Review of molecular interactions between epithelial γδ T cells and epithelial cells, and their function in homeostasis and repair.
Keywords: lymphocytes, epithelium, wound repair, dendritic epidermal T cells
Abstract
Intraepithelial γδ T cells play pivotal roles in homeostasis, tissue repair, inflammation, and protection from malignancy. In some tissues, γδ T cells are the only resident T cell population, whereas in others, they coexist with αβ T cells and other lymphocyte populations. γδ T cell function in the epithelium requires constant communication between cells in the form of cell-to-cell contacts and cell-to-matrix interactions. These interactions coordinate with the timely production of specific cytokines, chemokines, growth factors, and glycosaminoglycans, which have specialized effects on neighboring epithelial cells. Antigens that activate these T cells are not well-defined, and they do not express classic costimulatory or coreceptor molecules. As such, an understanding of the mechanisms used by epithelial γδ T cells to maintain homeostasis and facilitate wound repair has necessitated the identification of novel molecular interactions between γδ T cells and their neighboring epithelial cells.
Introduction
Epithelial tissues, such as skin, intestine, and lung, are under constant environmental exposure. As such, these tissues form an important first-line defense against invading microorganisms. This defense is carried out through continual surveillance by populations of resident T cells. In many epithelial sites, the majority of these resident T cells expresses the γδ TCR [1]. In the absence of these γδ IEL, epithelial dysregulation ensues. γδ TCR−/− mice have defects in wound healing, tumor rejection, and recovery from colitis, as well as earlier onset, and more severe, lung injury [2–5]. In addition, in the absence of γδ T cells, impaired homeostatic regulation of the epithelial environment is evident [6]. The location of IEL between epithelial cells, together with their critical role in homeostasis and repair, suggests continual cross-talk between epithelial cells and their neighboring IEL, as the IEL survey for signs of damage and disease. Recent studies have begun to shed light on the mechanisms for this surveillance, as well as the molecular interactions involved in the repair process following epithelial injury.
THE THYMUS
Cross-talk between T cells and neighboring cells begins in the thymus. Although not as well-defined for γδ T cells as it is for αβ T cells, intrathymic development and maturation of γδ T cells involve a number of check-points that likely entail strictly defined T cell–epithelial cell interactions. One such interaction involves the Skint1 molecule.
Skint1 is a prototypic member of the Skint Ig supergene family, closely related to the butyrophilin family of molecules [7]. It is a transmembrane protein expressed by thymic epithelial cells and keratinocytes [7]. In the presence of a nonfunctional Skint1 gene, as found in a substrain of FVB mice, Vγ3Vδ1 (nomenclature according to Garman et al. [8]), DETC precursors present in the thymus, remain immature in phenotype and do not populate the skin [9]. Transgenic expression of Skint1 is able to restore DETC maturation, and Vγ3Vδ1 T cells subsequently take up residence in the epidermis [7]. In addition, those cells that are able to develop in the absence of Skint1 interactions in the thymus express IL-17, whereas WT Vγ3Vδ1 T cells, upon engagement of Skint1, develop the propensity to produce IFN-γ [10]. This suggests that Skint1 interactions in the thymus imprint the functional capabilities of mature DETC.
The T cell ligand in this Skint1 interaction is less well-defined. Although antibody-mediated TCR ligation can induce maturation of Skint1−/− DETC precursors [9], no direct binding of Skint1 to the Vγ3Vδ1 TCR has been demonstrated. It is thus possible that the effects of Skint1 are through regulation of expression of another molecule that may bind to the TCR rather than Skint1 being in of itself a TCR ligand.
Early work suggested that another γδ T cell subset also requires ligand engagement during development. The KN6 γδ TCR recognizes the nonclassical MHC class1b molecule T22, and these KN6 T cells are found in peripheral LNs and the intestine [11]. Engagement of KN6 transgenic thymocytes by T22 promotes the development of a mature CD24lo γδ population [12]. In the absence of KN6 γδ TCR signaling, an αβ fate is favored [12]. These data suggest that ligand recognition is important for lineage choice and maturation of γδ T cells. This idea remains somewhat controversial, however, as more recent analysis in nontransgenic animals found no decrease in the number of T22-specifc γδ T cells in the absence of thymic T22 signals [13].
Nevertheless, epithelial γδ T cells undergo a series of phenotypic changes during their intrathymic development, including up-regulation of CD45RB and down-regulation of CD24 [14]. By analogy with αβ T cells, the conferring of maturation and tissue specificity to a γδ T cell likely involves intimate cross-talk between thymic epithelial cells and the developing γδ T cells surrounding them.
Vγ3Vδ1 γδ T cells are the first T cell population to develop in the thymus [1]. These cells begin their exit from the thymus around Day 16 of embryonic development [1]. Through mechanisms that are not well-characterized but likely involve acquisition of CCR10 [15] and down-regulation of CCR6 [16], mature Vγ3Vδ1 thymocytes all home to the skin, where they take up residence in the epidermis for the life of the animal.
