Chronic alcohol-induced neuroinflammation is mediated by the NLRP3 inflammasome, is augmented by TLR4, and involves HMGB1.
Keywords: HMGB1, TNF-α, MCP-1, cerebellum, CNS
Abstract
Alcohol-induced neuroinflammation is mediated by proinflammatory cytokines, including IL-1β. IL-1β production requires caspase-1 activation by inflammasomes—multiprotein complexes that are assembled in response to danger signals. We hypothesized that alcohol-induced inflammasome activation contributes to increased IL-1β in the brain. WT and TLR4-, NLRP3-, and ASC-deficient (KO) mice received an ethanol-containing or isocaloric control diet for 5 weeks, and some received the rIL-1ra, anakinra, or saline treatment. Inflammasome activation, proinflammatory cytokines, endotoxin, and HMGB1 were measured in the cerebellum. Expression of inflammasome components (NLRP1, NLRP3, ASC) and proinflammatory cytokines (TNF-α, MCP-1) was increased in brains of alcohol-fed compared with control mice. Increased caspase-1 activity and IL-1β protein in ethanol-fed mice indicated inflammasome activation. TLR4 deficiency protected from TNF-α, MCP-1, and attenuated alcohol-induced IL-1β increases. The TLR4 ligand, LPS, was not increased in the cerebellum. However, we found up-regulation of acetylated and phosphorylated HMGB1 and increased expression of the HMGB1 receptors (TLR2, TLR4, TLR9, RAGE) in alcohol-fed mice. NLRP3- or ASC-deficient mice were protected from caspase-1 activation and alcohol-induced IL-1β increase in the brain. Furthermore, in vivo treatment with rIL-1ra prevented alcohol-induced inflammasome activation and IL-1β, TNF-α, and acetylated HMGB1 increases in the cerebellum. Conversely, intracranial IL-1β administration induced TNF-α and MCP-1 in the cerebellum. In conclusion, alcohol up-regulates and activates the NLRP3/ASC inflammasome, leading to caspase-1 activation and IL-1β increase in the cerebellum. IL-1β amplifies neuroinflammation, and disruption of IL-1/IL-1R signaling prevents alcohol-induced inflammasome activation and neuroinflammation. Increased levels of acetylated and phosphorylated HMGB1 may contribute to alcoholic neuroinflammation.
Introduction
Innate immune signaling pathways play pivotal roles in the pathogenesis of neuroinfectious [1] and neurodegenerative diseases [2]. Inflammatory cytokines and mediators, including TNF-α, HMGB1, and IL-1β, affect neurons and are associated with disease symptoms in animal models [3]. TLR4 signaling involved in proinflammatory cytokine production has been proposed to trigger alcohol-induced neuroinflammation in rodents [4].
Inflammation and altered innate immune responses are hallmarks of alcohol-induced organ damage, affecting the liver, cardiovascular system, and brain [5–7]. Long-term alcohol intake results in neuroinflammation [8] and neurodegeneration in humans as well as animal models, as evidenced by increased expression of MCP-1, TNF-α, and caspase-3 in the brain [7, 9, 10]. Recently, increased levels of IL-1β were found in rodent brains after alcohol intake, but the mechanisms are undefined [7].
Pro-IL-1β levels are increased in response to TLR activation upon endogenous or exogenous danger signals, and the inflammasome processes pro-IL-1β to mature, active IL-1β via caspase-1 activation [11]. Inflammasomes are multiprotein complexes, containing NLRs (NLRP1, NLRP3, NLRC4), an adapter molecule (ASC), and procaspase-1 [11]. Inflammatory stimuli up-regulate the expression of these inflammasome components. For instance, NLRs are activated by PAMPs or DAMPs. NLRP3 inflammasome activation generally requires two signals: first, a priming signal to increase expression of inflammasome components and target proteins, procaspase-1 and pro-IL-1β, and a second, signal that leads to inflammasome activation and IL-1β secretion [12]. Upon inflammasome activation, procaspase-1 is converted to caspase-1 (a proteolytic, effector enzyme) that then cleaves pro-IL-1β to the mature, secretable form [11].
Inflammasome activation contributes to the pathogenesis of various neurologic disorders, including traumatic and thromboembolic brain injury and Alzheimer's disease [13–16]. PAMPs or DAMPs in sterile inflammation induce inflammasome activation [17]. DAMPs are molecules or cells that upon cellular danger, are actively excreted or passively released [18]. HMGB1 is a nuclear protein that upon cytoplasmic translocation, phosphorylation, or acetylation, represents a danger signal and activates TLRs and the inflammasome [19, 20].
We hypothesized that inflammasome activation contributes to IL-1β increase and neuroinflammation following chronic alcohol exposure in the brain. With a mouse model, we demonstrate for the first time that the inflammasome complex is activated in the cerebella of chronic alcohol-fed mice and that NLRP3- or ASC-deficient mice are protected from neuroinflammation. We show that inflammasome activation is TLR4-independent and identify increased phosphorylated and acetylated HMGB1 as potential alcohol-induced DAMPs in the brain. Finally, we demonstrate that pharmacologic disruption of IL-1R signaling with an IL-1ra can protect from alcohol-induced neuroinflammation.
MATERIALS AND METHODS
Animals
This study was conducted according to the regulations of the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School (Worcester, MA, USA). Six- to 8-week-old female C57BL/6J WT TLR4 KO, ASC KO, and NLRP3 KO mice (backcrossed on a C57BL/6J background) were used. For 5 weeks, the animals received 5% (v/v) ethanol (36% ethanol-derived calories) containing an EtOH or a PF diet with an equal amount of calories, where the alcohol-derived calories were substituted with dextran-maltose (Bio-Serv, Frenchtown, NJ, USA) [21]. The daily consumption of the diet was the same in the WT and all KO mouse strains, ∼10 ml/animal. The animals had access to water ad libitum, and the consumption was comparable among the different diet groups. BALs were measured using an alcohol analyzer (Analox Instruments, Lunenburg, MA, USA). BAL was significantly higher in EtOH compared with PF mice in all genotypes, and there was no statistically significant difference in BAL among the different genotypes (Supplemental Fig. 1). Some 8-week-old female C57BL/6J mice were anesthetized with isoflurane inhalation to effect and received 30 μl intracranial (posterior fontanelle) injection with mouse rIL-1β (100 ng/mouse; R&D Systems, Minneapolis, MN, USA) or equal amount of saline solution; these animals were sacrificed 6 h after the procedure.
Treatment
Where indicated, WT mice received daily i.p. injection of 25 mg/kg rIL-1ra (anakinra) or an equal amount of saline throughout the whole experiment.
Sample collection
Blood was collected, and animals were sacrificed by cervical dislocation. Cerebella and cerebra were collected immediately after cheek-bleeding and washed in ice-cold PBS. The tissue samples were snap-frozen or stored in RNAlater (Qiagen GmbH, Hilden, Germany) for protein or mRNA evaluation, respectively. Serum and brain samples were stored at −80°C.
PCR
RNA was extracted using RNeasy kit (Qiagen, Germantown, MD, USA). cDNA was transcribed from 1 μg total RNA by using the Reverse Transcription system (Promega, Madison, WI, USA) in a final volume of 30 μl. Sybr-Green-based real-time quantitative PCR was performed using the iCycler (Bio-Rad Laboratories, Hercules, CA, USA). A comparative threshold cycle method was used to calculate expressions relative to WT control groups. The final results were expressed as fold changes between the sample and the controls corrected with housekeeping gene 18S [22]. Primers that were used for the experiments are listed in Table 1.
