A novel method to ultra-purify unstimulated neutrophils by flow sorting and subsequent demonstration of the neutrophils' ability to release IL-1β following inflammatory stimulus.
Keywords: granulocyte, FACS, interleukin-1β
Abstract
The technical limitations of isolating neutrophils without contaminating leukocytes, while concurrently minimizing neutrophil activation, is a barrier to determining specific neutrophil functions. We aimed to assess the use of FACS for generating highly pure quiescent neutrophil populations in an antibody-free environment. Peripheral blood human granulocytes and murine bone marrow-derived neutrophils were isolated by discontinuous Percoll gradient and flow-sorted using FSC/SSC profiles and differences in autofluorescence. Postsort purity was assessed by morphological analysis and flow cytometry. Neutrophil activation was measured in unstimulated-unsorted and sorted cells and in response to fMLF, LTB4, and PAF by measuring shape change, CD62L, and CD11b expression; intracellular calcium flux; and chemotaxis. Cytokine production by human neutrophils was also determined. Postsort human neutrophil purity was 99.95% (sem=0.03; n=11; morphological analysis), and 99.68% were CD16+ve (sem=0.06; n=11), with similar results achieved for murine neutrophils. Flow sorting did not alter neutrophil activation or chemotaxis, relative to presorted cells, and no differences in response to agonists were observed. Stimulated neutrophils produced IL-1β, although to a lesser degree than CXCL8/IL-8. The exploitation of the difference in autofluorescence between neutrophils and eosinophils by FACS is a quick and effective method for generating highly purified populations for subsequent in vitro study.
Introduction
Neutrophils are key effector cells of the innate immune system that play an important role in the inflammatory cascade [1]. Delineating their functional, biochemical, and synthetic capabilities is essential in the understanding of the pathological basis of disease and the development of novel, anti-inflammatory agents. To perform robust in vitro studies, isolation of pure populations from peripheral blood is essential. Whereas conventional neutrophil isolation techniques provide a convenient method of purification, several important observations with regard to neutrophil function have only been revealed through use of highly purified neutrophil populations. For example, the effect of contaminating monocytes in the modulation of neutrophil survival [2] and cytokine production [3] has only been shown by subsequent purification following conventional isolation.
Given the ease with which neutrophils can become activated, the techniques used in the isolation of neutrophils from peripheral blood can profoundly influence subsequent in vitro and in vivo function [4–7]. Whereas use of Ficoll/Hypaque or dextran sedimentation with a subsequent discontinuous Percoll gradient is generally accepted as the isolation method least likely to activate neutrophils, purity of >95–97% is difficult to achieve [8, 9]. Mononuclear cell contamination, although possible to limit with good laboratory technique, cannot always be excluded. Subsequent purification of granulocytes by a variety of antibody cocktail/magnetic bead-based selection strategies has therefore been described for human and murine use [10, 11].
FACS is a well-established and widely available method of generating highly pure cell populations, usually through selection of antibody-labeled, cell-surface markers [12]. As a result of the sensitivity of neutrophils to antibody-mediated surface receptor cross-linking-induced activation, this approach is not suited for their purification. Furthermore, high levels of IgGR expression (FcγRIIa and FcγRIIIb) mean that despite these being low-affinity receptors, even negative selection strategies may expose neutrophils to antibodies that bind via the Fc portion, triggering neutrophil activation. However, using intrinsic differences in autofluorescence and granularity between cell types provides an alternative approach through flow cytometry-based isolation. The relative difference in granularity and nuclear size between mononuclear hemopoietic cells and granulocytes allows distinct separation in terms of SSC, whereas the unusually bright autofluorescence of eosinophils allows their differentiation from other granulocyte populations [13]. With the use of these intrinsic differences, we characterized and validated the potential role of antibody-free, autofluorescence-based flow sorting to generate highly purified, unactivated neutrophil populations for subsequent in vitro study and subsequently, examined neutrophil production of the proinflammatory cytokines IL-1β and CXCL8/IL-8.
MATERIALS AND METHODS
Human neutrophil isolation
Peripheral venous blood was taken from healthy human volunteers and mixed with 3.8% citrate prior to centrifugation. Granulocytes were subsequently isolated by dextran sedimentation and discontinuous Percoll gradient, as described [4]. Ethical approval was obtained from the Lothian Research Ethics Committee (Approval #08/S1103/38).
Isolated granulocytes were subsequently suspended at 10 × 106/ml in PBS without cations (PAA, Paisley, UK), supplemented with 1% autologous platelet poor plasma. The latter was made by layering 900 μl platelet-rich plasma atop 100 μl 90% Percoll, centrifuged at 1000 g for 20 min, and the cell-free plasma aspirated. Cells were flow-sorted using BD FACSAria II SORP (BD Biosciences, San Jose, CA, USA) with gates set around the granulocyte population, based on characteristic FSC and SSC profiles with doublets removed on the basis of FSC-area versus FSC-height. Neutrophils and eosinophils were easily separated by the increased autofluorescence of the latter population, visible at 450/50 nm (355 nm laser), 525/50 nm (488 nm laser), 488/10 nm (488 nm laser; SSC), and 585/15 nm (561 nm laser; Fig. 1A and C–E). Cells were sorted at 20°C through a 70-μm nozzle and collected into tubes coated with 1% platelet poor plasma. With 10 × 106 neutrophils isolated within 20 min of flow sorting, the time between initial venesection and obtaining highly purified neutrophils is ∼3 h, thus ensuring rapid availability for subsequent assays.