THE EPITHELIAL BARRIER
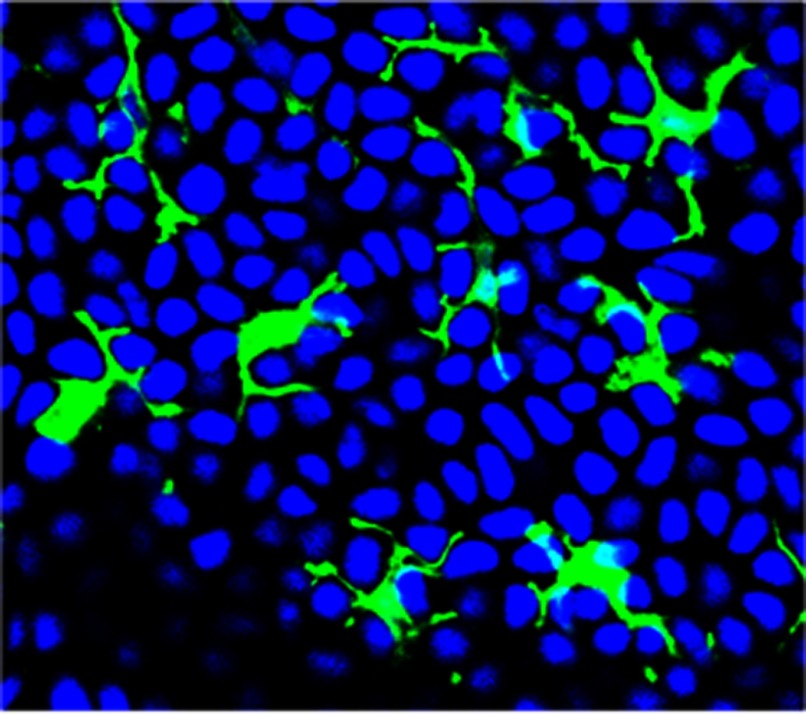
The skin provides a protective barrier essential for thermal and osmotic regulation. In addition, this barrier provides a first line of defense against environmental and pathogenic insults. γδ T cells in the mouse epidermis are essential for the correct function of the skin [17]. These γδ T cells, termed DETC, express a canonical Vγ3Vδ1 TCR and are positioned in the epidermis in intimate contact with neighboring keratinocytes, Langerhans cells, and melanocytes. DETC, as suggested by their name, exhibit a highly dendritic morphology. Their numerous dendritic projections extend between neighboring cells, allowing for simultaneous contact with multiple adjacent cells under homeostatic conditions (Fig. 1). The location and morphology of DETC thereby allow for the cross-talk between these cells and their neighbors. Increasing evidence is demonstrating that this cross-talk involves the coordinated interaction between multiple cell surface receptors and soluble molecules to maintain homeostasis in the skin, as well as to allow for rapid repair following damage or disease.
Figure 1. DETC are in constant contact with neighboring keratinocytes, surveying for signs of damage or disease.
Epidermal sheets from a WT mouse were stained with anti-γδ TCR mAb (green fluorescence) to visualize DETC and DAPI (blue nuclei) to visualize keratinocytes. Each DETC can simultaneously contact multiple keratinocytes through dendritic processes.
Similar evidence is emerging in other epithelial tissues, such as the intestine and the lung. Like the skin, the intestine is populated with IEL that reside intercalated between epithelial cells. These T cells include αβ and γδ TCR-bearing subsets that are crucial for the maintenance and repair of the protective barrier of the intestine, as well as for the initial defense against invading microorganisms [2, 18–20]. γδ T cells migrate dynamically in the intestinal epithelium, making extensive contacts with multiple epithelial cells, thereby facilitating surveillance of a large area of epithelium by a single γδ IEL [21]. Although not as well-defined, γδ T cells in the lung also appear to make frequent contacts with neighboring cells [22] and function in host resistance and damage repair [5, 23–26].
One of the unique features of epithelial resident γδ T cells is their lack of expression of CD4, CD8 (although some γδ IEL do express the CD8αα homodimer), and CD28 [27]—typical sources of molecules for αβ T cell cross-talk with nearby activating cells. This suggests that to communicate with their neighbors, epithelial γδ T cells likely use other molecular interactions to mediate homeostatic, activating, and inhibitory interactions in the epithelial compartments.
CROSS-TALK IN THE EPITHELIUM
TCR
The importance of the TCR in homeostasis and repair has been exemplified by studies in mice with disrupted γδ TCR gene expression. Disruption of the Vγ3 gene allows an alternate Vγ-expressing γδ T cell subset to populate the epidermis [28]. These epidermal γδ T cells, although not expressing the Vγ3 gene product, can, however, be recognized by a Vγ3Vδ1 clonotype-specific mAb [28], highlighting the importance of conformation of the γ and δ TCR pair for localization to and residence in the skin.
In mice lacking γδ T cells altogether (TCRδ−/−), wound repair is disrupted and delayed (as discussed below), and epidermal homeostasis is not maintained. The epidermis of TCRδ−/− mice is populated by T cells bearing diverse αβ TCRs [29]. In these animals, keratinocytes undergo increased apoptosis as a result of a deficiency in IGF-1 production [6]. Furthermore, the αβ T cell population is not maintained in the epidermis, and its numbers gradually decline over time, unlike what is seen in WT animals [29]. Together, these studies demonstrate the reliance on the correct TCR for epidermal homeostatic maintenance.
As mentioned above, in TCRδ−/− animals, wound-repair processes are disrupted [4]. Whereas studies using a soluble tetrameric Vγ3Vδ1 TCR to detect ligand in the epidermis demonstrate normal ligand expression in TCRδ−/− mice following epidermal wounding [30], many facets of the wound-repair process are delayed or absent. KGF-1 production, a hallmark feature of activated epidermal γδ T cells, is not produced by the resident αβ T cells present in the epidermis of TCRδ−/− mice. Thus, keratinocyte proliferation is reduced, and wound closure is delayed in these animals [4]. Furthermore, effective wound repair depends on the coordinated recruitment of specialized cells to the wound site. Whereas neutrophil migration is apparently unaffected by an absence of γδ T cells, macrophage entry into the wound site is delayed significantly in TCRδ−/− animals [31].
TCRδ−/− animals show a similar disruption to wound repair in the intestine in a DSS-induced mouse model of colitis [2]. In the absence of γδ T cells, there is increased severity of DSS-induced damage and a delay in tissue repair once DSS treatment is discontinued. In WT animals, following DSS treatment, γδ IEL localize to sites of epithelial cell damage and express KGF-1, resulting in vigorous epithelial cell proliferation to repair the damage [2]. This proliferation is impaired severely in γδ−/− animals [2]. Together, these studies highlight the importance of the communication between γδ TCR-bearing cells and epithelial cells for homeostatic tissue maintenance, as well as repair from epithelial damage.