Table 1. Real-Time PCR Primers.
| Target gene | Forward primer (5′>3′) | Reverse primer (5′>3′) |
|---|---|---|
| 18S | GTA ACC CGT TGA ACC CCA TT | CCA TCC AAT CGG TAG TAG CG |
| AIM2 | GGA GGT CAC CAG TTC CTC AG | TTT GTT TTG CTT GGG TTT CC |
| ASC | GAA GCT GCT GAC AGT GCA AC | GCC ACA GCT CCA GAC TCT TC |
| GFAP | GGA GAG GGA CAA CTT TGC AC | CCA GCG ATT CAA CCT TTC TC |
| HMGB1 | CGC GGA GGA AAA TCA ACT AA | TCA TAA CGA GCC TTG TCA GC |
| Iba1 | CCG AGG AGA CGT TCA GCT AC | GAC ATC CAC CTC CAA TCA GG |
| IL-1ra | GAG ACA GCC TGC CTG CCT GGG GGA | TCA CTG GCA TGG CCA CCT GC |
| NLRC4 | TGG TGA CAA TAG GGC TCC TC | CTG TTC CCT TTG CTC ACC TC |
| NLRP1 | TGG CAC ATC CTA GGG AAA TC | TCC TCA CGT GAC AGC AGA AC |
| NLRP3 | AGC CTT CCA GGA TCC TCT TC | CTT GGG CAG CAG TTT CTT TC |
| Pannexin-1 | TGT GGC TGC ACA AGT TCT TC | ACA GAC TCT GCC CCA CAT TC |
| Procaspase-1 | AGA TGG CAC ATT TCC AGG AC | GAT CCT CCA GCA GCA ACT TC |
| Pro-IL-1β | TCT TTG AAG TTG ACG GAC CC | TGA GTG ATA CTG CCT GCC TG |
| RAGE | GAA GGC TCT GTG GGT GAG TC | CCG CTT CCT CTG ACT GAT TC |
| TLR2 | ACA ATA GAG GGA GAC GCC TTT | AGT GTC TGG TAA GGA TTT CCC AT |
| TLR3 | GTG AGA TAC AAC GTA GCT GAC TG | TCC TGC ATC CAA GAT AGC AAG T |
| TLR4 | GCC TTT CAG GGA ATT AAG CTC C | AGA TCA ACC GAT GGA CGT GTA A |
| TLR9 | TGA AGT CTG TAC CCC GTT TCT | GTG GAC GAA GTC GGA GTT GT |
| TNF-α | CAC CAC CAT CAA GGA CTC AA | AGG CAA CCT GAC CAC TCT CC |
Real-time PCR primers—forward and reverse sequence of the primers used in real-time PCR.
ELISA
Tissue lysates were prepared from cerebella and cortices in RIPA buffer containing protease and phosphatase inhibitors (1 mM PMSF, 1 mM NaF, 2 mM Na3VO4, 20 mM Na4P2O7; Sigma-Aldrich, St.Louis, MO, USA) and protease and phosphatase inhibitor tablet (Roche Diagnostics, Indianapolis, IN, USA). First, the tissue was homogenized with stainless-steel bead (Qiagen, Germantown, MD, USA) in TissueLyser II (Qiagen, Germantown, MD, USA) and then clarified by centrifugation. The tissue lysate supernatant was stored at −80°C. Protein level was measured by an ELISA reader using Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories). TNF-α (BD Biosciences, San Diego, CA, USA), MCP-1 (BioLegend, San Diego, CA, USA), and IL-1β (R&D Systems) were measured in whole tissue lysates.
Enzyme-activity assay
Caspase-1 colorimetric assay was used to determine the enzymatic activity (R&D Systems) from cerebellar or cortical tissue lysates. Our group has shown earlier that the results of caspase-1 activity assay correspond to the Western blot results of cleaved capase-1 p10 protein in the liver [23]. We further evaluated the method for brain tissue using Western blots (see Fig. 4D).
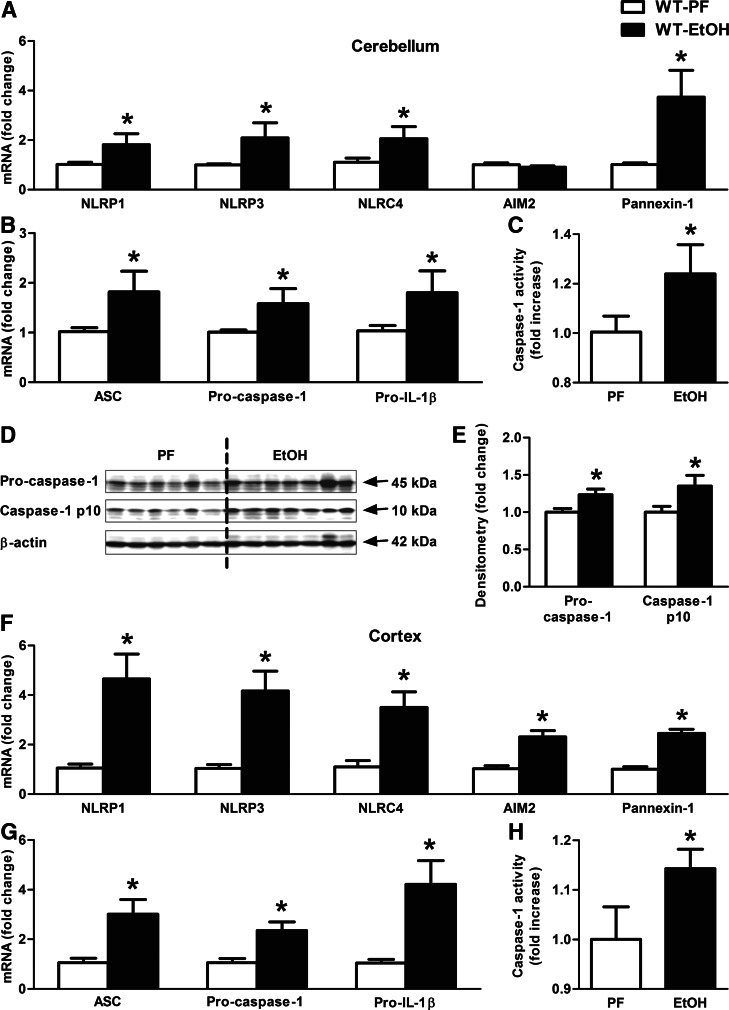
Figure 4. Inflammasome complex is up-regulated and activated in alcohol-fed mice in the cerebellum.
WT mice were fed with an EtOH (n=6) or a control (PF; n=8) diet for 5 weeks. Various inflammasome sensors (NLRP1, NLRP3, NLRC4, AIM2) and Pannexin-1 (A) and the inflammasome adaptor (ASC), the inflammasome effector (procaspase-1), and pro-IL-1β (B) were assessed by real-time PCR from whole cerebellar RNA extracts, normalized to 18S. Inflammasome activity was measured by a caspase-1 colorimetric assay (C) from whole cerebellar lysates. The caspase-1 p10 level (D) was visualized on a Western blot, using β-actin as a loading control, and quantified by densitometry (E). NLRP1, NLRP3, NLRC4, AIM2, and Pannexin-1 (F) and ASC, procaspase-1, and pro-IL-1β (G) were assessed by real-time PCR from whole cortical RNA extracts. Inflammasome activity was measured by a caspase-1 colorimetric assay (H) from whole cortical lysates. Bars represent mean ± sem (*P<0.05, relative to appropriate PF controls by the Kruskal-Wallis nonparametric test).
Endotoxin
Endotoxin levels were evaluated in serum and cerebellar lysates with LAL assay (Lonza Group, Basel, Switzerland).
Western blot
Tissue lysates were run on a 15% polyacrylamide gel. Proteins were transferred to nitrocellulose membrane overnight then blocked for 2 h in blocking buffer-1 or -2. Primary antibodies against IL-1β (R&D Systems), caspase-1 p10 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), HMGB1 (Abcam, Cambridge, MA, USA), and β-actin (Abcam) were used overnight at 4°C at different dilution rates varying from 1:100 to 1:10,000 in blocking buffer-1 or -2, followed by three washing steps. For detection, appropriate goat anti-rat, anti-rabbit, or anti-mouse secondary HRP-linked antibodies (Santa Cruz Biotechnology) were used for 1 h at a dilution rate of 1:5000 in blocking buffer-1 or -2. The immunoreactive bands were detected by chemiluminescence using Pierce ECL Western blotting substrate (Pierce Biotechnology, Rockford, IL, USA) and LAS-4000IR Ver.2.02 (Fujifilm, Valhalla, NY, USA). The results were quantified by densitometric analysis using Multi Gauge Version 3.2 Image software (Fujifilm). Correction for protein loading was made by dividing the OD of each protein sample with its corresponding β-actin OD. The densitometry fold change of each group was calculated by comparison to WT-PF mice, as WT-PF mice have a fold change of “one”. The sem of each group of fold changes would represent the variability seen within the group (blocking buffer-1: 0.1% Tween-20 TBS, 5% milk; blocking buffer-2: 0.1% Tween-20 TBS, 5% BSA).