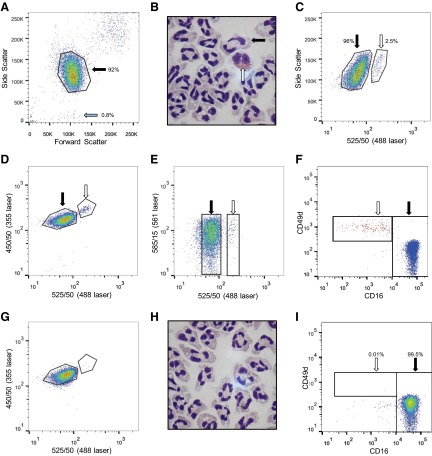
Figure 1. Neutrophils can be identified on the basis of size, granularity, and autofluorescence and can be separated from contaminating cells by flow sorting.
Human granulocytes, isolated by Dextran/Percoll gradient from whole blood, were analyzed by flow cytometry. FSC and SSC profiles separated granulocytes (black arrow; percentage of total events shown) from contaminating mononuclear cells (blue arrow; A). Morphological analysis of gated granulocytes confirmed the presence of neutrophils (black arrow) and eosinophils (white arrow; B; original magnification, ×1000). Eosinophils (white arrow) are easily distinguished from neutrophils (black arrow) when excited with 488 nm laser (525/50 nm) and SSC (488 nm laser, 488/10 nm; C); 488 nm laser (525/50 nm) and 355 nm laser (450/50 nm; D); or 488 nm laser (525/50 nm) and 561 nm laser (585/15 nm; E). The phenotypes of these distinct populations were confirmed by CD16 and CD49d staining (F), with a representative overlay of low- and high-autofluorescent populations (blue and red dots, respectively), with neutrophil population CD16+ve/CD49d−ve (black arrow) and eosinophil population CD16−ve/CD49d+ve (white arrow). Analysis of the sorted neutrophils demonstrated removal of autofluorescent cells [representative plot with 488 nm laser (525/50 nm) and 355 nm laser (450/50 nm); G]. Morphological analysis confirmed a highly pure neutrophil population with negligible numbers of contaminating eosinophils and no mononuclear cells seen (original magnification, ×1000; H). CD16/CD49d staining postsort confirmed neutrophil purity; all cells in FSC/SSC analyzed (I). Representative images and histograms from 11 separate donors.
Murine neutrophil isolation
Bone marrow-derived neutrophils were isolated as described [14]. Briefly, female CD1 mice were killed by cervical dislocation, femurs and tibias removed, and marrows flushed. Following hypotonic lysis (5 ml ice-cold 0.2% NaCl, added for 45 s, and then 5 ml 1.6% NaCl), granulocytes were separated from mononuclear cells by discontinuous Percoll gradient [61% Percoll overlaid with cells suspended in modified HBSS (PAA), containing 10% FCS and 20 mM sodium HEPES (Sigma-Aldrich, Poole, UK)]. Following centrifugation, the granulocyte layer was isolated and washed and cells resuspended at 10 × 106 cells/ml. Neutrophils were flow-sorted as described above (Fig. 2A and B).
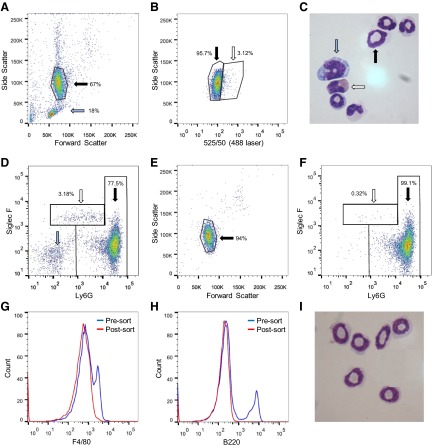
Figure 2. Murine bone marrow-derived neutrophils can be sorted by autofluorescence-based flow sorting.
Granulocytes isolated from mouse bone marrow by discontinuous Percoll gradient were analyzed by flow cytometry and sorted with FSC and SSC profiles, separating granulocytes (black arrow; percentage of total events shown) from contaminating mononuclear cells (blue arrow; A), and excited at 525/50 nm (488 nm laser) and SSC (488 nm laser, 488/10 nm), separating neutrophils (black arrow) from eosinophils (white arrow; B). Morphological analysis of isolated granulocytes confirmed the presence of neutrophils (black arrow), eosinophils (white arrow), and mononuclear cells (blue arrow; C; original magnification, ×1000). Siglec F and Ly6G staining corresponded to different autofluorescent populations, indicated by the black arrow (Ly6G+ve/Siglec F−ve) and white arrow (Ly6G−ve/Siglec F+ve), whereas mononuclear cells correspond to Ly6G−ve/Siglec F−ve cells (blue arrow; D; all cells in FSC/SSC analyzed). Postsort analysis of the collected neutrophils demonstrated removal of mononuclear cells (on FSC and SSC profiles; E) and the majority of eosinophils (representative plot of Ly6G/Siglec F; F). Effective removal of monocytes and lymphocytes was demonstrated by F4/80 and B220 staining (G and H), while morphological analysis confirmed a pure neutrophil population (I). Representative images and histograms from nine separate mice.