Analysis of skin wound repair in TCRδ−/− animals also identified a unique role for γδ T cell–keratinocyte interactions in the production of the glycosaminoglycan, hyaluronan, during wound repair [31]. It was found that not only did γδ T cells express hyaluronan synthases and produce hyaluronan, but also, activated γδ T cells were able to induce keratinocyte production of hyaluronan [31]. This may well be via γδ T cell- produced KGF-1, as KGF-1 has been shown to induce hyaluronan production by rat keratinocytes [32].
The identity of epithelial ligands for the distinct γδ TCRs that initiate the wound-repair functions in γδ T cells has been elusive. Skint1 represents an attractive candidate ligand for the Vγ3Vδ1 TCR in the epidermis, but as yet, no direct evidence of an interaction between Skint1 and the Vγ3Vδ1 TCR is available. Recent work has demonstrated rapid and transient expression of the unknown ligand following wounding, as well as a restricted distribution of expression to sites immediately adjacent to the wounds [30]. In this study, no ligand was detectable under steady-state conditions in nonwounded tissue. In contrast, another study using intravital microscopy found constitutive Vγ3Vδ1 TCR signaling from interaction with neighboring epithelial cells, with wounding eliciting a reorganization of TCR molecules and an increase in signal strength [33]. This suggested constitutive TCR–ligand interactions under homeostatic conditions. Skint1 has been proposed as a possible candidate molecule for a steady-state Vγ3Vδ1 TCR ligand, as it is constitutively expressed by keratinocytes [34]. However, as mentioned, no direct binding of Skint1 to the Vγ3Vδ1 TCR has been demonstrated. Until the identity of the Vγ3Vδ1 TCR ligand is firmly established, it cannot be concluded that this constitutive signaling in DETC in the steady-state is indeed ligand-induced. What is becoming increasingly clear, however, is that TCR–ligand interactions are not the sole communicators for epithelial γδ T cell interactions with their neighbors.
JAML
JAML is a type I transmembrane glycoprotein composed of a globular ectodomain, stalk, transmembrane helix, and cytoplasmic domain [35]. Expression of JAML can be found on a variety of effector cells of the innate and adaptive arms of the immune system. Most notably, JAML expression has been demonstrated on neutrophils, monocytes, and memory T cells [36, 37]. More recently, JAML was found to be expressed at low levels on epidermal γδ T cells under steady-state conditions and rapidly up-regulated upon stimulation [38]. With the exception of a population of activated CD8+ αβ T cells, JAML expression in the intestinal IEL populations is also restricted to the γδ subset. In vitro assays with isolated epidermal γδ T cells indicated a potential key role for JAML in γδ T cell costimulation [38]. Strikingly, this costimulatory function of JAML appears restricted to the epithelial subsets of γδ T cells.
JAML binds to CAR [37, 38], which is also a type I transmembrane glycoprotein [39] and is expressed on keratinocytes and intestinal epithelial cells [38]. CAR ligation of JAML recruits PI3K to JAML [40] and subsequently costimulates DETC proliferation and cytokine production, namely, IL-2, IFN-γ, and TNF-α [38]. Of note is that PI3K is also able to mediate costimulatory signals through the prototypic αβ T cell costimulatory molecule, CD28, through a binding motif similar to that found in JAML and another αβ costimulatory receptor, ICOS [41].
In the absence of JAML-CAR interactions in vivo in the skin, DETC activation, in response to wounding, is impaired, cytokine responses are diminished, and subsequent wound closure is delayed [38]. Thus, cross-talk between JAML and CAR is a key component of DETC activation and the wound-repair process. The expression pattern of JAML and CAR in the mouse intestine [38] suggests that these molecules may play a parallel role in IEL activation in the intestine. Whether this function is also vital for human skin and intestinal T cell activation and damage repair is still unknown.
NKG2D
The C-type, lectin-like NKG2D receptor is expressed as a homodimer on NK, γδ, and CD8+ T cells [3, 42–44]. Like JAML, NKG2D is thus expressed on effector cells of the innate and adaptive immune systems. A regulatory role for NKG2D in NK development has been reported [45]; however, NKG2D is best-characterized as providing activating signals upon ligation to one of its multiple ligands [44, 46, 47]. In humans, NKG2D ligands include MICA and MICB and members of the ULBP family of molecules. In the mouse, H60a-c, ULBP-like transcript 1, and RAE1 serve as NKG2D ligands [44, 48, 49]. NKG2D ligand expression is generally low under homeostatic conditions but can be up-regulated by a variety of signals of cellular stress, including infection, tumorigenesis, and tissue damage.
In the mouse, epidermal γδ T cells express NKG2D and engagement of NKG2D with NKG2D ligands activates DETC [3]. There is conflicting evidence as to whether this activation signal relies on concomitant TCR signaling or can directly stimulate DETC. H60c is a NKG2D ligand expressed in the epidermis upon skin damage and on cultured keratinocytes [50]. H60c engagement of NKG2D, in the absence of TCR-mediated signals, is unable to activate DETC in vitro. Instead, H60c provides a costimulatory signal to DETC through NKG2D [50]. Blockade of interactions between H60c and NKG2D impairs KGF production and the wound-repair response [51]. In contrast, keratinocyte-specific up-regulation of another NKG2D ligand, RAE1, is able to activate DETC directly without an apparent requirement for simultaneous TCR engagement [52, 53]. Whether this difference in TCR requirement could be a result of the nature of the damage and thus, the nature of the induced ligand and elicited DETC response is an intriguing question that remains unanswered.
In humans, the NKG2D ligands MICA and MICB can be recognized by intestinal epithelial T cells expressing the Vδ1 γδ TCR [54, 55]. As expression of MIC in the intestinal epithelium is apparently stress-induced, these NKG2D ligands have been proposed to be recognized by Vδ1 γδ T cells in their surveillance for signs of damaged, infected, or transformed intestinal epithelial cells [56]. Data suggest that MIC recognition can be directly through the TCR or via NKG2D and that recognition may, in fact, be sequential, using both molecules [57]. This idea, however, remains to be tested experimentally.