IP
Equal amounts of proteins from cerebellar tissue lysates were precleared with anti-rabbit Ig IP beads (eBioscience, San Diego, CA, USA). Beads were removed by centrifuge, and supernatants were incubated and rotated overnight at 4°C with 5 μg anti-HMGB1 or anti-acetyl lysine antibody (Abcam) or normal rabbit IgG (Santa Cruz Biotechnology) as a negative control. Samples were rotated partially for 1 h with anti-rabbit Ig IP beads at 4°C. The formed immuncomplexes were collected by centrifugation, washed three times, and boiled with 2× Laemmli's SDS sample buffer (Boston BioProducts, Ashland, MA, USA) to dissociate from the beads. The beads were then removed by centrifugation. Protein was separated from the supernatants by PAGE. Proteins were transferred to the nitrocellulose membrane and blocked for 2 h in blocking buffer-2 or TBS with 3% BSA, 0.1% gelatin, and 0.2% Tween-20.
The membrane was incubated overnight at 4°C with 3 μg/ml antiphosphoserine or 1 μg/ml anti-HMGB1 antibody (Abcam) in blocking buffer-2 or -3. The membrane was washed three times and incubated for 1 h with HRP-conjugated secondary anti-rabbit IgG antibody (eBioscience) at a 1:5000 dilution in blocking buffer-2 or -3. The immunoreactive bands were detected by chemiluminescence using Pierce ECL Western blotting substrate and LAS-4000IR. The results were quantified by densitometric analysis using Multi Gauge Version 3.2 Image software. The loading control was detected on a separate gel by monoclonal mouse anti-β-actin (blocking buffer-3: 0.2% Tween-20 TBS, containing 0.5% BSA).
Statistical analysis
As the data were not distributed normally, statistical analysis was performed using a Kruskal-Wallis nonparametric test. Data are shown as average ± sem, and differences were considered statistically significant at P ≤ 0.05. The experiments were performed a minimum of two times.
RESULTS
Chronic alcohol feeding results in neuroinflammation and increased inflammatory cytokine production in mice
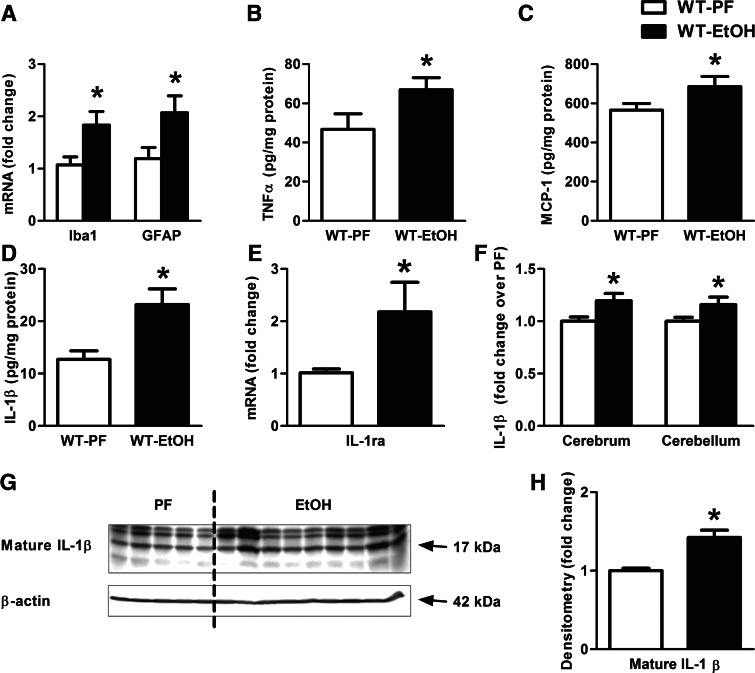
Previous studies showed that alcohol feeding in rodents leads to astrogliosis and microglia activation in the brain [7, 10, 24]. However, the effect of ethanol on the cerebellum has not yet been studied extensively. We found increased mRNA levels of the astrocyte-specific marker, GFAP [25, 26], and the microglial activation marker, Iba1, in the cerebellum of alcohol-fed mice (Fig. 1A). Consistent with neuroinflammation, we observed a significant increase in the protein levels of inflammatory mediators in the cerebellum, including TNF-α (Fig. 1B), MCP-1 (Fig. 1C), and IL-1β (Fig. 1D, G, and H) in EtOH-fed mice compared with PF controls. This was associated with the up-regulation of the endogenous IL-1ra (Fig. 1E), a natural inhibitor of IL-1β and IL-1α [27]. There was no increase in IL-1α protein in the cerebellum of alcohol-fed mice (data not shown). We determined that the increase in IL-1β production was identical in the cerebral cortex and the cerebellum of alcohol-fed mice, suggesting that investigation of the cerebellum is representative of the neuroinflammation in the brain (Fig. 1F).
Figure 1. Proinflammatory cytokines are increased in alcohol-induced brain injury.
WT mice were fed with an EtOH (n=6) or a control (PF; n=8) diet for 5 weeks. Astrocyte (GFAP) and microglia (Iba1) markers (A), as well as IL-1ra (E) were assessed by real-time PCR from whole cerebellar RNA extract, normalized to 18S. Inflammatory cytokines, TNF-α (B) and MCP-1 (C) of whole cerebellar lysates, and IL-1β (D and F) of cerebellar and cerebral lysates were measured by specific ELISAs. Mature IL-1β of whole cerebellar lysates were assessed by Western blot using β-actin as a loading control (G) and quantified further by densitometry (H). Bars represent mean ± sem (*P<0.05, relative to appropriate PF controls by the Kruskal-Wallis nonparametric test).
TLR4-KO mice are partially protected against alcohol-induced, neuroinflammatory cytokine production
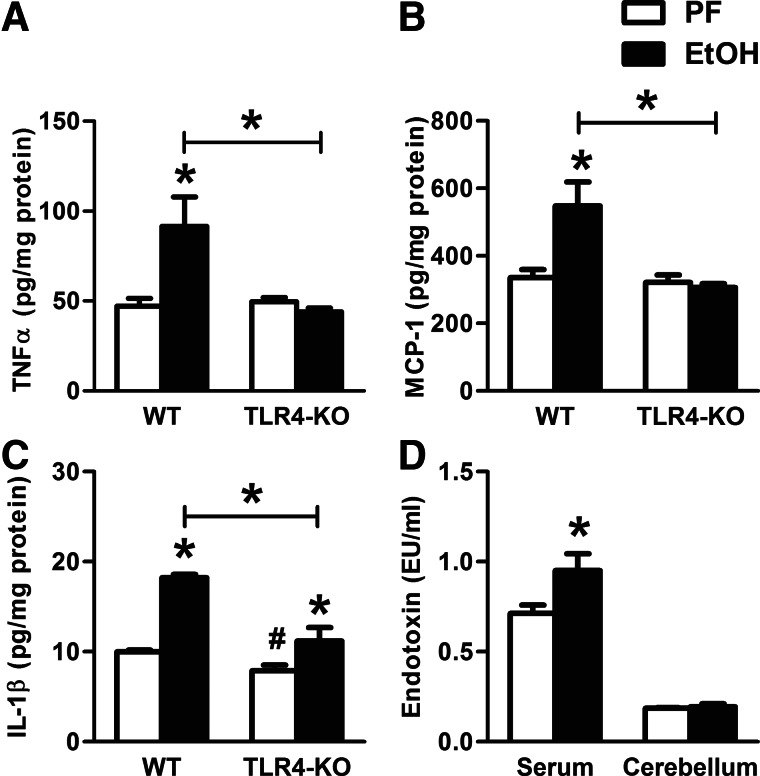
Previous studies indicated a role for TLR4 in alcohol-related, proinflammatory cytokine production by astrocytes and microglia [4, 24]. Thus, we evaluated the role of TLR4-mediated signals in alcohol-induced IL-1β production and neuroinflammation. In contrast to WT mice, alcohol-fed, TLR4-KO mice had no increase in TNF-α and MCP-1 protein levels in the cerebellum compared with PF controls (Fig. 2A and B). There was an overall decrease in IL-1β protein levels in TLR4-KO compared with WT mice; however, IL-1β remained induced by ethanol compared with PF TLR4-KO control mice (Fig. 2C), suggesting that IL-1β production was only partially dependent on TLR4 in the brain. PAMPs, including LPS, activate the TLR4 signaling pathway [28]. We found a significant increase in serum endotoxin (LPS) levels after alcohol feeding, but we found no detectable LPS in the cerebellum of alcohol-fed mice (Fig. 2D). These data suggested that first, TLR4-mediated pathways were responsible for the alcohol-induced TNF-α and MCP-1 increase in the cerebellum, even in the absence of detectable LPS in the brain; and second, increased IL-1β in the cerebellum after alcohol-feeding was not fully TLR4- or directly LPS-mediated.