Neutrophil purity
Cells were cytocentrifuged (300 rpm for 3 min), fixed in methanol, and stained with Diff-Quick (Gamidor, Oxford, UK), with 1000 cells counted to determine neutrophil purity, based on characteristic morphological appearance. Alternatively, human cells were incubated with CD16-APC-Cy7 (BioLegend, Cambridge, UK), CD49d-PE (BD Biosciences, Oxford, UK), CD3-FITC (BioLegend), HLA-DR-V450 (BD Biosciences), and CD14-PerCP-Cy5.5 (BioLegend). Murine neutrophils were incubated with Ly6G-Pacific Blue (BioLegend), B220-PerCP-Cy5.5 (eBioscience, Hatfield, UK), CD3-APC (eBioscience), Siglec F-PE (BioLegend), and F4/80-AF700 (AbD Serotec, Oxford, UK) and analyzed by flow cytometry.
Neutrophil and eosinophil activation
Unsorted granulocytes and sorted neutrophils and eosinophils were suspended at 10 × 106 cells/ml in PBS with cations (PAA) and incubated with fMLF, LTB4, PAF, or vehicle control (Sigma-Aldrich) for 15 min (eosinophils) or 30 min (neutrophils) at 37°C on a shaking heat block (300 rpm). Concentrations used are displayed in figure legends. Cells were then fixed in 2.5% glutaraldehyde (Sigma-Aldrich) to assess shape change or incubated on ice for 30 min with CD62L-PE (BD Biosciences) and CD11b-AF488 (eBioscience) for human neutrophils or CD62L-PE (BD Biosciences), CD11b-FITC (BioLegend), and Ly6G-Pacific Blue for mouse neutrophils. All samples were analyzed by flow cytometry. Superoxide anion production was assessed by DHR fluorescence, as described [9]. Briefly, cells (2×106/ml) were suspended in HBSS with cations and incubated with DHR (2 μM; Invitrogen, Carlsbad, CA, USA) for 10 min before PMA (300 nM; Sigma-Aldrich), or control was added for 15 min. DHR fluorescence was measured by flow cytometry (fluorescence-1).
Neutrophil chemotaxis
A 96-well chemotaxis chamber fitted with a 3-μm filter (Neuro Probe, Gaithersburg, MD, USA) was used to assess chemotaxis as described [15]. Unsorted human granulocytes or sorted neutrophils (3×106/ml in IMDM with 10% autologous serum) were loaded in the upper well with fMLF (100 nM) or vehicle control added to the lower well. Following incubation at 37°C for 90 min, the membrane was removed and nonadherent cells scraped from the upper surface and fixed with methanol before staining with Diff-Quick. OD, as an indicator of cell migration into the membrane, was measured at 570 nm on a Bio-Plex plate reader (Bio-Rad, Hertfordshire, UK).
Intracellular calcium flux
Neutrophils were loaded with fura-2/AM (2 μM; Invitrogen) for 30 min in HBSS without divalent cations, washed, and resuspended at 2 × 106/ml in HBSS with divalent cations. Intracellular calcium flux, in response to fMLF, LTB4, and PAF, was quantified using a spectrofluorimeter (Perkin Elmer, Waltham, MA, USA), as described [16].
Neutrophil apoptosis
Neutrophil apoptosis was assessed following incubation at 5 × 106/ml in IMDM (10% autologous serum) for 20 h at 37°C, in the presence or absence of LPS (100 ng/ml; Sigma-Aldrich) and GM-CSF (20 ng/ml; R&D Systems, Abingdon, UK). Cell viability, apoptosis, and necrosis were measured by Annexin V-FITC (Roche, West Sussex, UK) and PI (Sigma-Aldrich) staining and analyzed by flow cytometry, as described [9].
Cytokine expression
Highly pure human neutrophils or PBMCs were suspended at 10 × 106/ml in IMDM, supplemented with 10% autologous serum and incubated for 8 h at 37°C in the presence of fMLF (100 nM), GM-CSF (20 ng/ml), and LPS (100 ng/ml), with or without Imject Alum (125 μg/ml; Thermo Scientific, Loughborough, UK) added after 1 h incubation with LPS. Samples were centrifuged at 350 g for 5 min and supernatants frozen at −20°C. Freshly isolated neutrophils were lysed by nitrogen cavitation (400 psi for 5 min on ice) and lysates frozen immediately. Cytokine expression was measured by ELISA (R&D Systems), as per protocol, and cytokine bead array (human inflammation CBA kit; BD Biosciences). Cells were treated for 30 min with LPS (100 ng/ml), gliotoxin (0.1 μg/ml; Sigma-Aldrich), or in combination, and Western blotting for IκBα (Abcam, Cambridge, UK) and β-actin (Sigma-Aldrich) was performed, as described [17].
Statistical analysis
Flow cytometry analysis was performed using FlowJo v10.0.4 (Tree Star, Ashland, OR, USA). Results are presented as mean ± sem. Data were analyzed by one-way ANOVA with a Newman-Keuls multiple comparison post hoc test and CXCL8/IL-8 cytokine production analyzed by Student's t-test (GraphPad Prism v5; GraphPad Software, La Jolla, CA, USA); significance was accepted with P values: *P < 0.05, and **P < 0.01.
