperature. After injection of 7.5 mmol/kg 5′-AMP, body temperature gradually reduced from 34.5 ± 0.4°C to 23.4 ± 0.3°C at 4 h, after which, a biphasic increase toward euthermia was observed (Fig. 1A). At 4 h after injection of 5′-AMP, the number of circulating lymphocytes decreased significantly (P<0.01; Fig. 1B). Flow cytometric analysis revealed that the number of T lymphocytes (P<0.01; Fig. 1C) and B lymphocytes (P<0.05; Fig. 1D) was reduced significantly in the blood. The numbers of circulating T and B lymphocytes were restored by 10 h following 5′-AMP to values not significantly different from those measured prior to injection (Fig. 1B–D). In contrast to lymphocytes, 5′-AMP-induced torpor did not affect the number of circulating erythrocytes, monocytes, or neutrophils (Supplemental Fig. 1). Upon arousal, however, the number of neutrophils was increased strongly, whereas the number of erythrocytes and monocytes remained unaffected. Hence, injection of 5′-AMP leads to a significant drop in body temperature and is associated with a reversible reduction in the number of circulating T and B lymphocytes.
Figure 1. 5′-AMP-induced torpor results in the reversible reduction in the number of circulating T and B lymphocytes as a result of the activation of A2BR and independently of changes in body temperature.
Body temperature of mice prior to and following torpor induction by 5′-AMP (7.5 mmol/kg) or injection of the A2b agonist LUF6210 (3 mg/kg; A). The number of circulating total lymphocytes (B), T lymphocytes (C), or B lymphocytes (D) in mice at the indicated time following administration of 5′-AMP, the specific A2BR antagonist MRS1754 (12.5 mg/kg), and/or the A2b agonist LUF6210. Bars represent mean ± sem; */** = P < 0.05/0.01, respectively (n=6–9 animals/group).
Activation of A2BRs induces peripheral blood lymphopenia independent of lowered body temperature
Given that 5′-AMP can be dephosphorylated to adenosine in vivo, activation of adenosine receptors might play a role in the observed lymphopenia by 5′-AMP in mice [31]. The A2BR is the only adenosine receptor expressed on the HEV-like cell line KOP2.16 and reduces lymphocyte migration in vivo upon activation by adenosine [31]. To investigate whether activation of A2BRs might play a role in the induction of lymphopenia by 5′-AMP, we injected a selective A2BR antagonist (12.5 mg/kg MRS1754; binding constant KD=3.39±0.18 nM) [41] to block effects of 5′-AMP-derived adenosine mediated through A2BR, 15 min prior to injection of 5′-AMP. Pretreatment with MRS1754 did not affect the reduction of body temperature of mice induced by 5′-AMP, which was 24.8 ± 1.1°C at 4 h after injection of 5′-AMP (P>0.05 vs. 5′-AMP only). However, MRS1754 did prevent the induction of lymphopenia by 5′-AMP (P<0.05 vs. 5′-AMP only; Fig. 1B–D). To further examine the role of A2BRs in the induction of lymphopenia, we injected a selective A2B agonist (3 ng/kg LUF6210; commercially available as Bay60-6583; binding constant KD=0.33±0.08 nM) [41] in the absence of 5′-AMP and measured the number of circulating lymphocytes, 4 h after injection. Injection of the A2B agonist LUF6210 did not affect the body temperature (Fig. 1A), which was 37.7 ± 0.1°C at 4 h after injection and was not significantly different from the body temperature before injection. Similar to injection with 5′-AMP alone, administration of only LUF6210 reduced the total number of circulating lymphocytes (P<0.01; Fig. 1B), as a result of a decrease of T and B lymphocytes in the blood (P<0.01; Fig. 1C and D). In addition, MRS1754 pretreatment prevented the reduction of circulating lymphocytes induced by LUF6210 (Fig. 1B–D). The effects on circulating blood cell numbers following LUF6210 and/or MRS1754 administration are specific to lymphocytes, as neither LUF6210 alone nor MRS1754, combined with LUF6210 or 5′-AMP, induced a significant change in the number of circulating erythrocytes, neutrophils, or monocytes (Supplemental Fig. 1). To unravel whether effects of 5′-AMP might be mediated through A2BRs on lymphocytes or on other cells, such as endothelial cells, in HEVs, we incubated murine T and B lymphocytes in vitro with 5′-AMP (Supplemental Fig. 2). Although incubation of lymphocytes with 1 μM ionomycin induced a rapid and significant rise in intracellular calcium, incubation of lymphocytes with 7.5 μM 5′-AMP did not seem to induce a calcium influx. Likely, effects of 5′-AMP are mediated through activation of A2BRs on endothelial cells of HEVs rather than lymphocytes. Although 5′-AMP and adenosine can activate A1R, hydrolysis of 5′-AMP to adenosine is necessary for activation of A2BR [42]. Taken together, activation of A2BR, by a selective agonist or 5′-AMP-derived adenosine, resulted in the reduction of the number of circulating T and B lymphocytes, without impacting body temperature.