Figure 2. Cerebella of TLR4-deficient, alcohol-fed mice are partially protected against inflammatory cytokine production.
WT (n=8 or 7) and TLR4-KO (n=8 or 13) mice were fed with a control (PF) or EtOH diet for 5 weeks, respectively. Inflammatory cytokines, TNF-α (A), MCP-1 (B), and IL-1β (C) of whole cerebellar lysates, were measured by specific ELISAs. Endotoxin measurement was executed in serum and whole cerebellar lysates of WT mice by LAL assay (D). Bars represent mean ± sem (*P<0.05, relative to appropriate PF or WT-EtOH controls; #P<0.05, relative to appropriate WT-PF controls by the Kruskal-Wallis nonparametric test).
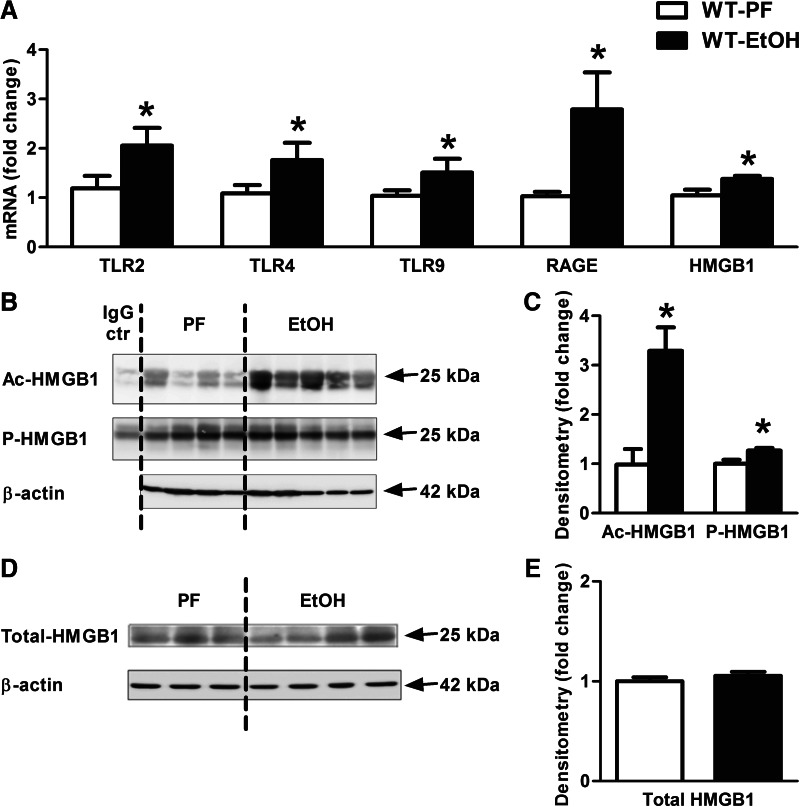
Chronic alcohol results in increased levels of acetylated and phosphorylated HMGB1 and HMGB1 receptors in the cerebellum
Based on the observation that IL-1β protein induction was only partially dependent of TLR4 and/or LPS, we searched for DAMPs that could induce inflammation. HMGB1 is recognized by several PRRs, including TLR2, TLR4, TLR9, and RAGE [19]. We observed a significant increase in the mRNA expression of TLR2, TLR4, TLR9, and RAGE receptors in the cerebellum of ethanol-fed compared with control mice (Fig. 3A). In addition, expression of HMGB1 mRNA was increased in the cerebellum of ethanol-fed mice (Fig. 3A). Post-translational modification by acetylation and phosphorylation is important for translocation of nuclear HMGB1 to the cytoplasm and its subsequent release [29], and acetylated or phosphorylated HMGB1 is recognized as a danger signal [30, 31]. With the use of IP, we detected significantly increased acetylated HMGB1, as well as phosphorylated HMGB1 (Fig. 3B and C) levels in the cerebellum in alcohol-fed compared with PF mice. Total HMGB1 levels were comparable between alcohol-fed and control mice (Fig. 3D and E).
Figure 3. Active endogenous danger molecule, HMGB1, and its receptors are elevated in alcohol-fed mice in the cerebellum.
WT mice were fed with an EtOH (n=6) or a control (PF; n=8) diet for 5 weeks. TLR2, TLR4, TLR9, and RAGE receptors and HMGB1 (A) were assessed by real-time PCR from whole cerebellar RNA extracts, normalized to 18S. Acetyl (Ac)- and phospho (P)-HMGB1 of whole cerebellar lysates were analyzed by IP (B) and assessed further by densitometry, using β-actin Western blot for loading control on a separate gel (C). Cerebellar lysate of one representative ethanol-fed mouse was applied for IgG control (ctr), using the same amount of protein. Total HMGB1 of whole cerebellar lysates was analyzed by Western blot using β-actin as a loading control (D) and quantified further by densitometry (E). Bars represent mean ± sem (*P<0.05, relative to appropriate PF controls by the Kruskal-Wallis nonparametric test).
Inflammasome expression and activation are increased in the cerebellum of alcohol-fed mice
Recent reports suggest that HMGB1 can contribute to the activation of the inflammasome complex to cleave pro-IL-1β into its mature (17 kD), biologically active form [20]. Our data indicated increased IL-1β in the brain after chronic alcohol administration (Fig. 1D and F–H). Thus, we evaluated the role of inflammasome activation in IL-1β production. We observed a significant increase in the expression of several inflammasome components, including NLRP1, NLRP3, NLRC4, and Pannexin-1, at the mRNA level in ethanol-fed compared with control mice (Fig. 4A). Expression of the inflammasome adaptor molecule, ASC, and the effector proteins, procaspase-1 and pro-IL-1β, were all increased at the mRNA level by alcohol feeding (Fig. 4B). Caspase-1 is the effector enzyme of the inflammasome complex, and when the 45-kDa procaspase-1 protein is cleaved into its active forms—caspase-1 p20- and p10-kDa subunits—it exerts its proteolytic effect on pro-IL-1β [11]. We found a significant increase in caspase-1 activity in the cerebella of alcohol-fed compared with PF mice (Fig. 4C), and this was verified by increased levels of the active, cleaved caspase-1 p10 on Western blots (Fig. 4D and E). Similarly, increased cortical mRNA expressions of the inflammasome receptors (Fig. 4F) and components, including ASC, procaspase-1, and pro-IL-1β (Fig. 4G), were observed in the cortex of ethanol-fed mice. Furthermore, caspase-1 activity was increased in the cortex of ethanol-fed compared with PF mice (Fig. 4H).
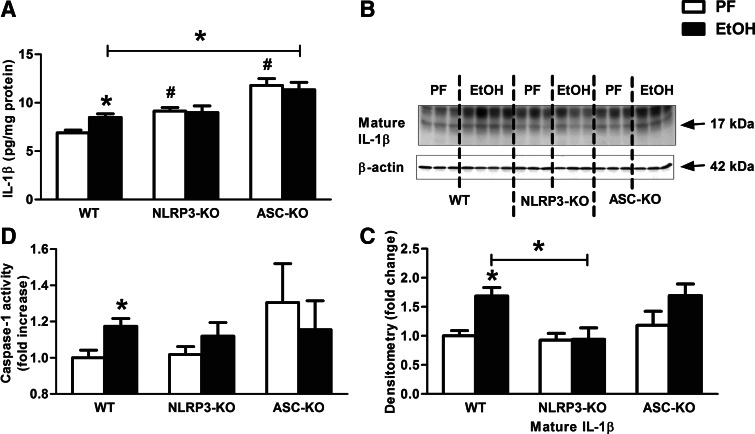
Deficiency in NLRP3 or ASC protects from an IL-1β increase in the brain after chronic ethanol feeding
To investigate further the mechanistic role of inflammasome activation in alcohol-induced IL-1β formation, we evaluated neuroinflammation in mice deficient in the inflammasome sensor, NLRP3, or the adaptor molecule, ASC. We found no increase in IL-1β protein levels (Fig. 5A–C) or caspase-1 activity (Fig. 5D) in alcohol-fed NLRP3-KO or ASC-KO mice compared with PF controls. This was in contrast with the significant increase in caspase-1 activity and mature IL-1β in alcohol-fed WT mice. The IL-1β mRNA expression was higher in NLRP3- and ASC-KO compared with WT mice that appears to be the result of phenotype. However, alcohol failed to induce a further increase in the NLRP3 and ASC-KO mice. These results suggested that deficiency in NLRP3 inflammasome or ASC can prevent the alcohol-induced increase in IL-1β production in the brain.