CD100
CD100, also known as Semaphorin 4D, is a member of the semaphorin family of molecules [58]. It has four known recptors: plexin B1, plexin B2, plexin C1, and CD72 [59–61]. The most well-characterized functions of semaphorins are as axon-guidance molecules in the developing nervous system, through their interactions with plexins [62]. CD100 is also widely expressed outside of the nervous system, including skeletal muscle, thymus, and T and B cells [63]. A role for CD100 in the immune system has been known for some time. Early in vitro work suggested a costimulatory function for CD100 on human T cells [64]. More recent in vivo studies using CD100−/− mice have demonstrated a role for CD100 in the activation of B and T cells, including epithelial resident γδ T cells [65, 66].
One of the most striking features of the epidermal γδ T cell response to keratinocyte damage is the rapid retraction of numerous dendritic projections and assumption of a round morphology [4]. A recent study has implicated the semaphorin, CD100, and one of its ligands, Plexin B2, in this process [66]. Mice deficient in the CD100 molecule were found to exhibit delayed DETC rounding upon wounding. A direct role for CD100 and plexin B2 in this morphology change was confirmed by in vitro ligation of CD100, leading to ERK and cofilin activation, concurrent with rapid DETC rounding. The importance of the CD100-plexin B2-mediated rounding in epithelial wound repair was demonstrated by the delayed wound closure observed in animals deficient for the CD100 molecule [66].
Plexin B2 is broadly expressed on many epithelial tissues, where CD100-expressing γδ T cells reside, suggesting a more general role for CD100-plexin B2 in epithelial cell–T cell interactions. Indeed, a more severe colitis and similar delay in repair are seen in the absence of CD100 in a mouse model of DSS-induced colitis (unpublished results).
AhR
The AhR is a member of the family of basic helix-loop-helix transcription factors [67]. Until recently, the most well-known function of AhR was in mediating the toxic effects of the environmental pollutant, dioxin [68]. Several recent studies have shown an immunoregulatory role for AhR in αβ and γδ T cells [67, 69, 70]. Epithelial resident T cells express high levels of AhR and depend on its activity for local maintenance [69, 70]. A complete dependence on AhR for DETC and IEL maintenance in the epidermis and intestine, respectively, has been demonstrated recently [69, 70].
In the epidermis, AhR is expressed by keratinocytes, Langerhans cells, melanocytes, and DETC [69]. In the absence of AhR, DETC undergo apparently normal intrathymic development and are able to home to the epidermis [69, 70]. However, DETC in AhR−/− animals do not exhibit their normal dendritic morphology [69]. They do not extend dendrites to neighboring epithelial cells; instead, remain round. Furthermore, DETC do not take up residence in the epidermis but decline steadily in number in the first weeks after their initial homing to the epidermis [69, 70]. Conditional knockout animals have demonstrated that it is specifically a deficiency in AhR in the DETC themselves that is responsible for the lack of retention in the epidermis [70]. Specific deletion of AhR in keratinocytes or Langerhans cells has no effect on DETC numbers [69]. This suggests that AhR−/− DETC may be unable to make the necessary contacts with keratinocytes and possibly Langerhans cells, which are required for maintenance in the epidermal compartment. A defect in c-kit interaction with its ligand, stem cell factor, may play a role in this DETC loss [69].
A similar loss of intestinal epithelial T cells in the absence of AhR has been described [70]. Whereas normal numbers of γδ T cells were found in LN, spleen, and thymus, AhR−/− animals were virtually devoid of small intestinal TCRαβCD8αα and γδ IEL. There was no defect evident in the proliferation or tissue homing of IEL. As in the epidermis, AhR−/− activity was found to be responsible for a lack of maintenance of these cells in the intestine. Cruciferous vegetables were found to be an important source of AhR ligands in the intestine [70], highlighting the importance of diet in the maintenance of epithelial integrity. A reduction in AhR ligands or AhR deficiency itself resulted in increased immunopathology in DSS-induced colitis [70]. Although clearly important for epithelial homeostasis, just how AhR signals maintain DETC and IEL at epithelial sites is unknown. In addition, the role of AhR in the activation of these cells during the wound-repair process still requires investigation. Again, these AhR signals likely require coordinated interactions between resident γδ T cells and their neighboring epithelial cells.
Other molecules
A number of other molecules have been implicated in the maintenance of epidermal homeostasis and repair following injury, although their roles are not well-defined at this point. Epithelial expressed CD98hc has been shown recently to be important in the regulation of skin homeostasis and wound healing [71]. Whether this is through a direct interaction with DETC is unknown but likely involves signals through the integrin family of molecules, most notably, β1.
First noted in transcripts of intestinal IEL [72], Tβ4 splice variants are also produced by DETC and function to prevent neutrophil infiltration and inhibit inflammation in models of contact hypersensitivity [73]. In addition, wound closure in vitro and in vivo has been reported to be promoted by Tβ4 [74], although the precise interactions mediating this enhanced wound closure are unknown.
Numerous molecules, such as integrins, adhesion molecules, cytokine receptors, and known markers of activation are expressed by DETC and other γδ IEL and are modulated in vitro and/or in vivo by activation signals [27, 72, 75]. Future studies designed at elucidating the precise role of these various molecules in the cross-talk between epithelial cells and their neighboring T cells should shed light on their functions in the repair process.
SOLUBLE MEDIATORS OF EPITHELIAL CROSS-TALK
Chemokines, cytokines, and growth factors
Upon stimulation, DETC secrete a variety of cytokines and chemokines that play important roles in the cross-talk among DETC, keratinocytes, Langerhans cells, and other immune cells recruited to the site of damage during the wound-repair response. CCL-3 (MIP1a), CCL-4 (MIP1b), CCL5 (RANTES), and XCL1 (lymphotactin) are all expressed in abundance by DETC following activation [76]. The recruitment of specialized cells, such as macrophages and αβ T cells, is choreographed by these chemoattractants and notably delayed in TCRδ−/− animals [31].