5′-AMP-induced peripheral blood lymphopenia occurs even in the absence of reductions in body temperature
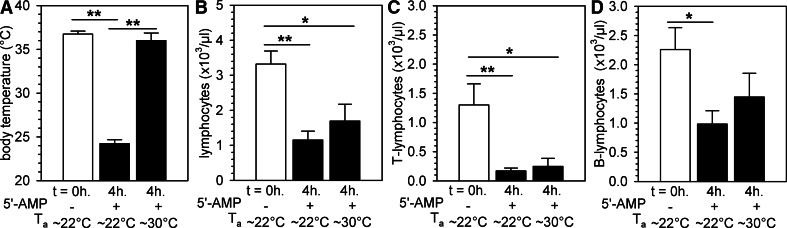
As data from the experiment described above suggest that lymphopenia, during 5′-AMP-induced torpor, might be secondary to temperature-independent activation of A2BR, we further elucidated the role of body temperature on lymphopenia during 5′-AMP-induced torpor in mice. To do so, we housed animals injected with 5′-AMP at an Ta of ∼30°C to preclude the reduction in body temperature by 5′-AMP. Numb behavior of the animals was readily observed upon 5′-AMP injection but lasted only ∼2 h at an Ta of ∼30°C, possibly caused by more rapid metabolism of 5′-AMP. To allow torpor to continue for up to 4 h at this higher Ta, we injected a second dose of 1.9 mmol/kg 5′-AMP i.p. (25% of the initial dose), 2 h after injection of the initial dose. In animals kept at ∼30°C, we found that although 5′-AMP did not affect body temperature (Fig. 2A), the number of circulating lymphocytes was reduced compared with baseline (P<0.05; Fig. 2B). This was largely a result of a decrease in the number of T lymphocytes (Fig. 2C), as the number of B lymphocytes was not affected significantly (Fig. 2D). Furthermore, the number of circulating erythrocytes and monocytes was unaltered, and we observed increased neutrophil blood counts in animals housed at a higher Ta following injection of 5′-AMP (Supplemental Fig. 3). In summary, the reduction in the number of circulating T lymphocytes as a consequence of injection of 5′-AMP is likely the result of the activation of A2BRs and can occur independently of lowered body temperature.
Figure 2. 5′-AMP-induced peripheral blood lymphopenia occurs independent of hypothermia.
Body temperature prior to (0 h) and 4 h following torpor induction by 5′-AMP administration in mice kept at room temperature (∼22°C) or at the higher Ta of ∼30°C (A). Total lymphocyte (B), T lymphocyte (C), and B lymphocyte (D) counts in peripheral blood of mice kept at Ta of ∼22°C or 30°C prior to and 4 h after 5′-AMP administration. Mice kept at Ta = 30°C were given two doses of 5′-AMP at the start of the experiment (t=0; 7.5 mmol/kg) and 2 h (1.9 mmol/kg). Bars represent mean ± sem; */** = P < 0.05/0.01, respectively (n=3–9 animals/group).
Forced hypothermia leads to a concomitant decrease in the number of circulating lymphocytes
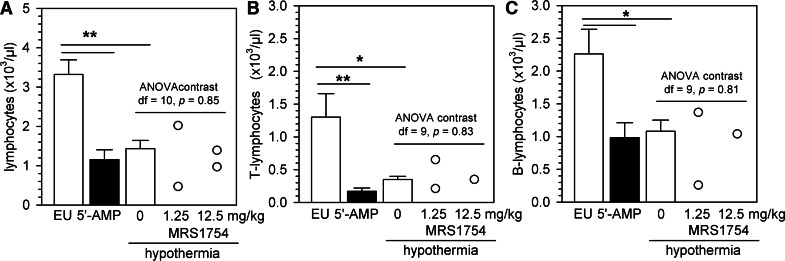
Although activation of A2BRs induced peripheral blood lymphopenia without affecting body temperature, this finding does not necessarily imply that a drop in body temperature cannot cause lymphopenia in the absence of A2BR engagement. To assess whether a reduction in body temperature affects the number of blood lymphocyte numbers, we cooled anesthetized mice using ice packs and measured the number of circulating lymphocytes, 20 min after a body temperature of ∼24°C was reached. Similar to what was observed after injection of 5′-AMP, forced hypothermia led to a reduction in the number of lymphocytes, which seemed more prominent for T lymphocytes than for B lymphocytes (P<0.05; Fig. 3A–C). Cooling did not affect the number of circulating erythrocytes, neutrophils, and monocytes (Supplemental Fig. 4). To assess whether A2BRs might be involved in cooling-induced lymphopenia, we injected animals with 1.25 mg/kg or 12.5 mg/kg of the A2BR antagonist MRS1754, 30 min prior to forced hypothermia. We found no significant effects of MRS1754 on the reduction in the number of circulating lymphocytes in hypothermic animals (Fig. 3A–C), demonstrating that A2BRs are not involved in the peripheral blood lymphopenia induced by hypothermia in the absence of 5′-AMP administration.
Figure 3. Forced hypothermia leads to a decrease in the number of circulating lymphocytes.
The peripheral blood lymphocyte (A), T lymphocyte (B), and B lymphocyte (C) counts were measured in euthermic controls (EU), 5′-AMP-treated mice, or anesthetized mice, whose body temperature was lowered to ±24°C. Mice in forced hypothermia were injected with saline or with an A2BR antagonist (1.25 mg/kg and 12.5 mg/kg MRS1754), 30 min prior to cooling. Bars represent mean ± sem. Circles represent data from individual mice; */** = P < 0.05/0.01, respectively (n=7–9 animals/group where bars are shown). df, Degrees of freedom.