Figure 5. NLRP3 or ASC deficiency prevents caspase-1 activity and IL-1β production in alcohol-fed mice in the cerebellum.
WT (n=7 or 14), NLRP3 (n=8 or 10), and ASC-KO (n=9 or 12) mice were fed with a control (PF) or an EtOH diet for 5 weeks, respectively. IL-1β protein level of whole cerebellar lysates was measured by specific ELISA (A). Mature IL-1β of whole cerebellar lysates was assessed by Western blot using β-actin as a loading control (B) and quantified further by densitometry (C). Inflammasome activity was evaluated by a caspase-1 colorimetric assay from whole cerebellar lysates (D). Bars represent mean ± sem (*P<0.05, relative to appropriate PF or WT-EtOH controls; #P<0.05, relative to appropriate WT-PF controls by the Kruskal-Wallis nonparametric test).
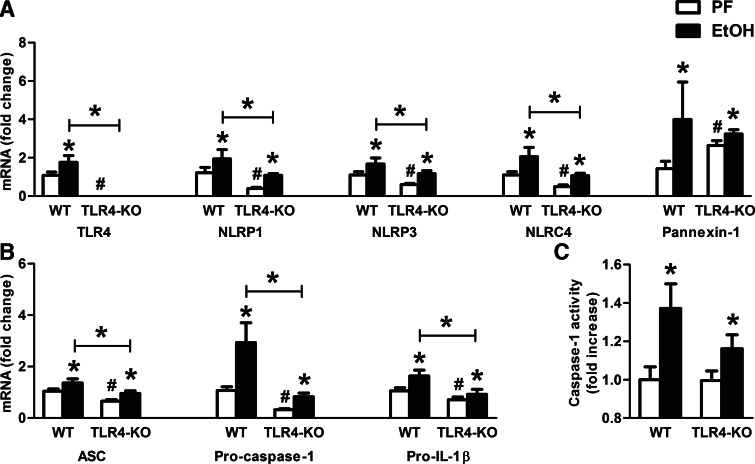
TLR4 deficiency attenuates inflammasome mRNA expression but not activation by alcohol
NLRP3 inflammasome activation involves two steps, priming via TLRs to up-regulate the expression of inflammasome components and a second signal to activate the inflammasome complex to cleave caspase-1 [12]. Therefore, we investigated the role of TLR4 as a primer in inflammasome up-regulation in the brain. We found significantly attenuated expression of the inflammasome components (NLRP1, NLRP3, NLRC4, ASC) and effector molecules (procaspase-1, pro-IL-1β) in alcohol-fed TLR4-KO compared with WT mice (Fig. 6A and B); however, the levels remained induced upon alcohol challenge with similar fold-change induction as in WT controls.
Figure 6. TLR4 deficiency does not decrease caspase-1 activity in alcohol-fed mice in the cerebellum.
WT (n=8 or 7) and TLR4-KO (n=8 or 13) mice were fed with a control (PF) or an EtOH diet for 5 weeks, respectively. TLR4, various inflammasome receptors (NLRP1, NLRP3, NLRC4), and Pannexin-1 (A) and the inflammasome adaptor ASC, the inflammasome effector procaspase-1, and pro-IL-1β (B) were assessed by real-time PCR from whole cerebellar RNA extracts, normalized to 18S. Inflammasome activity was evaluated by the caspase-1 colorimetric assay from whole cerebellar lysates (C). Bars represent mean ± sem (*P<0.05, relative to appropriate PF or WT-EtOH controls; #P<0.05, relative to appropriate WT-PF controls by the Kruskal-Wallis nonparametric test).
To assess inflammasome activation, we tested caspase-1 activity and found a significant increase in TLR4-KO and WT mice after alcohol feeding (Fig. 6C), suggesting that activation of the inflammasome was independent of TLR4. This observation was also consistent with the alcohol-induced up-regulation of IL-1β protein in the same TLR4-KO mice seen in Fig. 2. The results suggest first, that TLR4 contributes to the baseline and alcohol-induced mRNA expression of inflammasome components. Second, data in Fig. 2C suggest that IL-1β protein production is attenuated in the absence of TLR4.
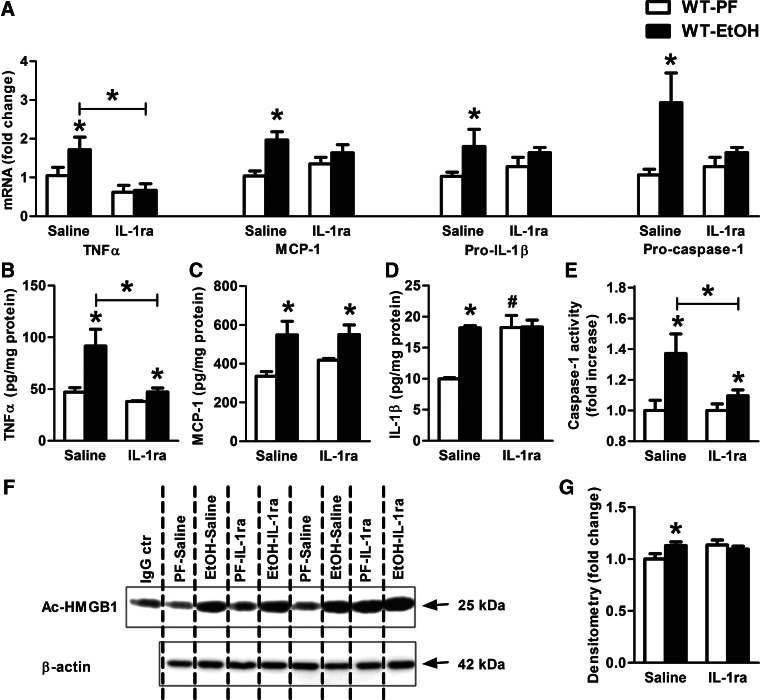
IL-1ra, anakinra, treatment prevents alcohol-induced activation of the inflammasome and inflammatory markers in the cerebellum
IL-1β exerts its biologic function via the IL-1R and amplifies inflammation through autocrine and paracrine effects [32]. The IL-1ra is a natural inhibitor of IL-1α and IL-1β; IL-1ra occupies the IL-1R without transducing activation [27]. To evaluate the involvement of IL-1/IL-1R signaling in alcohol-induced neuroinflammation, we treated mice with daily doses of 25 mg/kg rIL-1ra, anakinra, during alcohol feeding, which resulted in prevention of alcoholic liver disease [23]. IL-1ra treatment prevented the increase in TNF-α, MCP-1, pro-IL-1β, and procaspase-1 mRNA in the cerebellum of alcohol-fed mice (Fig. 7A). Furthermore, IL-1ra administration significantly attenuated TNF-α protein levels in alcohol-fed mice (Fig. 7B), suggesting interruption of the positive-feedback loop in inflammatory cytokine production. Interestingly, the alcohol-induced increase in MCP-1 protein was not affected by IL-1ra treatment in the brain (Fig. 7C). Importantly, there was no increase in IL-1β protein levels, and caspase-1 activity was significantly attenuated in IL-1ra-treated, alcohol-fed compared with PF control mice (Fig. 7D and E). Finally, there was no increase in acetylated HMGB1 levels in alcohol-fed, IL-1ra-treated mice (Fig. 7F and G).
Figure 7. IL-1ra, anakinra, treatment attenuates the effect of alcohol-feeding on caspase-1 activity and IL-1β production in murine cerebella.