Similarly, activated DETC are rich sources of numerous cytokines, including IL-2, IL-3, GM-CSF, IFN-γ, and TNF-α [38, 77, 78]. Somewhat more controversial has been the role of DETC in IL-17 production in the skin [79–82]. Whereas some studies have shown significant amounts of IL-17 production by DETC upon cutaneous infection, others have found IL-17A production exclusively by dermal γδ T cells [83, 84]. IL-17A production by γδ T cells is an area of active investigation and additional information in different disease models are sure to be forthcoming. What is becoming increasingly evident is that disruption of cross-talk between keratinocytes and DETC cell-surface molecules, as described earlier, can lead to reduced cytokine production by DETC and subsequently, delayed wound closure.
Growth factors are important regulators of epidermal homeostasis and repair mechanisms. IGF-1, as mentioned above, is vital to keratinocyte maintenance in the epidermis [6]. TCRδ−/− mice are deficient in DETC-derived IGF-1. These animals have increased keratinocyte apoptosis and show reduced IGF-1R phosphorylation at wound borders. In addition, activated DETC and intestinal γδ IEL inducibly secrete KGFs [2, 4, 51, 85], potent epithelial mitogens. KGF-1 acts specifically on epithelial cells, including keratinocytes, to stimulate proliferation and migration of these cells during wound healing. Thus, intraepithelial γδ T cell-derived growth factors play an important role in epithelial homeostasis under steady-state conditions and following perturbation. It should be noted that some studies have failed to detect KGF-1 message in intraepithelial γδ T cells [72, 86]. This may reflect differences in the mode of activation or activation state of these cells.
AMPs
One of the most important functions of epithelial barrier tissues is to maintain homeostasis with commensal microorganisms, while limiting exposure to those that are pathogenic. Epithelial resident γδ T cells of the skin, gut, respiratory tract, and other epithelial surfaces are important mediators of this homeostasis through the regulation of AMPs [19, 20, 87]. Through cross-talk with neighboring epithelial cells, these T cells are also able to detect the presence of invading bacteria. A major function of AMPs is to respond rapidly to epithelial disruption and establish a temporary protective shield against infection. The rapid response of epithelial γδ T cells is thus ideal in facilitating this early protection against tissue invasion.
CONCLUDING REMARKS
γδ T cells form intimate contacts with neighboring epithelial cells in barrier tissues. Effective communication between these cells is essential for maintenance of homeostasis and for the return to steady-state conditions following disruption of this protective epithelial barrier. Recent studies have shed light on the molecular mechanisms directing the cross-talk between these unique T cells and their neighbors and how individual receptor–ligand pairs function in this process (Fig. 2). Future studies should provide a more thorough understanding of these interactions, opening the door for their modulation in the clinic to provide more effective treatment of diseases, resulting from epithelial dysregulation, such as chronic nonhealing wounds, colitis, and asthma.
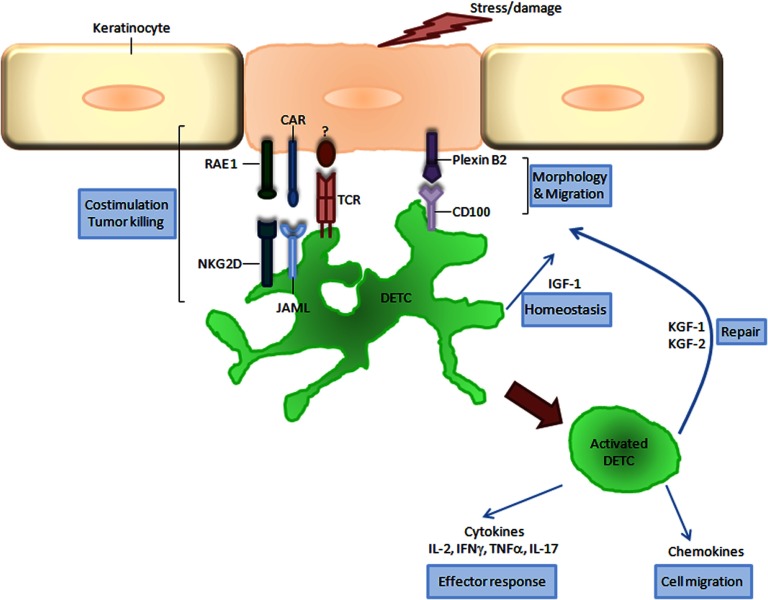
Figure 2. Cross-talk in epidermal γδ T cell activation.
γδ DETC recognize stress or damage signals delivered by keratinocytes. This recognition involves the coordinated interaction between the TCR and its unknown ligand, together with the costimulatory molecules, JAML and NKG2D, with their keratinocyte ligands, CAR and H60c/RAE 1, respectively. Additional signals are generated through CD100 on DETC and plexin B2 on keratinocytes to initiate DETC rounding prior to complete activation. Activated DETC exhibit a round morphology and secrete KGFs (KGF-1 and KGF-2) that facilitate keratinocyte proliferation and re-epithelialization. Simultaneous secretion of a battery of cytokines and chemokines ensures an appropriate effector response and recruitment of inflammatory cells. Together, these coordinated events allow the efficient functioning of all facets of the wound-repair process.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grants AI36964, AI064811, and AI095823.
We thank Olivia Garijo for assistance with figures.