Restoration of lymphocyte counts during return to euthermia after torpor requires lymphocyte egress from secondary lymphoid organs
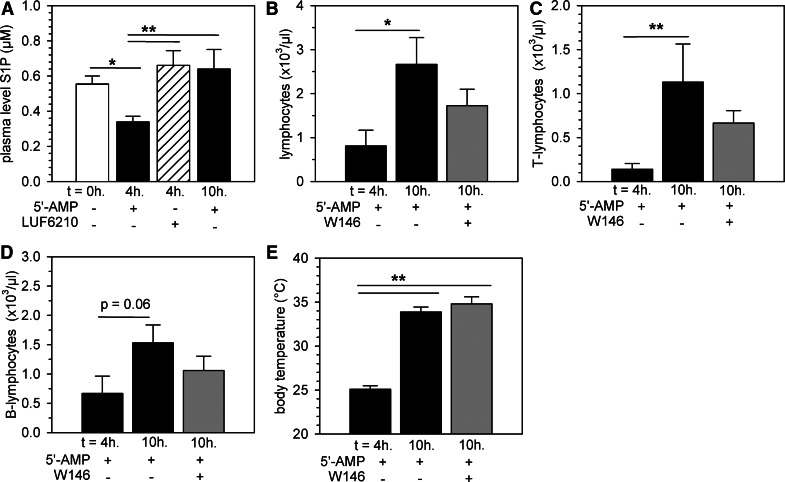
Plasma S1P levels influence lymphocyte egress from lymphoid organs through S1P1Rs expressed on lymphocytes [43] and thus, can impact the number of circulating lymphocytes. Previously, we showed that the drop in the number of circulating lymphocytes during deep torpor in hibernating hamsters is associated with a lowered plasma level of S1P [20]. For this reason, we measured the plasma S1P levels after injection of 5′-AMP in mice and determined whether restoration of normal numbers of circulating lymphocytes is associated with changes in plasma concentrations of S1P. Injection of 5′-AMP in mice significantly reduced the plasma S1P level by ∼40% at 4 h after injection (P<0.05 compared with baseline). S1P levels were fully normalized during return to euthermia after torpor at 10-h post-5′-AMP administration (P<0.05 compared with 4 h; Fig. 4A). Injection of the A2BR agonist LUF6210 alone did not affect S1P levels (Fig. 4A), suggesting that S1P-level decreases might be the result of decreases in metabolic rate during torpor rather than a result of the activation of A2BRs. To test whether lymphocyte egress from secondary lymphoid organs via chemotactic responses to S1P [43] might play a role in the restoration of the number of circulating lymphocytes upon arousal, we injected the S1P1-selective antagonist W146 at 6 h following injection of 5′-AMP, i.e., in the phase where mice return to euthermia. Injection of W146 partly precluded the restoration of circulating lymphocyte numbers, as the number of circulating lymphocytes upon arousal, following injection of W146, was not significantly different from torpor or arousal in the absence of W146 (Fig. 4B). Restoration of a normal number of lymphocytes upon rewarming was mainly a result of an increase in the number of circulating T lymphocytes (Fig. 4C), as the number of B lymphocytes was not yet increased significantly at 10 h compared with 4 h after injection of 5′-AMP (Fig. 4D). As expected, the S1P1-selective antagonist did not affect restoration of body temperature (Fig. 4E) or the number of circulating erythrocytes or monocytes (Supplemental Fig. 5A and C), although it prevented the increase in the number of circulating neutrophils upon arousal (Supplemental Fig. 5B). Taken together, these data suggest that during 5′-AMP-induced torpor, lymphocytes are trapped within secondary lymphoid organs as a result of activation of A2BRs and potentially, additional effects induced by the lowered S1P plasma level, whereas restoration of lymphocyte counts following a return to euthermia after torpor requires lymphocyte egress via S1P sensing.
Figure 4. Restoration of lymphocyte counts during return to euthermia after torpor requires lymphocyte egress from secondary lymphoid organs.
Plasma S1P levels in mice prior to (0 h), 4 h, or 10 h following torpor induction with 5′-AMP (7.5 mmol/kg) or at 4 h after administration of the A2BR agonist LUF6210 (3 mg/kg; A). Peripheral blood lymphocyte counts (B), T lymphocyte counts (C), and B lymphocyte counts (D) in mice during torpor (4 h post-5′-AMP administration) or following return to euthermia in mice (10 h post-5′-AMP administration) that were given the S1P1-specific antagonist W146 (10 mg/kg) compared with untreated controls. Bars represent mean ± sem; */** = P < 0.05/0.01, respectively (n=6–9 animals/group).
5′-AMP-induced torpor reduces lymphocyte recirculation rates and causes the retention of lymphocytes within secondary lymphoid organs
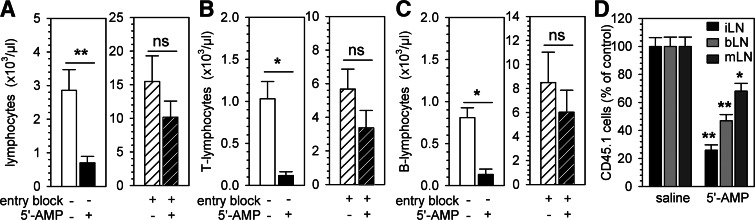
To further examine whether retention of lymphocytes in secondary lymphoid organs is necessary for the induction of peripheral blood lymphopenia after injection of 5′-AMP, we blocked homing to LNs by injecting neutralizing antibodies against α4/αL-integrins prior to the induction of torpor. α4/αL-Integrins are expressed on lymphocytes and are required for LN entry [34]. Blocking lymphocyte homing to LNs resulted in an increased number of circulating T and B lymphocytes, 2 h later, prior to the injection of 5′-AMP. The subsequent induction of torpor in mice pretreated with anti-α4/αL-integrin antibodies did not lead to a reduction in the number of circulating lymphocytes at 4 h following injection of 5′-AMP (P<0.05; Fig. 5A–C). Therefore, sequestration of T and B lymphocytes in secondary lymphoid organs, which is precluded by blocking lymphocyte homing, as described above, is essential for the induction of lymphopenia by 5′-AMP.