WT mice were fed with a PF or an EtOH diet for 5 weeks and received daily i.p. IL-1ra (anakinra: 25 mg/kg) or an equal amount of saline injections. Inflammatory cytokines (TNF-α, MCP-1, and pro-IL-1β) and procaspase-1 (A) were assessed by real-time PCR from whole cerebellar RNA extract, normalized to 18S. Proinflammatory cytokines, TNF-α (B), MCP-1 (C), and IL-1β (D) of whole cerebellar lysates were measured by specific ELISAs. Inflammasome activity was measured by the caspase-1 colorimetric assay from whole cerebellar lysates (E). Acetyl-HMGB1 of whole cerebellar lysates were analyzed by IP (F) and assessed by densitometry, using β-actin Western blot for loading control on a separate gel (G). The cerebellar lysate of one representative ethanol-fed, IL-1ra-treated mouse was applied for IgG control, using the same amount of protein. Bars represent mean ± sem (*P<0.05, relative to appropriate PF or saline treated-EtOH controls; #P<0.05, relative to appropriate saline-treated PF controls by the Kruskal-Wallis nonparametric test. PF-saline, n = 8; PF-IL-1ra, n = 5; EtOH-saline, n = 7; EtOH-IL-1ra, n=9).
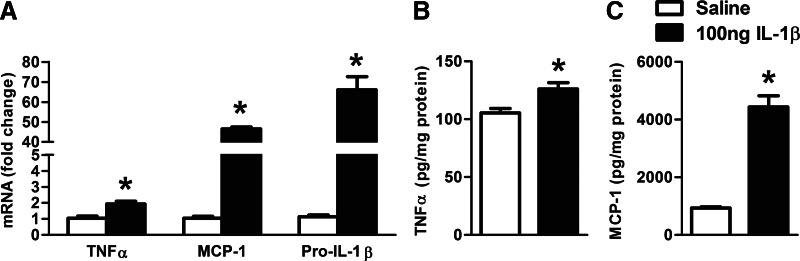
Intracranial IL-1β administration induces TNF-α and MCP-1 in the cerebellum
To evaluate whether a positive, proinflammatory feedback loop was induced by IL-1β in the brain, some mice received intracranial injection of mouse rIL-1β. We found a significant increase in TNF-α, MCP-1, and IL-1β mRNA (Fig. 8A), as well as TNF-α (Fig. 8B) and MCP-1 (Fig. 8C) protein levels in the cerebellum of mice after intracranial IL-1β injection compared with saline-treated controls.
Figure 8. IL-1β induces TNF-α and MCP-1 in the cerebellum.
WT mice received 100 ng (30 μl) intracranial mouse rIL-1β or an equal amount of saline solution (n=7 or 8). Inflammatory cytokines (TNF-α, MCP-1, and pro-IL-1β) were assessed by real-time PCR from whole cerebellar RNA extract, normalized to 18S (A). Proinflammatory cytokines, TNF-α (B) and MCP-1 (C), of whole cerebellar lysates were measured by specific ELISAs. Bars represent mean ± sem (*P<0.05, relative to appropriate saline-treated controls by the Kruskal-Wallis nonparametric test).
DISCUSSION
A previous study showed that short-term ethanol administration in mice resulted in increased brain MCP-1 and TNF-α levels, whereas IL-1β was only increased following i.p. LPS challenge [7]. Here, we report that chronic alcohol-fed mice exhibit increased IL-1β, MCP-1, and TNF-α levels in the brain without any additional stimulation. Our results demonstrate a pivotal role for NLRP3 inflammasome activation that mediates an IL-1β increase in alcohol-related neuroinflammation. We report a feed-forward amplification loop among proinflammatory cytokines, IL-1β, TNF-α, and MCP-1, as we found that intracranial IL-1β injection increases TNF-α, MCP-1, and IL-1β mRNA, as well as MCP-1 and TNF-α protein levels in the cerebellum, and IL-1R blockade prevents alcohol-induced neuroinflammation. Our data confirm an alcohol-induced amplification loop among inflammatory cytokines in the brain.
TLR4 ligands promote TNF-α and MCP-1 production that contribute to microglia accumulation, astrogliosis, and inflammatory cytokine production in alcoholic neuroinflammation [4, 24]. Our results indicate that TLR4 mediates the increase in TNF-α and MCP-1 in the brain in a LPS-independent manner, as ethanol-fed TLR4-KO mice were protected from TNF-α and MCP-1 increases. We also found attenuation but not complete protection from alcohol-induced IL-1β mRNA and protein increase in TLR4-KO mice in the cerebellum. Our mechanistic studies revealed that inflammasome activation, indicated by IL-1β release, was partially independent of TLR4; and the presence of TLR4 contributed to up-regulation of the various components of the inflammasome complex at the mRNA level [33]. In particular, the NLRP3 inflammasome can be induced by MyD88 or Toll/IL-1R domain-containing adapter-inducing IFN-β signaling pathways that are downstream of TLRs [12]. Our observation that TLR4-KO mice are not completely protected from the alcohol-induced neuroinflammatory changes is in alignment with a significantly higher GFAP staining found in TLR4-KO ethanol-fed mice compared with the nonalcohol-exposed controls in a previous study [4]. Alfonso-Loeches et al. [4] observed protection from alcohol-induced IL-1β induction, a central mediator of neuroinflammation [34], in the cerebral cortex of TLR4-KO mice. Some of the differences between our data and the study by Alfonso-Loeches et al. [4] could be attributed to differences between the alcohol administration models and the brain regions studied.
Several studies underscore the importance of the inflammasome in neuroinflammation. NLRP3 activation is involved in neuroinflammation and tissue damage in response to amyloid-β in Alzheimer's [13]. Attenuation of ASC or the NLRP1 receptor improves traumatic brain injury [16, 35]. Caspase-1 is involved in the pathogenesis of neurodegenerative and neuroinfectious diseases [14, 15, 36]. Molecules, such as NLRC4 [11, 37], AIM2 receptors [38], and the pannexin-1 channel [39], facilitate intracellular DAMP transport, stimulate the NLRP3 inflammasome, and can be activated in the neural system [11, 37]. In our animal model, NLRP3 and ASC were necessary for inflammasome activation, and our data suggest that the NLRP3 inflammasome complex is active and necessary for IL-1β production in the cerebella of mice with chronic ethanol feeding (Fig. 5). It has been postulated that two separate signals are required for NLRP3 inflammasome activation: priming that induces the inflammasome components and an activating step [12]. We investigated the role of LPS, a TLR4 ligand, in alcohol-induced inflammasome activation.
LPS mediates alcoholic liver disease and is increased in the serum of chronic alcohol-fed mice [21], as well as in patients with alcoholic cirrhosis [40]. LPS could increase IL-1β directly via the TLR4-mediated pathway in the brain; however, despite increased endotoxin levels in the serum, we found no detectable LPS in the brain of alcohol-fed mice. Our observation suggests that LPS is unlikely to be a direct cause of inflammation in the brain after alcohol feeding. Previous studies are controversial on the effect of alcohol on the integrity of the blood-brain-barrier. Some have found no increase in permeability [41–43], whereas others suggest impairment [44, 45]. Reports also indicate an indirect role for LPS in neuroinflammation [3]. Neuroimmune reflexes have been shown to sense and respond to peripheral insults [3]. Vagotomy diminished the IL-1β response in the brain but not on the periphery or the pituitary gland after i.p. injection with LPS [46]. Although we cannot rule out indirect effects of LPS via extracerebral targets, our data suggest that direct effects of LPS are not involved in neuroinflammation induced by alcohol. This led us to explore other danger molecules and/or PRRs that could be involved locally in the proinflammatory process in alcohol-induced neuroinflammation.
HMGB1, a DAMP, was shown to mediate ischemic brain damage [47]. Furthermore, nasal introduction of siRNA targeting HMGB1 in a postischemic brain injury model resulted in significant neuroprotection [48]. We found that the acetylated and phosphorylated forms of HMGB1 are increased in our model and therefore, could induce signaling through TLRs to augment the inflammasome response and IL-1β production in alcoholic neuroinflammation. HMGB1 exerts its effect, partially via inducing the multiprotein inflammasome complex to enhance IL-1β production [20, 49]. NLRP3 and ASC, independent of caspase-1, are necessary for HMGB1 release [50] and IL-1β can induce HMGB1 release from monocytes and macrophages [51, 52]. Although HMGB1 itself does not exert proinflammatory effects, upon phosphorylation, HMGB1 can enhance inflammation by binding to other cytokines and initiating or promoting signaling through TLR2, TLR4, TLR9, RAGE, or IL-1R [19, 30, 31, 53]. Similar to alcoholic steatohepatitis [54], we found increased mRNA expression levels of HMGB1 receptors (TLR2, TLR4, TLR9, and RAGE), as well as phosphorylated and acetylated HMGB1 levels in the cerebella of alcohol-treated mice. HMGB1 also exerts neuronal apoptosis via caspase-3 and induces iNOS, TNF-α, COX2, and IFN-γ expression in primary microglia [55]. Increases in caspase-3 activity, iNOS, COX2, and IFN-γ are observed in alcohol consumption [4]. Thus, it is possible that in addition to activation of the inflammasome-caspase-1 complex, increased phosphorylated and acetylated HMGB1 could also contribute to caspase-3 activation in the chronic alcoholic model in the brain. HMGB1 acetylation and phosphorylation are important for its activation, leading to translocation from the nucleus to the cytoplasm [29]. There is increasing evidence for a feed-forward activation between acetylated HMGB1 and IL-1, as IL-1β can induce HMGB1 acetylation and therefore, contribute to its release in monocytes [52]. Consistent with this notion, IL-1ra treatment prevented the increase in acetylated HMGB1 in alcohol-fed mice in our experiments. Recent studies showing increased HMGB1 expression in alcoholic human and rodent brains and its contribution to IL-1β production further corroborate the hypothesis of an existing, proinflammatory, feed-forward loop [56, 57].