RESULTS AND DISCUSSION
Dextran sedimentation and discontinuous Percoll gradient, as a means to isolate neutrophils from peripheral venous blood, result in cell purity of ≥95%, with between 1% and 5% eosinophils and 1–2% contaminating mononuclear cells (Fig. 1A and B). In individuals with atopic conditions, such as hay fever and asthma, eosinophils can represent >5–10% of the granulocyte population. Whereas the frequency of contaminating mononuclear cells can be limited by good experimental technique, their presence cannot be excluded completely by density gradient centrifugation. A variety of other methods are used to isolate neutrophils from human whole blood, although some, most notably, those that involve hypotonic lysis, are associated with greater levels of cell activation [6, 7]. To remove contaminating mononuclear cells, magnetic bead-based negative selection has been used to achieve purity of >99% [10, 18], although it is worth noting that some antibody cocktails do not concomitantly remove eosinophils [19]. CD15- and CD16-positive selection methods have been used, but antibody binding of these molecules can influence neutrophil function. For example, several antibodies against CD16 (FcγRIII) inhibit microcrystal-induced neutrophil tyrosine phosphorylation [20], whereas mAb against CD16 and CD15 influence neutrophil intracellular calcium flux and degranulation [21]. Hence, we investigated the use of antibody-free FACS to study the synthetic capacity of neutrophils, devoid of contaminating mononuclear cells and eosinophils, while minimizing concomitant activation or priming.
Intracellular birefringent granules within human eosinophils have greater depolarized SSC than neutrophils allowing differentiation of granulocyte populations relative to polarized SSC [22]. The use of this method alone, however, does not allow clear delineation of neutrophil and eosinophil populations. With the use of eosinophil autofluorescence, attributed to granule-associated flavin adenine dinucleotide [23], along with polarized SSC, separation of these cell populations is possible and has been used previously to distinguish granulocyte populations. This approach has been used in a variety of contexts, including assessment of differential responses of neutrophils and eosinophils to a variety of agonists, including eotaxin and IL-8 [24], the isolation and assessment of autofluorescent properties of eosinophils [13], and the detection of eosinophils within histological sections by confocal microscopy [25].
Excitation maxima for eosinophils occur at ∼380 nm and ∼450 nm, with maximum emission at 520 nm [23]. Delineation of the eosinophil population was therefore possible on multiple lasers. Greatest separation of the cell types was seen using 488 nm (525/50 nm) laser excitation in conjunction with SSC (488/10 nm; Fig. 1C) or a 355-nm (450/50-nm) laser (Fig. 1D). Although the 561-nm (585/15-nm) laser does not separate the populations, well-defined populations are obtained with the 488 laser alone, and this was used throughout (Fig. 1E). The nature of these two distinct populations was confirmed by CD16 and CD49d staining—neutrophil- and eosinophil-specific markers, respectively. The weakly autofluorescent granulocyte population was exclusively CD16+ve/CD49d−ve, whereas the highly autofluorescent cells were predominantly CD16−ve/CD49d+ve (Fig. 1F).
Any of the above gating strategies removed mononuclear cells and autofluorescent eosinophils, allowing isolation of a highly pure neutrophil population with purity of 99.95% (sem=0.03; n=11), based on morphological analysis, and 99.68% (sem=0.06; n=11), as assessed by flow cytometry (CD16+ve/CD49d−ve; Fig. 1G–I and Table 1). Mononuclear cell-specific markers (CD3, HLA-DR, CD14) were undetectable in all postsort samples, with only 0.13% of cells CD49d-positive (Table 1). Importantly, any contaminating cells that were observed by morphological analysis were eosinophils and not mononuclear cells.
Table 1. Assessment of Neutrophil Purity by Morphological Analysis and Flow Cytometry after Flow Sorting.
| Measure | % Cells | sem | Events counted | n |
|---|---|---|---|---|
| Human | ||||
| Morphological analysis | 99.95% | 0.03 | 1000 | 11 |
| CD16 | 99.68% | 0.06 | 10,000 | 11 |
| CD49d | 0.13% | 0.04 | 10,000 | 7 |
| CD3 | <0.05% | N/A | 10,000 | 7 |
| HLA-DR | <0.05% | N/A | 10,000 | 7 |
| CD14 | <0.05% | N/A | 10,000 | 7 |
| Mouse | ||||
| Morphological analysis | 99.2% | 0.15 | 1000 | 8 |
| Ly6G | 98.8% | 0.20 | 10,000 | 9 |
| Siglec F | 1.0% | 0.19 | 10,000 | 7 |
| F4/80 | 0.07% | 0.19 | 10,000 | 7 |
| CD3 | <0.05% | N/A | 10,000 | 9 |
| B220 | <0.05% | N/A | 10,000 | 9 |
Murine bone marrow-derived neutrophils were similarly flow-sorted with eosinophils, again being highly autofluorescent, confirmed by Ly6G and Siglec F staining (Fig. 2A–D). Purity of postsort samples was 99.2% (sem=0.15; n=8), based on morphological analysis, and 98.8% (sem=0.2; n=9) were Ly6G+ve. Lymphocyte markers CD3 and B220 were undetectable, with the majority of contaminating cells Siglec F+ve eosinophils (1.0% sem=0.19; n=7), and a small fraction F4/80+ve (Fig. 2E–I and Table 1). This technique has been described previously in murine cells [26], achieving 97% neutrophil purity; however, by removing most mononuclear cells by discontinuous Percoll gradient, using more stringent gating strategies and using a modern flow sorter, we were able to further enrich this population to acquire a purer neutrophil population. Preliminary experiments, investigating neutrophil isolation from mouse blood and spleen, were attempted, but both yield and purity were limited. However, we feel that this does not preclude future modifications of our technique to allow greater enrichment of this population. The small discrepancy in purity between human peripheral blood neutrophils and murine bone marrow-derived neutrophils may well lie in the latter population's more heterogeneous nature of immature and mature cells of different lineages.