Figure 5. 5′-AMP-induced torpor reduces lymphocyte recirculation rates and causes retention of lymphocytes in secondary lymphoid organs.
LN homing of lymphocytes was blocked by the administration of anti-α4- and anti-αL-integrin antibodies, 2 h prior to 5′-AMP (7.5 mmol/kg) or saline injection and the number of total lymphocytes (A), T lymphocytes (B), or B lymphocytes (C) measured in the peripheral blood. To assess the change in homing efficiency of T cells during torpor, congenic CD45.1+ T cells were adoptively transferred to recipients given 5′-AMP and the number of CD45.1+ cells counted, 2 h post-transfer in inguinal (iLN), axillary (aLN), and mesenteric (mLN) LNs; the homing efficiency of adoptively transferred T cells is shown as the percent of cells relative to saline controls (D). Bars represent mean ± sem; */** = P < 0.05/0.01, respectively (n=4–6 animals/group).
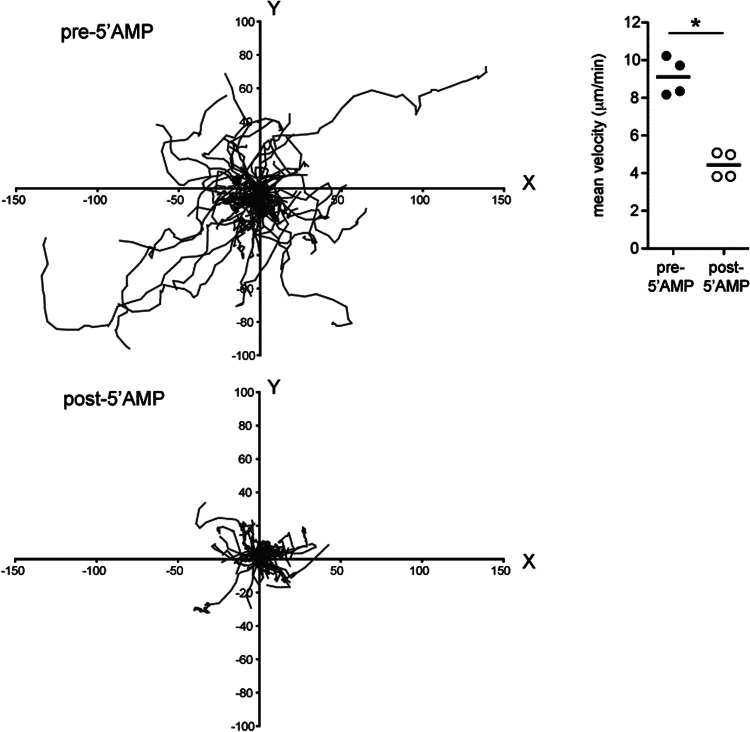
Given that the augmentation of lymphocyte homing to lymphoid organs or the decreased egress of lymphocytes from lymphoid organs back into the blood, or both, could lead to a reduction in the number of circulating lymphocytes, we investigated these two processes separately to assess how lymphocyte recirculation is altered during torpor. First, to quantify the effects of 5′-AMP on LN homing of lymphocytes, we adoptively transferred CD45.1+ T lymphocytes at the same time that 5′-AMP or saline was administered and assessed the number of transferred cells that was found in the LNs of recipients, 2 h after injection in torpid versus euthermic mice. We found that 5′-AMP significantly reduced the homing of CD45.1+ T lymphocytes to peripheral and mesenteric LNs (P<0.05; Fig. 5D). Thus, although lymphopenia is a result of sequestration of T lymphocytes within secondary lymphoid organs following administration of 5′-AMP (and therefore, depends on homing), sequestration of lymphocytes is unlikely to result from an increased homing rate of T lymphocytes. These data suggest that sequestration of lymphocytes within secondary lymphoid organs during torpor is the result of reduction in the egress of lymphocytes from secondary lymphoid organs. In part, this may be a result of changes in S1P plasma concentrations, as described above. Alternatively, we hypothesized that a lowered migration rate of lymphocytes inside LNs might impair the migration of lymphocytes toward exit sites and hence, lead to a reduced egress rate. To investigate this, we used two-photon microscopy and examined the dynamic behavior of fluorescently labeled T lymphocytes within the inguinal LN prior to and following the injection of 5′-AMP. To rule out effects of temperature, mice were kept at ∼35°C during intravital imaging. Injection of 5′-AMP significantly reduced the motility of T lymphocytes inside inguinal LNs (P<0.01; Fig. 6 and Supplemental Movie 1). The change in the dynamic behavior of T lymphocytes after 5′-AMP administration occurred within a few minutes of torpor induction and likely impacts the probability of cells migrating to cortical and medullary sinuses to exit back into the lymph [44]. Taken together, lymphopenia induced by 5′-AMP is a result of retention of lymphocytes in LNs because of a decreased egress rate. Likely, the reduced motility of lymphocytes in LNs and potentially also, the lowered S1P plasma level inhibit lymphocyte egress after injection of 5′-AMP.