Footnotes
- −/−
- deficient
- AhR
- aryl hydrocarbon receptor
- AMP
- antimicrobial protein
- CAR
- coxsackie and adenovirus receptor
- DETC
- dendritic epidermal T cell(s)
- DSS
- dextran sulfate sodium
- IEL
- intraepithelial lymphocyte(s)
- JAML
- junctional adhesion molecule-like
- KGF
- keratinocyte growth factor
- MIC
- MHC class I-related
- NKG2D
- NK group 2, member D
- RAE1
- retinoic acid early-inducible 1
- Tβ4
- thymosin-β4
- ULBP
- UL16-binding protein
AUTHORSHIP
D.A.W. and W.L.H. contributed to the writing of this review.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Allison J. P., Havran W. L. (1991) The immunobiology of T cells with invariant γδ antigen receptors. Annu. Rev. Immunol. 9, 679–705 [DOI] [PubMed] [Google Scholar]
- 2. Chen Y., Chou K., Fuchs E., Havran W. L., Boismenu R. (2002) Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 99, 14338–14343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Girardi M., Oppenheim D. E., Steele C. R., Lewis J. M., Glusac E., Filler R., Hobby P., Sutton B., Tigelaar R. E., Hayday A. C. (2001) Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 [DOI] [PubMed] [Google Scholar]
- 4. Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W. L. (2002) A role for skin γδ T cells in wound repair. Science 296, 747–749 [DOI] [PubMed] [Google Scholar]
- 5. King D. P., Hyde D. M., Jackson K. A., Novosad D. M., Ellis T. N., Putney L., Stovall M. Y., Van Winkle L. S., Beaman B. L., Ferrick D. A. (1999) Cutting edge: protective response to pulmonary injury requires γδ T lymphocytes. J. Immunol. 162, 5033–5036 [PubMed] [Google Scholar]
- 6. Sharp L. L., Jameson J. M., Cauvi G., Havran W. L. (2005) Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 [DOI] [PubMed] [Google Scholar]
- 7. Boyden L. M., Lewis J. M., Barbee S. D., Bas A., Girardi M., Hayday A. C., Tigelaar R. E., Lifton R. P. (2008) Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40, 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garman R. D., Doherty P. J., Raulet D. H. (1986) Diversity, rearrangement, and expression of murine T cell γ genes. Cell 45, 733–742 [DOI] [PubMed] [Google Scholar]
- 9. Lewis J. M., Girardi M., Roberts S. J., Barbee S. D., Hayday A. C., Tigelaar R. E. (2006) Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7, 843–850 [DOI] [PubMed] [Google Scholar]
- 10. Turchinovich G., Hayday A. C. (2011) Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells. Immunity 35, 59–68 [DOI] [PubMed] [Google Scholar]
- 11. Meyer C., Zeng X., Chien Y. H. (2010) Ligand recognition during thymic development and γδ T cell function specification. Semin. Immunol. 22, 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haks M. C., Lefebvre J. M., Lauritsen J. P., Carleton M., Rhodes M., Miyazaki T., Kappes D. J., Wiest D. L. (2005) Attenuation of γδ TCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity 22, 595–06 [DOI] [PubMed] [Google Scholar]
- 13. Jensen K. D., Shin S., Chien Y. H. (2009) Cutting edge: γδ intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J. Immunol. 182, 7348–7351 [DOI] [PubMed] [Google Scholar]
- 14. Leclercq G., Plum J., Nandi D., De Smedt M., Allison J. P. (1993) Intrathymic differentiation of Vγ3 T cells. J. Exp. Med. 178, 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin Y., Xia M., Sun A., Saylor C. M., Xiong N. (2010) CCR10 is important for the development of skin-specific γδ T cells by regulating their migration and location. J. Immunol. 185, 5723–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu S., Xiong N. (2013) Programmed downregulation of CCR6 is important for establishment of epidermal γδT cells by regulating their thymic egress and epidermal location. J. Immunol. 190, 3267–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jameson J., Havran W. L. (2007) Skin γδ T-cell functions in homeostasis and wound healing. Immunol. Rev. 215, 114–122 [DOI] [PubMed] [Google Scholar]
- 18. Inagaki-Ohara K., Chinen T., Matsuzaki G., Sasaki A., Sakamoto Y., Hiromatsu K., Nakamura-Uchiyama F., Nawa Y., Yoshimura A. (2004) Mucosal T cells bearing TCRγδ play a protective role in intestinal inflammation. J. Immunol. 173, 1390–1398 [DOI] [PubMed] [Google Scholar]
- 19. Ismail A. S., Behrendt C. L., Hooper L. V. (2009) Reciprocal interactions between commensal bacteria and γδ intraepithelial lymphocytes during mucosal injury. J. Immunol. 182, 3047–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ismail A. S., Severson K. M., Vaishnava S., Behrendt C. L., Yu X., Benjamin J. L., Ruhn K. A., Hou B., DeFranco A. L., Yarovinsky F., Hooper L. V. (2011) γδ Intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA 108, 8743–8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edelblum K. L., Shen L., Weber C. R., Marchiando A. M., Clay B. S., Wang Y., Prinz I., Malissen B., Sperling A. I., Turner J. R. (2012) Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. USA 109, 7097–7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wands J. M., Roark C. L., Aydintug M. K., Jin N., Hahn Y. S., Cook L., Yin X., Dal Porto J., Lahn M., Hyde D. M., Gelfand E. W., Mason R. J., O'Brien R. L., Born W. K. (2005) Distribution and leukocyte contacts of γδ T cells in the lung. J. Leukoc. Biol. 78, 1086–1096 [DOI] [PubMed] [Google Scholar]
- 23. Born W., Cady C., Jones-Carson J., Mukasa A., Lahn M., O'Brien R. (1999) Immunoregulatory functions of γδ T cells. Adv. Immunol. 71, 77–144 [PubMed] [Google Scholar]
- 24. Born W. K., Lahn M., Takeda K., Kanehiro A., O'Brien R. L., Gelfand E. W. (2000) Role of γδ T cells in protecting normal airway function. Respir. Res. 1, 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahn Y. S., Taube C., Jin N., Takeda K., Park J. W., Wands J. M., Aydintug M. K., Roark C. L., Lahn M., O'Brien R. L., Gelfand E. W., Born W. K. (2003) Vγ4+ γδ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J. Immunol. 171, 3170–3178 [DOI] [PubMed] [Google Scholar]
- 26. Lahn M., Kanehiro A., Takeda K., Konowal A., O'Brien R. L., Gelfand E. W., Born W. K. (2001) γδ T cells as regulators of airway hyperresponsiveness. Int. Arch. Allergy Immunol. 125, 203–210 [DOI] [PubMed] [Google Scholar]