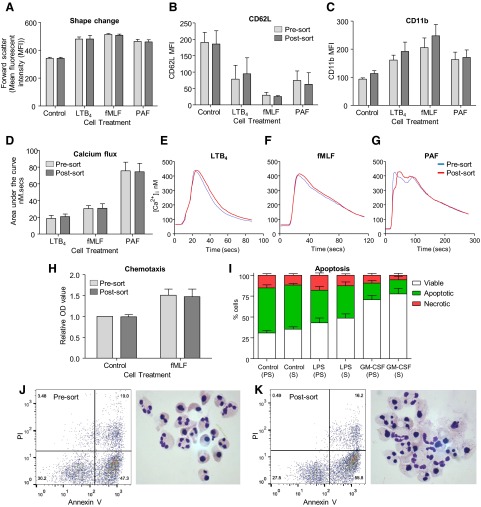
Although shear stress associated with hydrodynamic cell sorting is minimized in modern cell sorters, it remains a concern for isolation of neutrophils, which activate easily. Given this risk, we sought to determine the effect of cell sorting on neutrophil function and survival. Shape change, CD62L, and CD11b expression, as well as chemotaxis, have been shown previously to be affected during neutrophil isolation, with activated cells less able to migrate in response to chemotactic stimuli, having less CD62L expression, greater CD11b expression, and a blunted response to neutrophil agonists, such as fMLF [4]. Importantly, there were no differences between unsorted and sorted cells regarding shape change, CD62L, or CD11b expression or in their response to the classic neutrophil agonists fMLF, PAF, and LTB4 in human neutrophils (Fig. 3A–C). Similarly, no differences were observed in basal intracellular calcium levels, measured by spectrofluorimetry of Fura-2-loaded cells, and calcium flux, following stimulation with LTB4, fMLF, or PAF (Fig. 3D–G). Migration toward fMLF was equal in both cell populations, and importantly, basal levels of chemotaxis were equal in both populations (Fig. 3H). Activated neutrophils exhibit delayed apoptosis in vivo and in vitro [27]; however, there was no difference in constitutive apoptosis or in LPS- or GM-CSF-treated cells between sorted and unsorted cells following 8 h of culture (Fig. 3I–K).
Figure 3. Autofluorescence-based flow sorting does not alter human neutrophil activation, rates of apoptosis, or their response to proinflammatory stimuli.
Shape change (A), CD62L (B), and CD11b expression (C) were unaltered by flow sorting, and no differences in response to the agonists LTB4, fMLF, or PAF were seen relative to unsorted cells from the same donor. No difference in intracellular calcium flux was observed in response to LTB4, fMLF, or PAF (D) traces shown for each agonist (E–G, respectively; mean of n=3 donors/trace). Basal- and fMLF-induced neutrophil chemotaxis were unaffected by cell sorting (H). Apoptosis following 20 h culture was determined by Annexin V (AnnV)/PI binding (Viable, AnnV−ve/PI−ve; Apoptotic, AnnV+ve/PI−ve; Necrotic, AnnV+ve/PI+ve) following treatment with LPS (100 ng/ml), GM-CSF (20 ng/ml), or vehicle control (PS, presort cells; S, sorted cells; I). Representative flow plots (AnnV vs. PI) and cytocentrifuge preparations of control cells (×1000 original magnification) of presorted (J) and postsorted (K) cells shown; five to nine separate donors/group.
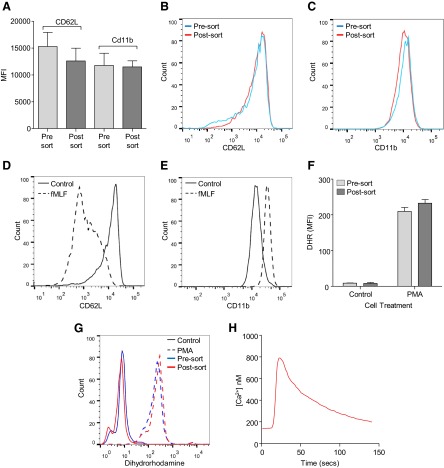
Similarly, flow-sorting murine neutrophils did not alter their expression of CD11b following flow sorting, whereas there was a small but nonsignificant reduction in CD62L expression (Fig. 4A–C), while they remained responsive to a range of agonists. fMLF induced CD62L loss and CD11b up-regulation (Fig. 4D and E), whereas PMA induced superoxide anion production at comparable levels with unsorted cells (Fig. 4F and G). An increase in intracellular calcium following PAF stimulation was also observed (Fig. 4H). In light of these findings, we conclude that autofluorescence-based flow sorting provided a satisfactory means to highly purify human and mouse neutrophils in an inactivated state suitable for further in vitro culture and study.