Figure 6. In vivo decrease in motility of T lymphocytes within the LN following 5′-AMP-induced torpor.
Tracks of 75 CD4 T cells in the inguinal LN, prior to and following torpor induction by 5′-AMP (7.5 mmol/kg) administration in mice kept at a body temperature of ∼35°C. x and y coordinates (given in μm) are shown for each cell, normalized such that all starting coordinates are set at 0,0 (left). Summary of mean velocity of CD4 and CD8 T cells in mice prior to and following 5′-AMP injection. Data points represent mean of 80–670 T cell tracks from four mice and two independent experiments (right); *P < 0.05.
DISCUSSION
Hibernating animals are able to withstand physiological extreme conditions without signs of organ injury [10–15]. Changes in key physiological parameters occur during natural torpor, including a profound, reversible reduction in the number of circulating lymphocytes [20]. In this study, we used the ability to pharmacologically induce a torpor-like state in nonhibernators by injecting 5′-AMP into mice to further study the impact of torpor on lymphocyte recirculation. Following injection of 5′-AMP, the number of circulating T and B lymphocytes is reduced, without significant effects on other blood cells. 5′-AMP-induced lymphopenia depends on the activation of the adenosine A2BR, as coinfusion of an A2B-selective antagonist precluded lymphopenia but did not affect the reduction in body temperature. Interestingly, the effect of MRS1754 on the induction of lymphopenia through A2BRs seem to be more pronounced for B lymphocytes than for T lymphocytes. We speculate that this might be a result of differences in LN transition times of T and B lymphocytes or A2BR density. Furthermore, lymphopenia could also be induced by injecting an A2B-selective agonist, which in contrast to 5′-AMP, did not reduce the body temperature. In addition, lowering of the body temperature to reach a similar value, as induced by 5′-AMP, led to a similar decrease in the number of total, T, and B lymphocytes, which was not prevented by A2BR antagonism. Together, these observations imply that the induction of lymphopenia during 5′-AMP-induced torpor in mice occurs independently of body temperature and is governed by A2BR.
Upon induction of torpor using 5′-AMP, the induction of lymphopenia was prevented by blocking lymphocyte homing receptors in vivo that enable entry into secondary lymphoid organs. This finding suggests that retention of lymphocytes in peripheral LNs during torpor leads to a reduction in the number of circulating lymphocytes, similar to what is seen during natural deep torpor in the Syrian hamster [20]. The sequestration of lymphocytes within secondary lymphoid organs was not the result of increased homing to these sites upon 5′-AMP administration but resulted from reductions in the egress rate from LNs into the lymph and back into the blood. Inhibition of homing by 5′-AMP may be secondary to activation of A2BRs, as activation of A2BRs expressed by HEVs has been shown to restrict lymphocyte migration into the LN parenchyma [31]. Two factors that were measured in torpid mice could contribute to the reduced egress of lymphocytes from secondary lymphoid organs. We showed that injection of 5′-AMP results in lowered plasma S1P levels, and it has been shown previously that changes in the gradient of S1P concentrations between blood or lymph and lymphoid organs can impair lymphocyte egress substantially [45]. The change we describe here in S1P plasma concentration during 5′-AMP-induced torpor is similar to our previous observation that in naturally hibernating hamsters, when animals enter deep torpor, and the body temperature is lowered, the plasma of S1P level drops, which is likely a result of a diminished release from erythrocytes [20]. During the return to euthermia of torpid mice, S1P levels returned to pretorpor levels, concomitantly with the restoration of circulating lymphocyte numbers, whereby T cell counts returned to normal more rapidly than B cell counts, likely as a result of the longer LN dwell time of B cells [46]. As would be expected if lymphocyte egress from secondary lymphoid organs is required for the re-establishment of normal blood lymphocyte counts, administration of a S1PR1-selective antagonist, prior to rewarming, prevented the reversal of torpor-associated lymphopenia. Notably, injection of an A2B-selective agonist, which induced lymphopenia without changes in body temperature, did not impact plasma S1P levels, suggesting that activation of A2BRs by 5′-AMP induces additional temperature-independent effects on egress from secondary lymphoid organs that are not governed by changes in S1P concentration. Consistent with this idea, we found that 5′-AMP administration (at 37°C) resulted in an immediate and almost complete reduction in lymphocyte motility within the LN. We speculate that the reduced motility of lymphocytes impairs their ability to reach the medullary and cortical lymphatic sinuses and hence, to exit from the LNs back into the lymph [47].
Whereas 5′-AMP-induced torpor displays profound effects on lymphocyte recirculation, it also induces substantial neutrocytosis upon arousal. This increase in number of circulating neutrophils was also precluded by blocking the S1P1R during arousal. In vitro experiments show that S1P stimulates FcγR signal transduction [48] and IL-8 production [49], which both promote neutrophil chemotaxis. Further, S1P lyase−/− mice have a higher plasma level S1P, an augmented granulopoeisis, and a higher number of circulating neutrophils [50]. Thus, the rise in the number of circulating neutrophils above baseline may be caused by S1P as well. Previously, we demonstrated that low body temperature inhibits the release of S1P from erythrocytes into the plasma through ATP-binding cassette transporters, which can be stimulated by (ex vivo) rewarming of erythrocytes [20]. In conclusion, rewarming following 5′-AMP-induced torpor in mice leads to an increased plasma level of S1P, which leads to an increased number of circulating neutrophils.