The rIL-1ra, anakinra, is a disease-modifying, anti-inflammatory medication for active rheumatoid arthritis and has been suggested for treating a variety of diseases with excessive IL-1β production, including recurring-fever syndromes, gout, or diabetes mellitus [58]. In short-term ethanol administration in mice, IL-1ra pretreatment efficiently reduced the sedation and motor-impairment recovery time [59], suggesting that IL-1β or IL-1α might be involved in alcohol-induced neuroinflammatory changes. We found increased IL-1β and not IL-1α in the brain after alcohol feeding. Our in vivo experiments suggested further that IL-1ra interrupted the circuit of neuroinflammation at the level of IL-1R and prevented the excessive activation of the inflammasome complex and neuroinflammation in the cerebellum of chronic ethanol-fed mice.
Our study focused mainly on changes in the cerebellum after chronic alcohol intake. The immunological aspect of ethanol-induced cerebellar damage has not been studied extensively, and the high cellular density and easy accessibility of cerebellum provide a preferable candidate for research. Alcohol intake impairs integration of sensory perception, coordination, and motor control, which are functions of the cerebellum [60, 61]. In long term, alcohol consumption can lead to cerebellar atrophy, which is, in part, a result of malnutrition. A recent study showing cerebellar ataxia in approximately two-third of well-nourished alcoholics [62] along with our observation of inflammation suggest that immunological pathways might be involved. Furthermore, researchers found a high frequency of alcoholic cerebellar degeneration without Wernicke's encephalopathy and suggested that an early intervention might prevent progression [63]. Alcoholism causes distinctive Purkinje cell loss and molecular cell-layer changes in cerebellum [63]. Similarly to the changes in the cerebellum, we found induced proinflammatory mediators (IL-1β, TNF-α), inflammasome mRNA expression, and increased caspase-1 activation in the cerebral cortex of mice after chronic ethanol intake.
In summary, we show for the first time that increased IL-1β production after chronic alcohol administration is mediated by activation of the NLRP3/ASC inflammasome and caspase-1 and that it amplifies expression of inflammatory mediators in the brain. Our novel data suggest that chronic alcohol administration increases the levels of the phosphorylated and acetylated forms of HMGB1. Interestingly, HMGB1 antibodies or siRNA targeting HMGB1 were used effectively in arthritis, sepsis, cancer, or postischemic brain-injury models, all of which are associated with induction of the inflamamsome-IL-1β cascade [50]. ROS can also activate the NLRP3 inflammasome complex [49], and in an alcohol model, ROS has been shown to play a role in tissue damage [57]. This is supported by our findings that without the complete NADPH-oxidase complex, the alcohol-induced up-regulation of the inflammasome complex is impaired in the brain (data not shown). Finally, our innovative therapeutic intervention with a rIL-1ra prevented alcohol-induced neuroinflammation by inhibiting inflammasome activation, IL-1β production, and TNF-α increase in the brain. Based on our observation of attenuation of neuroinflammation and the beneficial effect on motor impairment and sedation reported previously [59], IL-1ra should be explored as a therapeutic intervention in alcohol-induced neuroinflammation.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by U.S. National Institutes of Health grants AA017729 and AA011576.
We thank Anna Cerny and Terence Bukong for providing technical assistance.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- AIM2
- absent in melanoma 2
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain
- BAL
- blood alcohol level
- DAMP
- danger-associated molecular pattern
- EtOH
- Lieber-DeCarli ethanol diet
- GFAP
- glial fibrillary acidic protein
- HMGB1
- high-mobility group box-1
- Iba1
- ionized calcium-binding adaptor molecule-1
- IL-1ra
- IL-1R antagonist
- IP
- immunoprecipitation
- KO
- knockout
- LAL
- Limulus amoebocyte lysate
- NLR
- nucleotide-binding oligomerization domain-like receptor
- NLRC4
- nucleotide-binding oligomerization-like receptor family caspase recruitment domain-containing protein 4
- NLRP
- nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing protein
- PF
- pair-fed (isocaloric diet)
- RAGE
- receptor for advanced glycation endoproducts
- siRNA
- small interfering RNA
AUTHORSHIP
G.S. was the principal investigator, takes primary responsibility for the paper, designed and supervised the research, and critically reviewed the manuscript. D.L. wrote the manuscript and designed, performed, and analyzed experiments. S.B. and T.C. performed experiments. J.P. and I.L. carried out animal experiments. E.A.K-J. kindly provided KO animals, critically reviewed the manuscript, and provided general advice. All authors contributed to the editing of the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Holley M. M., Kielian T. (2012) Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J. Immunol. 188, 1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tracey K. J. (2010) Understanding immunity requires more than immunology. Nat. Immunol. 11, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alfonso-Loeches S., Pascual-Lucas M., Blanco A. M., Sanchez-Vera I., Guerri C. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 30, 8285–8295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szabo G., Mandrekar P., Petrasek J., Catalano D. (2011) The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin. Exp. Res. 35, 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawaguchi M., Takahashi M., Hata T., Kashima Y., Usui F., Morimoto H., Izawa A., Takahashi Y., Masumoto J., Koyama J., Hongo M., Noda T., Nakayama J., Sagara J., Taniguchi S., Ikeda U. (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594–604 [DOI] [PubMed] [Google Scholar]
- 7. Qin L., He J., Hanes R. N., Pluzarev O., Hong J. S., Crews F. T. (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valles S. L., Blanco A. M., Pascual M., Guerri C. (2004) Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 14, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crews F. T., Nixon K. (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 44, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He J., Crews F. T. (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 210, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mankan A. K., Kubarenko A., Hornung V. (2012) Immunology in clinic review series; focus on autoinflammatory diseases: inflammasomes: mechanisms of activation. Clin. Exp. Immunol. 167, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross O., Thomas C. J., Guarda G., Tschopp J. (2011) The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 [DOI] [PubMed] [Google Scholar]
- 13. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jha S., Srivastava S. Y., Brickey W. J., Iocca H., Toews A., Morrison J. P., Chen V. S., Gris D., Matsushima G. K., Ting J. P. (2010) The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 30, 15811–15820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoegen T., Tremel N., Klein M., Angele B., Wagner H., Kirschning C., Pfister H. W., Fontana A., Hammerschmidt S., Koedel U. (2011) The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J. Immunol. 187, 5440–5451 [DOI] [PubMed] [Google Scholar]
- 16. Abulafia D. P., de Rivero Vaccari J. P., Lozano J. D., Lotocki G., Keane R. W., Dietrich W. D. (2009) Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 29, 534–544 [DOI] [PubMed] [Google Scholar]
- 17. Menu P., Vince J. E. (2011) The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin. Exp. Immunol. 166, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubartelli A., Lotze M. T. (2007) Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 [DOI] [PubMed] [Google Scholar]
- 19. Bianchi M. E. (2009) HMGB1 loves company. J. Leukoc. Biol. 86, 573–576 [DOI] [PubMed] [Google Scholar]
- 20. Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., Perfettini J. L., Schlemmer F., Tasdemir E., Uhl M., Genin P., Civas A., Ryffel B., Kanellopoulos J., Tschopp J., Andre F., Lidereau R., McLaughlin N. M., Haynes N. M., Smyth M. J., Kroemer G., Zitvogel L. (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 [DOI] [PubMed] [Google Scholar]