Figure 4. Flow sorting does not alter mouse neutrophil activity or response to proinflammatory stimuli.
Neutrophil CD62L and CD11b expression was unaltered by flow sorting (A). Representative histograms of presort and postsort cell CD62L (B) and CD11b expression (C; n=7). Sorted cells remained functionally active with CD62L loss (D) and increased CD11b expression (E) in response to fMLF (control, solid line; 5 μM fMLF, dashed line; representative trace from two separate mouse preparations). Basal neutrophil superoxide anion release and response to PMA were unaltered by flow sorting (F). Representative histogram of superoxide anion production in unsorted and sorted cells (G; solid line, control; dashed line, 300 nM PMA; n=3). Increased intracellular calcium flux was observed in postsorted neutrophils following stimulation with 1 μM PAF (H; representative trace from two separate mouse preparations).
With the collection of the autofluorescent granulocytes, we were able to simultaneously enrich a functionally active eosinophil population (Fig. 5A–C). The yield was, however, lower than with other methods of eosinophil isolation [28, 29], and therefore, although useful, flow sorting may not lend itself to the generation of large numbers of eosinophils with which to perform a subsequent in vitro study. As demonstrated by CD49d staining of unsorted granulocytes, some neutrophils have greater autofluorescence (Fig. 1F); therefore, we were unable to generate a population of eosinophils with comparable purity with flow-sorted human neutrophils. Purity of sorted eosinophils was 97.38% (sem=0.55; n=5), based on morphological analysis with 96.7% (sem=0.82; n=8) CD16−ve, as assessed by flow cytometry. No cells were HLA-DR-, CD14-, or CD3-positive (n=5). Isolated eosinophils remained functionally active following flow sorting, undergoing shape change and CD11b up-regulation in response to PAF stimulation (Fig. 5D and E) and generating superoxide anion following incubation with PMA (Fig. 5F).
Figure 5. Eosinophils isolated by autofluorescence-based flow sorting remain functionally active.
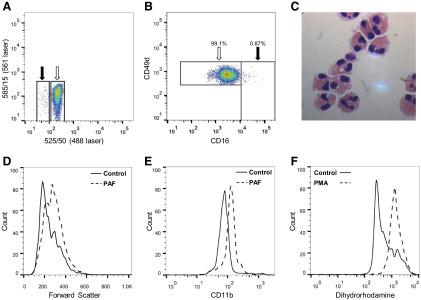
Sorted eosinophils analyzed by flow cytometry show enrichment of the autofluorescent population (white arrow) relative to presorted cells (A; representative postsort sample). A pure population of eosinophils is isolated, indicated by CD16/CD49d staining (B; representative flow plot; all viable cells in FSC/SSC analyzed; n=7). Morphological analysis confirmed a pure eosinophil population with few contaminating neutrophils (C; original magnification, ×1000). Eosinophils remained functionally active after flow sorting, as demonstrated by shape change and CD11b up-regulation, induced by 100 nM PAF (D and E; representative histograms) and superoxide anion generation following stimulation with 300 nM PMA (F).
Neutrophils, as short-lived and terminally differentiated cells, have limited transcriptional capacity with the majority of secretory proteins formed during maturation within the bone marrow. Significant CXCL8/IL-8 production in response to cytokines, growth factors, pathogens, and other inflammatory mediators is well-recognized with release of other cytokines and chemokines, such as IL-1Rα and IL-12, also described [30, 31]. A recent description of IL-1β production, regulated by the NLRP3 inflammasome, was demonstrated in human neutrophils using the potassium ionophore nigericin [19]. IL-1β expression is dependent on activation of the inflammasome, allowing caspase-1-mediated cleavage of pro-IL-1β to active IL-1β. We therefore sought to determine whether alum, another agent known to activate the inflammasome, was able to induce IL-1β expression in human neutrophils purified by flow sorting.
Prior to IL-1β release, NF-κB activation is required for the synthesis of pro-IL-1β. We found that highly pure neutrophils abundantly express IκBα, the physiological inhibitor of NF-κB, and rapid down-regulation occurs following stimulation with LPS in keeping with NF-κB activation (Fig. 6A). Inhibition of this process by gliotoxin, a known NF-κB inhibitor [32], is included for comparison. With the use of cells purified by flow sorting, we found neutrophil IL-1β production to be ∼160-fold lower than PBMCs (Fig. 6B and C). Following incubation with LPS alone for 4 h or 8 h, low levels of IL-1β were detected (26.43 pg/ml, sem=13.27, n=3; 14.85 pg/ml, sem=8.01, n=7, respectively), whereas significant amounts of CXCL8/IL-8 were released (Fig. 6D). Cells were primed with LPS and coincubated with alum. While alum alone did not induce IL-1β expression (data not shown) IL-1β was detected following pretreatment with LPS, thereby supporting previous findings of neutrophil NLRP3-dependent IL-1β production [19]. Alternative stimulation with GM-CSF or fMLF did not induce significant IL-1β expression, whereas TNF-α, IL-6, and IL-10 were undetectable in all treatment groups (data not shown). Furthermore, whereas some CXCL8/IL-8 release following inflammatory stimulus is attributed to preformed cytokine [33], this does not appear to be evident for IL-1β, as cell lysates obtained immediately postisolation do not contain IL-1β, suggesting de novo synthesis following activation (Fig. 6B).