Whereas we demonstrate in this study that activation of A2BRs reduces the number of circulating lymphocytes, A2BRs can exert additional immunomodulatory actions upon activation. A2BRs can be activated by (endogenous) adenosine, whereas A2B-mediated signaling can be inhibited by IFN-γ [51]. Formation of endogenous adenosine is regulated by the inducible enzymes CD73 (ecto-5′-nucleotidase; 5′-NT) and CD39 (nucleoside triphosphate diphosphohydrolose-1), which are expressed on subsets of T and B lymphocytes, follicular DCs, thymic medullary reticular fibroblasts, epithelial cells, and afferent lymphatic vessels [30, 52, 53]. Subsequent metabolism of adenosine can be mediated through ADA. Interestingly, ADA−/− mice exhibit increased levels of adenosine and purine metabolites, leading to severe immunodeficiency with recurrent infections [54]. The role of adenosine in lymphocyte migration is illustrated by the reduced homing rate [31] and increased influx into tissues in CD73−/− mice compared with WT animals, following experimental cerebral ischemia [55] or following cardiac allotransplantation [56]. Activation of A2BRs can reduce lymphocyte homing [31] and decrease the barrier function of vascular endothelium [30, 56]. The importance of A2B-mediated signaling for the adaptive immune system is illustrated by the fact that genetic ablation of CD73 or A2B antagonism leads to increased lymphocyte transmigration into tissues, whereas agonism of A2BR prolongs graft survival in a cardiac allograft model [56]. As (5′-)AMP can be metabolized into adenosine by ectonucleotidases, its administration can therefore have diverse, immune-suppressive properties and result in changes in lymphocyte migration. The immune-suppressive effects of 5′-AMP might be of relevance to the treatment of infectious diseases, autoimmune diseases, and tumor immunology [57–59].
The potential to pharmacologically induce a torpor-like state with increased resistance to ischemia/reperfusion and hypothermia combined with immune suppression might be of major clinical relevance. One of the applications might be optimization of the application of therapeutic hypothermia, as currently used during brain and cardiac surgery [60]. Although hypothermia during surgery is thought to be crucial in limiting neuronal injury during periods of low oxygen supply by reducing cerebral metabolism, hypothermia is associated with kidney injury postoperatively [61], which represents an important risk factor for in-hospital and long-term mortality following cardiac surgery [62].
Conclusion
Injection of 5′-AMP leads to the induction of a torpor-like state in mice, which is characterized by a drop in body temperature and is associated with a substantial, transient lymphopenia through activation of A2BRs. Inhibition of lymphocyte motility by activation of A2BRs (e.g., by 5′-AMP or LUF6210) diminishes egress of cells and induces retention of lymphocytes in LNs, followed by rapid restoration upon arousal. The signaling pathways activated by 5′-AMP might represent an important pharmacological target to safely suppress metabolism and the immune system, leading to optimization of outcome following major surgery and hence, reduction of mortality during follow-up.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- −/−
- deficient
- ADA
- adenosine deaminase
- ESI
- electrospray ionization
- HEV
- high endothelial venule
- LC
- liquid chromatography
- MRM
- multiple reaction monitoring
- MS
- mass spectrometry
- N2
- nitrogen gas
- S1P
- sphingosine-1-phosphate
- Ta
- ambient temperature
AUTHORSHIP
H.R.B. and J.N.M. designed and performed experiments, analyzed data, and wrote the manuscript. A.M.S. and A.S.B. designed and performed experiments and assisted in writing the manuscript. J-W.K. and A.v.D. set up the method to measure sphingolipids and analyzed data. A.I.J. provided specific pharmacological compounds and assisted in writing the paper. F.G.M.K. and R.H.H. designed the experiments and assisted in writing the paper.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Melvin R. G., Andrews M. T. (2009) Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol. Metab. 20, 490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kenagy G. J., Sharbaugh S. M., Nagy K. A. (1989) Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia 78, 269. [DOI] [PubMed] [Google Scholar]
- 3. Twente J. W., Twente J. A. (1965) Effects of core temperature upon duration of hibernation Citellus lateralis. J. Appl. Physiol. 20, 411–416 [DOI] [PubMed] [Google Scholar]
- 4. Carey H. V., Andrews M. T., Martin S. L. (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181 [DOI] [PubMed] [Google Scholar]
- 5. Heldmaier G., Ortmann S., Elvert R. (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329 [DOI] [PubMed] [Google Scholar]
- 6. Andjus R. K., Olivera M., Petrovic V., Rajevski V. (1964) Influence of hibernation and of intermittent hypothermia on the formation of immune hemagglutinins in the ground squirrel. Ann. Acad. Sci. Fenn. Biol. 71, 26–36 [Google Scholar]
- 7. Hut R. A., Barnes B. M., Daan S. (2002) Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J. Comp. Physiol. B 172, 47–58 [DOI] [PubMed] [Google Scholar]
- 8. Heldmaier G., Klingenspor M., Werneyer M., Lampi B. J., Brooks S. P., Storey K. B. (1999) Metabolic adjustments during daily torpor in the Djungarian hamster. Am. J. Physiol. 276, E896–E906 [DOI] [PubMed] [Google Scholar]