- 27. Hayday A., Theodoridis E., Ramsburg E., Shires J. (2001) Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2, 997–1003 [DOI] [PubMed] [Google Scholar]
- 28. Mallick-Wood C. A., Lewis J. M., Richie L. I., Owen M. J., Tigelaar R. E., Hayday A. C. (1998) Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science 279, 1729–1733 [DOI] [PubMed] [Google Scholar]
- 29. Jameson J. M., Cauvi G., Witherden D. A., Havran W. L. (2004) A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 172, 3573–3579 [DOI] [PubMed] [Google Scholar]
- 30. Komori H. K., Witherden D. A., Kelly R., Sendaydiego K., Jameson J. M., Teyton L., Havran W. L. (2012) Cutting edge: dendritic epidermal γδ T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J. Immunol. 188, 2972–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jameson J. M., Cauvi G., Sharp L. L., Witherden D. A., Havran W. L. (2005) γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201, 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karvinen S., Pasonen-Seppanen S., Hyttinen J. M., Pienimaki J. P., Torronen K., Jokela T. A., Tammi M. I., Tammi R. (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J. Biol. Chem. 278, 49495–49504 [DOI] [PubMed] [Google Scholar]
- 33. Chodaczek G., Papanna V., Zal M. A., Zal T. (2012) Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 13, 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayday A., Tigelaar R. (2012) Casting new light on the TCR. Nat. Immunol. 13, 209–211 [DOI] [PubMed] [Google Scholar]
- 35. Moog-Lutz C., Cave-Riant F., Guibal F. C., Breau M. A., Di Gioia Y., Couraud P. O., Cayre Y. E., Bourdoulous S., Lutz P. G. (2003) JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood 102, 3371–3378 [DOI] [PubMed] [Google Scholar]
- 36. Luissint A. C., Lutz P. G., Calderwood D. A., Couraud P. O., Bourdoulous S. (2008) JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by α4β1 integrin activation. J. Cell Biol. 183, 1159–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zen K., Liu Y., McCall I. C., Wu T., Lee W., Babbin B. A., Nusrat A., Parkos C. A. (2005) Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol. Biol. Cell 16, 2694–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Witherden D. A., Verdino P., Rieder S. E., Garijo O., Mills R. E., Teyton L., Fischer W. H., Wilson I. A., Havran W. L. (2010) The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science 329, 1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bergelson J. M., Cunningham J. A., Droguett G., Kurt-Jones E. A., Krithivas A., Hong J. S., Horwitz M. S., Crowell R. L., Finberg R. W. (1997) Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323 [DOI] [PubMed] [Google Scholar]
- 40. Verdino P., Witherden D. A., Havran W. L., Wilson I. A. (2010) The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science 329, 1210–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudd C. E., Schneider H. (2003) Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat. Rev. Immunol. 3, 544–556 [DOI] [PubMed] [Google Scholar]
- 42. Bauer S., Groh V., Wu J., Steinle A., Phillips J. H., Lanier L. L., Spies T. (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 [DOI] [PubMed] [Google Scholar]
- 43. Jamieson A. M., Diefenbach A., McMahon C. W., Xiong N., Carlyle J. R., Raulet D. H. (2002) The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 17, 19–29 [DOI] [PubMed] [Google Scholar]
- 44. Raulet D. H. (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3, 781–790 [DOI] [PubMed] [Google Scholar]
- 45. Zafirova B., Wensveen F. M., Gulin M., Polic B. (2011) Regulation of immune cell function and differentiation by the NKG2D receptor. Cell. Mol. Life Sci. 68, 3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coudert J. D., Held W. (2006) The role of the NKG2D receptor for tumor immunity. Semin. Cancer Biol. 16, 333–343 [DOI] [PubMed] [Google Scholar]
- 47. Lodoen M. B., Lanier L. L. (2006) Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18, 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Champsaur M., Lanier L. L. (2010) Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235, 267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eagle R. A., Trowsdale J. (2007) Promiscuity and the single receptor: NKG2D. Nat. Rev. Immunol. 7, 737–744 [DOI] [PubMed] [Google Scholar]
- 50. Whang M. I., Guerra N., Raulet D. H. (2009) Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J. Immunol. 182, 4557–4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshida S., Mohamed R. H., Kajikawa M., Koizumi J., Tanaka M., Fugo K., Otsuka N., Maenaka K., Yagita H., Chiba H., Kasahara M. (2012) Involvement of an NKG2D ligand H60c in epidermal dendritic T cell-mediated wound repair. J. Immunol. 188, 3972–3979 [DOI] [PubMed] [Google Scholar]
- 52. Nitahara A., Shimura H., Ito A., Tomiyama K., Ito M., Kawai K. (2006) NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J. Invest. Dermatol. 126, 1052–1058 [DOI] [PubMed] [Google Scholar]
- 53. Strid J., Roberts S. J., Filler R. B., Lewis J. M., Kwong B. Y., Schpero W., Kaplan D. H., Hayday A. C., Girardi M. (2008) Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 [DOI] [PubMed] [Google Scholar]
- 54. Das H., Groh V., Kuijl C., Sugita M., Morita C. T., Spies T., Bukowski J. F. (2001) MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity 15, 83–93 [DOI] [PubMed] [Google Scholar]
- 55. Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K. H., Spies T. (1999) Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 96, 6879–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Groh V., Steinle A., Bauer S., Spies T. (1998) Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 [DOI] [PubMed] [Google Scholar]