- 21. Hritz I., Mandrekar P., Velayudham A., Catalano D., Dolganiuc A., Kodys K., Kurt-Jones E., Szabo G. (2008) The critical role of Toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48, 1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oak S., Mandrekar P., Catalano D., Kodys K., Szabo G. (2006) TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J. Immunol. 176, 7628–7635 [DOI] [PubMed] [Google Scholar]
- 23. Petrasek J., Bala S., Csak T., Lippai D., Kodys K., Menashy V., Barrieau M., Min S. Y., Kurt-Jones E. A., Szabo G. (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Lizarbe S., Pascual M., Guerri C. (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 183, 4733–4744 [DOI] [PubMed] [Google Scholar]
- 25. Otani N., Nawashiro H., Fukui S., Ooigawa H., Ohsumi A., Toyooka T., Shima K., Gomi H., Brenner M. (2006) Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J. Clin. Neurosci. 13, 934–938 [DOI] [PubMed] [Google Scholar]
- 26. Maragakis N. J., Rothstein J. D. (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2, 679–689 [DOI] [PubMed] [Google Scholar]
- 27. Dinarello C. A. (1991) Interleukin-1 and interleukin-1 antagonism. Blood 77, 1627–1652 [PubMed] [Google Scholar]
- 28. Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451 [DOI] [PubMed] [Google Scholar]
- 29. Evankovich J., Cho S. W., Zhang R., Cardinal J., Dhupar R., Zhang L., Klune J. R., Zlotnicki J., Billiar T., Tsung A. (2010) High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J. Biol. Chem. 285, 39888–39897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oh Y. J., Youn J. H., Ji Y., Lee S. E., Lim K. J., Choi J. E., Shin J. S. (2009) HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J. Immunol. 182, 5800–5809 [DOI] [PubMed] [Google Scholar]
- 31. Youn J. H., Shin J. S. (2006) Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J. Immunol. 177, 7889–7897 [DOI] [PubMed] [Google Scholar]
- 32. Attur M. G., Dave M., Cipolletta C., Kang P., Goldring M. B., Patel I. R., Abramson S. B., Amin A. R. (2000) Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J. Biol. Chem. 275, 40307–40315 [DOI] [PubMed] [Google Scholar]
- 33. Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Nunez G. (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 34. Simi A., Tsakiri N., Wang P., Rothwell N. J. (2007) Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 35, 1122–1126 [DOI] [PubMed] [Google Scholar]
- 35. De Rivero Vaccari J. P., Lotocki G., Alonso O. F., Bramlett H. M., Dietrich W. D., Keane R. W. (2009) Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maroso M., Balosso S., Ravizza T., Iori V., Wright C. I., French J., Vezzani A. (2011) Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8, 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chakraborty S., Kaushik D. K., Gupta M., Basu A. (2010) Inflammasome signaling at the heart of central nervous system pathology. J. Neurosci. Res. 88, 1615–1631 [DOI] [PubMed] [Google Scholar]
- 38. Wu J., Fernandes-Alnemri T., Alnemri E. S. (2010) Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 30, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silverman W. R., de Rivero Vaccari J. P., Locovei S., Qiu F., Carlsson S. K., Scemes E., Keane R. W., Dahl G. (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284, 18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keshavarzian A., Holmes E. W., Patel M., Iber F., Fields J. Z., Pethkar S. (1999) Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am. J. Gastroenterol. 94, 200–207 [DOI] [PubMed] [Google Scholar]
- 41. Banks W. A., Robinson S. M. (2010) Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 24, 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh A. K., Jiang Y., Gupta S., Benlhabib E. (2007) Effects of chronic ethanol drinking on the blood brain barrier and ensuing neuronal toxicity in alcohol-preferring rats subjected to intraperitoneal LPS injection. Alcohol Alcohol. 42, 385–399 [DOI] [PubMed] [Google Scholar]
- 43. Elmas I., Kucuk M., Kalayci R. B., Cevik A., Kaya M. (2001) Effects of profound hypothermia on the blood-brain barrier permeability in acute and chronically ethanol treated rats. Forensic Sci. Int. 119, 212–216 [DOI] [PubMed] [Google Scholar]
- 44. Haorah J., Knipe B., Leibhart J., Ghorpade A., Persidsky Y. (2005) Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 78, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 45. Haorah J., Heilman D., Knipe B., Chrastil J., Leibhart J., Ghorpade A., Miller D. W., Persidsky Y. (2005) Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin. Exp. Res. 29, 999–1009 [DOI] [PubMed] [Google Scholar]
- 46. Laye S., Bluthe R. M., Kent S., Combe C., Medina C., Parnet P., Kelley K., Dantzer R. (1995) Subdiaphragmatic vagotomy blocks induction of IL-1 β mRNA in mice brain in response to peripheral LPS. Am. J. Physiol. 268, R1327–R1331 [DOI] [PubMed] [Google Scholar]
- 47. Muhammad S., Barakat W., Stoyanov S., Murikinati S., Yang H., Tracey K. J., Bendszus M., Rossetti G., Nawroth P. P., Bierhaus A., Schwaninger M. (2008) The HMGB1 receptor RAGE mediates ischemic brain damage. J. Neurosci. 28, 12023–12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim I. D., Shin J. H., Kim S. W., Choi S., Ahn J., Han P. L., Park J. S., Lee J. K. (2012) Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol. Ther. 20, 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiang M., Shi X., Li Y., Xu J., Yin L., Xiao G., Scott M. J., Billiar T. R., Wilson M. A., Fan J. (2011) Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J. Immunol. 187, 4809–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Willingham S. B., Allen I. C., Bergstralh D. T., Brickey W. J., Huang M. T., Taxman D. J., Duncan J. A., Ting J. P. (2009) NLRP3 (NALP3, cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 183, 2008–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., Manogue K. R., Faist E., Abraham E., Andersson J., Andersson U., Molina P. E., Abumrad N. N., Sama A., Tracey K. J. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- 52. Gardella S., Andrei C., Ferrera D., Lotti L. V., Torrisi M. R., Bianchi M. E., Rubartelli A. (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 3, 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klune J. R., Dhupar R., Cardinal J., Billiar T. R., Tsung A. (2008) HMGB1: endogenous danger signaling. Mol. Med. 14, 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gustot T., Lemmers A., Moreno C., Nagy N., Quertinmont E., Nicaise C., Franchimont D., Louis H., Deviere J., Le Moine O. (2006) Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43, 989–1000 [DOI] [PubMed] [Google Scholar]
- 55. Kim S. W., Lim C. M., Kim J. B., Shin J. H., Lee S., Lee M., Lee J. K. (2011) Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox. Res. 20, 159–169 [DOI] [PubMed] [Google Scholar]
- 56. Crews F. T., Qin L., Sheedy D., Vetreno R. P., Zou J. (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol. Psychiatry 73, 602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qin L., Crews F. T. (2012) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflammation 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goldbach-Mansky R. (2012) Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin. Exp. Immunol. 167, 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Y., Lousberg E. L., Moldenhauer L. M., Hayball J. D., Robertson S. A., Coller J. K., Watkins L. R., Somogyi A. A., Hutchinson M. R. (2011) Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav. Immun. 25 (Suppl. 1), S155–S164 [DOI] [PubMed] [Google Scholar]
- 60. Diener H. C., Dichgans J., Bacher M., Guschlbauer B. (1984) Improvement of ataxia in alcoholic cerebellar atrophy through alcohol abstinence. J. Neurol. 231, 258–262 [DOI] [PubMed] [Google Scholar]
- 61. Motoki K., Kishi H., Hori E., Tajiri K., Nishijo H., Muraguchi A. (2009) The direct excitatory effect of IL-1β on cerebellar Purkinje cell. Biochem. Biophys. Res. Commun. 379, 665–668 [DOI] [PubMed] [Google Scholar]
- 62. Fitzpatrick L. E., Jackson M., Crowe S. F. (2012) Characterization of cerebellar ataxia in chronic alcoholics using the international cooperative ataxia rating scale (ICARS). Alcohol. Clin. Exp. Res. 36, 1942–1951 [DOI] [PubMed] [Google Scholar]
- 63. Yokota O., Tsuchiya K., Terada S., Oshima K., Ishizu H., Matsushita M., Kuroda S., Akiyama H. (2006) Frequency and clinicopathological characteristics of alcoholic cerebellar degeneration in Japan: a cross-sectional study of 1,509 postmortems. Acta Neuropathol. 112, 43–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.