- 9. Geiser F. (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274 [DOI] [PubMed] [Google Scholar]
- 10. Sandovici M., Henning R. H., Hut R. A., Strijkstra A. M., Epema A. H., van Goor H., Deelman L. E. (2004) Differential regulation of glomerular and interstitial endothelial nitric oxide synthase expression in the kidney of hibernating ground squirrel. Nitric Oxide 11, 194–200 [DOI] [PubMed] [Google Scholar]
- 11. Zancanaro C., Malatesta M., Mannello F., Vogel P., Fakan S. (1999) The kidney during hibernation and arousal from hibernation. A natural model of organ preservation during cold ischemia and reperfusion. Neprol. Dial. Transplant. 14, 1982–1990 [DOI] [PubMed] [Google Scholar]
- 12. Fleck C. C., Carey H. V. (2005) Modulation of apoptotic pathways in intestinal mucosa during hibernation. Am. J. Physiol. 289, R586–R595 [DOI] [PubMed] [Google Scholar]
- 13. Talaei F., Hylkema M. N., Bouma H. R., Boerema A. S., Strijkstra A. M., Henning R. H., Schmidt M. (2011) Reversible remodelling of lung tissue during hibernation in the Syrian hamster. J. Exp. Biol. 214, 1276–1282 [DOI] [PubMed] [Google Scholar]
- 14. Arendt T., Stieler J., Strijkstra A. M., Hut R. A., Rudiger J., van der Zee E. A., Harkany T., Holzer M., Hartig W. (2003) Reversible paired helical filament-like phosphorylation of τ is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 23, 6972–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouma H. R., Verhaag E. M., Otis J. P., Heldmaier G., Swoap S. J., Strijkstra A. M., Henning R. H., Carey H. V. (2012) Induction of torpor: mimicking natural metabolic suppression for biomedical applications. J. Cell. Physiol. 227, 1285–1290 [DOI] [PubMed] [Google Scholar]
- 16. Frerichs K. U., Kennedy C., Sokoloff L., Hallenbeck J. M. (1994) Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J. Cereb. Blood Flow Metab. 14, 193–205 [DOI] [PubMed] [Google Scholar]
- 17. Storey K. B. (2004) Cold ischemic organ preservation: lessons from natural systems. J. Investig. Med. 52, 315–322 [DOI] [PubMed] [Google Scholar]
- 18. Lindell S. L., Klahn S. L., Piazza T. M., Mangino M. J., Torrealba J. R., Southard J. H., Carey H. V. (2005) Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G473–G480 [DOI] [PubMed] [Google Scholar]
- 19. Bouma H. R., Carey H. V., Kroese F. G. (2010) Hibernation: the immune system at rest? J. Leukoc. Biol. 88, 619–624 [DOI] [PubMed] [Google Scholar]
- 20. Bouma H. R., Kroese F. G., Kok J. W., Talaei F., Boerema A. S., Herwig A., Draghiciu O., van Buiten A., Epema A. H., van Dam A., Strijkstra A. M., Henning R. H. (2011) Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 108, 2052–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aslami H., Juffermans N. P. (2010) Induction of a hypometabolic state during critical illness-a new concept in the ICU? Neth. J. Med. 68, 190–198 [PubMed] [Google Scholar]
- 22. Maathuis M. H., Leuvenink H. G., Ploeg R. J. (2007) Perspectives in organ preservation. Transplantation 83, 1289–1298 [DOI] [PubMed] [Google Scholar]
- 23. Zhang J., Kaasik K., Blackburn M. R., Lee C. C. (2006) Constant darkness is a circadian metabolic signal in mammals. Nature 439, 340–343 [DOI] [PubMed] [Google Scholar]
- 24. Lee C. C. (2008) Is human hibernation possible? Annu. Rev. Med. 59, 177–186 [DOI] [PubMed] [Google Scholar]
- 25. Lindsley J. E., Rutter J. (2004) Nutrient sensing and metabolic decisions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 543–559 [DOI] [PubMed] [Google Scholar]
- 26. Bouma H. R., Ketelaar M. E., Yard B. A., Ploeg R. J., Henning R. H. (2010) AMPK as target for preconditioning in transplantation medicine. Transplantation 90, 619–624 [DOI] [PubMed] [Google Scholar]
- 27. Swoap S. J., Rathvon M., Gutilla M. (2007) AMP does not induce torpor. Am. J. Physiol. 293, R468–R473 [DOI] [PubMed] [Google Scholar]
- 28. Aymerich I., Foufelle F., Ferre P., Casado F. J., Pastor-Anglada M. (2006) Extracellular adenosine activates AMP-dependent protein kinase (AMPK). J. Cell Sci. 119, 1612–1621 [DOI] [PubMed] [Google Scholar]
- 29. Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Muller C. E. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol. Rev. 63, 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linden J., Cekic C. (2012) Regulation of lymphocyte function by adenosine. Arterioscler. Thromb. Vasc. Biol. 32, 2097–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takedachi M., Qu D., Ebisuno Y., Oohara H., Joachims M. L., McGee S. T., Maeda E., McEver R. P., Tanaka T., Miyasaka M., et al. (2008) CD73-generated adenosine restricts lymphocyte migration into draining lymph nodes. J. Immunol. 180, 6288–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Briggs C., Harrison P., Grant D., Staves J., MacHin S. J. (2000) New quantitative parameters on a recently introduced automated blood cell counter—the XE 2100. Clin. Lab. Haematol. 22, 345–350 [DOI] [PubMed] [Google Scholar]
- 33. Ruzicka K., Veitl M., Thalhammer-Scherrer R., Schwarzinger I. (2001) The new hematology analyzer Sysmex XE-2100: performance evaluation of a novel white blood cell differential technology. Arch. Pathol. Lab. Med. 125, 391–396 [DOI] [PubMed] [Google Scholar]
- 34. Lo C. G., Xu Y., Proia R. L., Cyster J. G. (2005) Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller M. J., Wei S. H., Cahalan M. D., Parker I. (2003) Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA 100, 2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 [DOI] [PubMed] [Google Scholar]