Figure 6. Highly pure human neutrophils secrete small amounts of IL-1β in response to inflammatory stimuli.
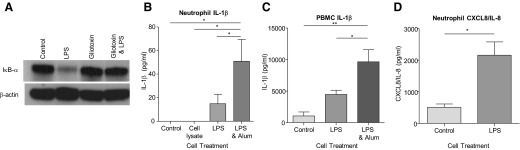
Neutrophils were incubated at 5 × 106/ml and incubated in the presence of LPS (100 ng/ml), with or without gliotoxin (0.1 μg/ml) for 30 min prior to Western blotting for IκBα (A); β-actin loading control (representative blots, n=4 separate donors). Neutrophils and PBMCs were incubated at 10 × 106/ml for 8 h in the presence of LPS (100 ng/ml), with or without alum (125 μg/ml), and IL-1β and CXCL8/IL-8 release measured (B–D). The presence of preformed IL-1β was measured in cell lysates obtained by nitrogen cavitation; n = 4–7 separate donors; *P < 0.05; **P < 0.01.
Here, we demonstrate a simple methodology using intrinsic differences in granulocyte autofluorescence to isolate highly purified human and murine neutrophils by flow sorting. This process has no effect on neutrophil activity or survival, therefore, allowing study of these cells in a variety of in vitro applications. Importantly, we subsequently demonstrate that although neutrophils possess the ability to secrete the proinflammatory cytokine IL-1β, the quantity released is markedly lower than that released by mononuclear cells. Within the context of neutrophil-dominant inflammatory conditions, however, neutrophil-derived IL-1β may well be biologically significant.
ACKNOWLEDGMENTS
The authors acknowledge funding from the Wellcome Trust WT096497 (D.A.D.) and MRC G0601481 (A.G.R.) and thank Xiaofeng Zhao for help with intracellular calcium flux.
Footnotes
- APC
- allophycocyanin
- CD62L
- CD62 ligand
- DHR
- dihydrorhodamine
- FSC
- forward-scatter
- LTB4
- leukotriene B4
- NLRP3
- nucleotide-binding oligomerization-like receptor family, pyrin domain-containing 3
- PAF
- platelet-activating factor
- SSC
- side-scatter
AUTHORSHIP
D.A.D., C.D.L., A.L.A., J.A.M., F.R., and A.G.R. performed experiments. A.G.R., I.D., K.D., and C.H. provided guidance and critical review of methods. D.A.D., C.D.L., and A.G.R. wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Dorward D. A., Lucas C. D., Rossi A. G., Haslett C., Dhaliwal K. (2012) Imaging inflammation: molecular strategies to visualize key components of the inflammatory cascade, from initiation to resolution. Pharmacol. Ther. 135, 182–199 [DOI] [PubMed] [Google Scholar]
- 2. Sabroe I., Prince L. R., Dower S. K., Walmsley S. R., Chilvers E. R., Whyte M. K. (2004) What can we learn from highly purified neutrophils? Biochem. Soc. Trans. 32, 468–469 [DOI] [PubMed] [Google Scholar]
- 3. Davey M. S., Tamassia N., Rossato M., Bazzoni F., Calzetti F., Bruderek K., Sironi M., Zimmer L., Bottazzi B., Mantovani A., Brandau S., Moser B., Eberl M., Cassatella M. A. (2011) Failure to detect production of IL-10 by activated human neutrophils. Nat. Immunol. 12, 1017–1018 [DOI] [PubMed] [Google Scholar]
- 4. Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr., Henson P. M. (1985) Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119, 101–110 [PMC free article] [PubMed] [Google Scholar]
- 5. Lane T. A., Bergum P. W., Lichter J. P., Spragg R. G. (1982) The labeling of rabbit neutrophils with [111In]oxine. J. Immunol. Methods 51, 293–305 [DOI] [PubMed] [Google Scholar]
- 6. Youssef P. P., Mantzioris B. X., Roberts-Thomson P. J., Ahern M. J., Smith M. D. (1995) Effects of ex vivo manipulation on the expression of cell adhesion molecules on neutrophils. J. Immunol. Methods 186, 217–224 [DOI] [PubMed] [Google Scholar]
- 7. Macey M. G., McCarthy D. A., Vordermeier S., Newland A. C., Brown K. A. (1995) Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J. Immunol. Methods 181, 211–219 [DOI] [PubMed] [Google Scholar]
- 8. Hu Y. (2012) Isolation of human and mouse neutrophils ex vivo and in vitro. Methods Mol. Biol. 844, 101–113 [DOI] [PubMed] [Google Scholar]
- 9. Lucas C. D., Allen K. C., Dorward D. A., Hoodless L. J., Melrose L. A., Marwick J. A., Tucker C. S., Haslett C., Duffin R., Rossi A. G. (2013) Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. 27, 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parker L. C., Prince L. R., Buttle D. J., Sabroe I. (2009) The generation of highly purified primary human neutrophils and assessment of apoptosis in response to Toll-like receptor ligands. Methods Mol. Biol. 517, 191–204 [DOI] [PubMed] [Google Scholar]
- 11. Cotter M. J., Norman K. E., Hellewell P. G., Ridger V. C. (2001) A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 159, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaye D. L., Bray R. A., Gebel H. M., Harris W. A., Waller E. K. (2012) Translational applications of flow cytometry in clinical practice. J. Immunol. 188, 4715–4719 [DOI] [PubMed] [Google Scholar]