- 57. Xu B., Pizarro J. C., Holmes M. A., McBeth C., Groh V., Spies T., Strong R. K. (2011) Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl. Acad. Sci. USA 108, 2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hall K. T., Boumsell L., Schultze J. L., Boussiotis V. A., Dorfman D. M., Cardoso A. A., Bensussan A., Nadler L. M., Freeman G. J. (1996) Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc. Natl. Acad. Sci. USA 93, 11780–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumanogoh A., Watanabe C., Lee I., Wang X., Shi W., Araki H., Hirata H., Iwahori K., Uchida J., Yasui T., Matsumoto M., Yoshida K., Yakura H., Pan C., Parnes J. R., Kikutani H. (2000) Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 13, 621–631 [DOI] [PubMed] [Google Scholar]
- 60. Masuda K., Furuyama T., Takahara M., Fujioka S., Kurinami H., Inagaki S. (2004) Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells 9, 821–829 [DOI] [PubMed] [Google Scholar]
- 61. Tamagnone L., Artigiani S., Chen H., He Z., Ming G. I., Song H., Chedotal A., Winberg M. L., Goodman C. S., Poo M., Tessier-Lavigne M., Comoglio P. M. (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 [DOI] [PubMed] [Google Scholar]
- 62. Kruger R. P., Aurandt J., Guan K. L. (2005) Semaphorins command cells to move. Nat. Rev. Mol. Cell. Biol. 6, 789–800 [DOI] [PubMed] [Google Scholar]
- 63. Kumanogoh A., Kikutani H. (2004) Biological functions and signaling of a transmembrane semaphorin, CD100/Sema4D. Cell. Mol. Life Sci. 61, 292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bougeret C., Mansur I. G., Dastot H., Schmid M., Mahouy G., Bensussan A., Boumsell L. (1992) Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J. Immunol. 148, 318–323 [PubMed] [Google Scholar]
- 65. Shi W., Kumanogoh A., Watanabe C., Uchida J., Wang X., Yasui T., Yukawa K., Ikawa M., Okabe M., Parnes J. R., Yoshida K., Kikutani H. (2000) The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity 13, 633–642 [DOI] [PubMed] [Google Scholar]
- 66. Witherden D. A., Watanabe M., Garijo O., Rieder S. E., Sarkisyan G., Cronin S. J., Verdino P., Wilson I. A., Kumanogoh A., Kikutani H., Teyton L., Fischer W. H., Havran W. L. (2012) The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity 37, 314–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Esser C., Rannug A., Stockinger B. (2009) The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 [DOI] [PubMed] [Google Scholar]
- 68. Fernandez-Salguero P., Pineau T., Hilbert D. M., McPhail T., Lee S. S., Kimura S., Nebert D. W., Rudikoff S., Ward J. M., Gonzalez F. J. (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268, 722–726 [DOI] [PubMed] [Google Scholar]
- 69. Kadow S., Jux B., Zahner S. P., Wingerath B., Chmill S., Clausen B. E., Hengstler J., Esser C. (2011) Aryl hydrocarbon receptor is critical for homeostasis of invariant γδ T cells in the murine epidermis. J. Immunol. 187, 3104–3110 [DOI] [PubMed] [Google Scholar]
- 70. Li Y., Innocentin S., Withers D. R., Roberts N. A., Gallagher A. R., Grigorieva E. F., Wilhelm C., Veldhoen M. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 [DOI] [PubMed] [Google Scholar]
- 71. Boulter E., Estrach S., Errante A., Pons C., Cailleteau L., Tissot F., Meneguzzi G., Feral C. C. (2013) CD98hc (SLC3A2) regulation of skin homeostasis wanes with age. J. Exp. Med. 210, 173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shires J., Theodoridis E., Hayday A. C. (2001) Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15, 419–434 [DOI] [PubMed] [Google Scholar]
- 73. Girardi M., Sherling M. A., Filler R. B., Shires J., Theodoridis E., Hayday A. C., Tigelaar R. E. (2003) Anti-inflammatory effects in the skin of thymosin-β4 splice-variants. Immunology 109, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Malinda K. M., Sidhu G. S., Mani H., Banaudha K., Maheshwari R. K., Goldstein A. L., Kleinman H. K. (1999) Thymosin β4 accelerates wound healing. J. Invest. Dermatol. 113, 364–368 [DOI] [PubMed] [Google Scholar]
- 75. Uchida Y., Kawai K., Ibusuki A., Kanekura T. (2011) Role for E-cadherin as an inhibitory receptor on epidermal γδ T cells. J. Immunol. 186, 6945–6954 [DOI] [PubMed] [Google Scholar]
- 76. Boismenu R., Feng L., Xia Y. Y., Chang J. C., Havran W. L. (1996) Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J. Immunol. 157, 985–992 [PubMed] [Google Scholar]
- 77. Matsue H., Cruz P. D., Jr., Bergstresser P. R., Takashima A. (1993) Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J. Invest. Dermatol. 101, 537–542 [DOI] [PubMed] [Google Scholar]
- 78. Taylor K. R., Mills R. E., Costanzo A. E., Jameson J. M. (2010) γδ T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFα in mouse models of obesity and metabolic disease. PLoS One 5, e11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V. R., Zhang H. G., Wang T., Zheng J., Yan J. (2011) Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gray E. E., Suzuki K., Cyster J. G. (2011) Cutting edge: identification of a motile IL-17-producing γδ T cell population in the dermis. J. Immunol. 186, 6091–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M. (2009) Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 [DOI] [PubMed] [Google Scholar]
- 82. Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 [DOI] [PubMed] [Google Scholar]
- 83. Cho J. S., Pietras E. M., Garcia N. C., Ramos R. I., Farzam D. M., Monroe H. R., Magorien J. E., Blauvelt A., Kolls J. K., Cheung A. L., Cheng G., Modlin R. L., Miller L. S. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sumaria N., Roediger B., Ng L. G., Qin J., Pinto R., Cavanagh L. L., Shklovskaya E., Fazekas de St Groth B., Triccas J. A., Weninger W. (2011) Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 208, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang H., Antony P. A., Wildhaber B. E., Teitelbaum D. H. (2004) Intestinal intraepithelial lymphocyte γδ-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J. Immunol. 172, 4151–4158 [DOI] [PubMed] [Google Scholar]
- 86. Fahrer A. M., Konigshofer Y., Kerr E. M., Ghandour G., Mack D. H., Davis M. M., Chien Y. H. (2001) Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl. Acad. Sci. USA 98, 10261–10266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gallo R. L., Hooper L. V. (2012) Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12, 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]