- 37. Egen J. G., Rothfuchs A. G., Feng C. G., Winter N., Sher A., Germain R. N. (2008) Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 39. Sullards M. C., Merrill A. H., Jr., (2001) Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci. STKE 2001, pI1. [DOI] [PubMed] [Google Scholar]
- 40. Sullards M. C., Wang E., Peng Q., Merrill A. H., Jr., (2003) Metabolomic profiling of sphingolipids in human glioma cell lines by liquid chromatography tandem mass spectrometry. Cell. Mol. Biol. (Noisy -le-grand) 49, 789–797 [PubMed] [Google Scholar]
- 41. Auchampach J. A., Kreckler L. M., Wan T. C., Maas J. E., van der Hoeven D., Gizewski E., Narayanan J., Maas G. E. (2009) Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J. Pharmacol. Exp. Ther. 329, 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rittiner J. E., Korboukh I., Hull-Ryde E. A., Jin J., Janzen W. P., Frye S. V., Zylka M. J. (2012) AMP is an adenosine A1 receptor agonist. J. Biol. Chem. 287, 5301–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., et al. (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 [DOI] [PubMed] [Google Scholar]
- 44. Grigorova I. L., Schwab S. R., Phan T. G., Pham T. H., Okada T., Cyster J. G. (2009) Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 10, 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 46. Tomura M., Yoshida N., Tanaka J., Karasawa S., Miwa Y., Miyawaki A., Kanagawa O. (2008) Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl. Acad. Sci. USA 105, 10871–10876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sinha R. K., Park C., Hwang I. Y., Davis M. D., Kehrl J. H. (2009) B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity 30, 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Florey O., Haskard D. O. (2009) Sphingosine 1-phosphate enhances Fc γ receptor-mediated neutrophil activation and recruitment under flow conditions. J. Immunol. 183, 2330–2336 [DOI] [PubMed] [Google Scholar]
- 49. Milara J., Mata M., Mauricio M. D., Donet E., Morcillo E. J., Cortijo J. (2009) Sphingosine-1-phosphate increases human alveolar epithelial IL-8 secretion, proliferation and neutrophil chemotaxis. Eur. J. Pharmacol. 609, 132–139 [DOI] [PubMed] [Google Scholar]
- 50. Allende M. L., Bektas M., Lee B. G., Bonifacino E., Kang J., Tuymetova G., Chen W., Saba J. D., Proia R. L. (2011) Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kolachala V., Asamoah V., Wang L., Srinivasan S., Merlin D., Sitaraman S. V. (2005) Interferon-γ down-regulates adenosine 2b receptor-mediated signaling and short circuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. J. Biol. Chem. 280, 4048–4057 [DOI] [PubMed] [Google Scholar]
- 52. Algars A., Karikoski M., Yegutkin G. G., Stoitzner P., Niemela J., Salmi M., Jalkanen S. (2011) Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood 117, 4387–4393 [DOI] [PubMed] [Google Scholar]
- 53. Resta R., Yamashita Y., Thompson L. F. (1998) Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol. Rev. 161, 95–109 [DOI] [PubMed] [Google Scholar]
- 54. Sauer A. V., Brigida I., Carriglio N., Hernandez R. J., Scaramuzza S., Clavenna D., Sanvito F., Poliani P. L., Gagliani N., Carlucci F., et al. (2012) Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood 119, 1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrovic-Djergovic D., Hyman M. C., Ray J. J., Bouis D., Visovatti S. H., Hayasaki T., Pinsky D. J. (2012) Tissue-resident ecto-5′ nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain. J. Immunol. 188, 2387–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hasegawa T., Bouis D., Liao H., Visovatti S. H., Pinsky D. J. (2008) Ecto-5′ nucleotidase (CD73)-mediated adenosine generation and signaling in murine cardiac allograft vasculopathy. Circ. Res. 103, 1410–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beldi G., Wu Y., Banz Y., Nowak M., Miller L., Enjyoji K., Haschemi A., Yegutkin G. G., Candinas D., Exley M., et al. (2008) Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology 48, 841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mandapathil M., Hilldorfer B., Szczepanski M. J., Czystowska M., Szajnik M., Ren J., Lang S., Jackson E. K., Gorelik E., Whiteside T. L. (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 285, 7176–7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sun X., Wu Y., Gao W., Enjyoji K., Csizmadia E., Muller C. E., Murakami T., Robson S. C. (2010) CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139, 1030–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arrich J., Holzer M., Herkner H., Mullner M. (2009) Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 9, CD004128. [DOI] [PubMed] [Google Scholar]
- 61. Kourliouros A., Valencia O., Phillips S. D., Collinson P. O., van Besouw J. P., Jahangiri M. (2010) Low cardiopulmonary bypass perfusion temperatures are associated with acute kidney injury following coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 37, 704–709 [DOI] [PubMed] [Google Scholar]
- 62. Loef B. G., Epema A. H., Navis G., Ebels T., Stegeman C. A. (2009) Postoperative renal dysfunction and preoperative left ventricular dysfunction predispose patients to increased long-term mortality after coronary artery bypass graft surgery. Br. J. Anaesth. 102, 749–755 [DOI] [PubMed] [Google Scholar]