- 13. Weil G. J., Chused T. M. (1981) Eosinophil autofluorescence and its use in isolation and analysis of human eosinophils using flow microfluorometry. Blood 57, 1099–1104 [PubMed] [Google Scholar]
- 14. Boxio R., Bossenmeyer-Pourie C., Steinckwich N., Dournon C., Nusse O. (2004) Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75, 604–611 [DOI] [PubMed] [Google Scholar]
- 15. Ward C., Wong T. H., Murray J., Rahman I., Haslett C., Chilvers E. R., Rossi A. G. (2000) Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem. Pharmacol. 59, 305–314 [DOI] [PubMed] [Google Scholar]
- 16. O'Flaherty J. T., Rossi A. G. (1993) 5-Hydroxyicosatetraenoate stimulates neutrophils by a stereospecific, G protein-linked mechanism. J. Biol. Chem. 268, 14708–14714 [PubMed] [Google Scholar]
- 17. Leitch A. E., Riley N. A., Sheldrake T. A., Festa M., Fox S., Duffin R., Haslett C., Rossi A. G. (2010) The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur. J. Immunol. 40, 1127–1138 [DOI] [PubMed] [Google Scholar]
- 18. Calafat J., Janssen H., Stahle-Backdahl M., Zuurbier A. E., Knol E. F., Egesten A. (1997) Human monocytes and neutrophils store transforming growth factor-α in a subpopulation of cytoplasmic granules. Blood 90, 1255–1266 [PubMed] [Google Scholar]
- 19. Mankan A. K., Dau T., Jenne D., Hornung V. (2012) The NLRP3/ASC/caspase-1 axis regulates IL-1β processing in neutrophils. Eur. J. Immunol. 42, 710–715 [DOI] [PubMed] [Google Scholar]
- 20. Barabe F., Gilbert C., Liao N., Bourgoin S. G., Naccache P. H. (1998) Crystal-induced neutrophil activation VI. Involvment of FcγRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 12, 209–220 [DOI] [PubMed] [Google Scholar]
- 21. Mackenzie S. J., Kerr M. A. (1995) IgM monoclonal antibodies recognizing Fc α R but not Fc γ RIII trigger a respiratory burst in neutrophils although both trigger an increase in intracellular calcium levels and degranulation. Biochem. J. 306, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shapiro H. M. (2003) Practical Flow Cytometry, 4th Ed Wiley Liss, New York, NY, USA [Google Scholar]
- 23. Mayeno A. N., Hamann K. J., Gleich G. J. (1992) Granule-associated flavin adenine dinucleotide (FAD) is responsible for eosinophil autofluorescence. J. Leukoc. Biol. 51, 172–175 [DOI] [PubMed] [Google Scholar]
- 24. Sabroe I., Hartnell A., Jopling L. A., Bel S., Ponath P. D., Pease J. E., Collins P. D., Williams T. J. (1999) Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 162, 2946–2955 [PubMed] [Google Scholar]
- 25. Eversole R. R., Mackenzie C. D., Beuving L. J. (2003) A photoreactive fluorescent marker for identifying eosinophils and their cytoplasmic granules in tissues. J. Histochem. Cytochem. 51, 253–257 [DOI] [PubMed] [Google Scholar]
- 26. Watt S. M., Burgess A. W., Metcalf D., Battye F. L. (1980) Isolation of mouse bone marrow neutrophils by light scatter and autofluorescence. J. Histochem. Cytochem. 28, 934–946 [DOI] [PubMed] [Google Scholar]
- 27. Lee A., Whyte M. K., Haslett C. (1993) Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54, 283–288 [PubMed] [Google Scholar]
- 28. Alessandri A. L., Duffin R., Leitch A. E., Lucas C. D., Sheldrake T. A., Dorward D. A., Hirani N., Pinho V., de Sousa L. P., Teixeira M. M., Lyons J. F., Haslett C., Rossi A. G. (2011) Induction of eosinophil apoptosis by the cyclin-dependent kinase inhibitor AT7519 promotes the resolution of eosinophil-dominant allergic inflammation. PLoS One 6, e25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akuthota P., Shamri R., Weller P. F. (2012) Isolation of human eosinophils. Curr. Protoc. Immunol. Chapter 7, Unit 7.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scapini P., Lapinet-Vera J. A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M. A. (2000) The neutrophil as a cellular source of chemokines. Immunol. Rev. 177, 195–203 [DOI] [PubMed] [Google Scholar]
- 31. Cassatella M. A. (1999) Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73, 369–509 [DOI] [PubMed] [Google Scholar]
- 32. Ward C., Chilvers E. R., Lawson M. F., Pryde J. G., Fujihara S., Farrow S. N., Haslett C., Rossi A. G. (1999) NF-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274, 4309–4318 [DOI] [PubMed] [Google Scholar]
- 33. Schroder A. K., Uciechowski P., Fleischer D., Rink L. (2006) Crosslinking of CD66B on peripheral blood neutrophils mediates the release of interleukin-8 from intracellular storage. Hum. Immunol. 67, 676–682 [DOI] [PubMed] [Google Scholar]