Abstract
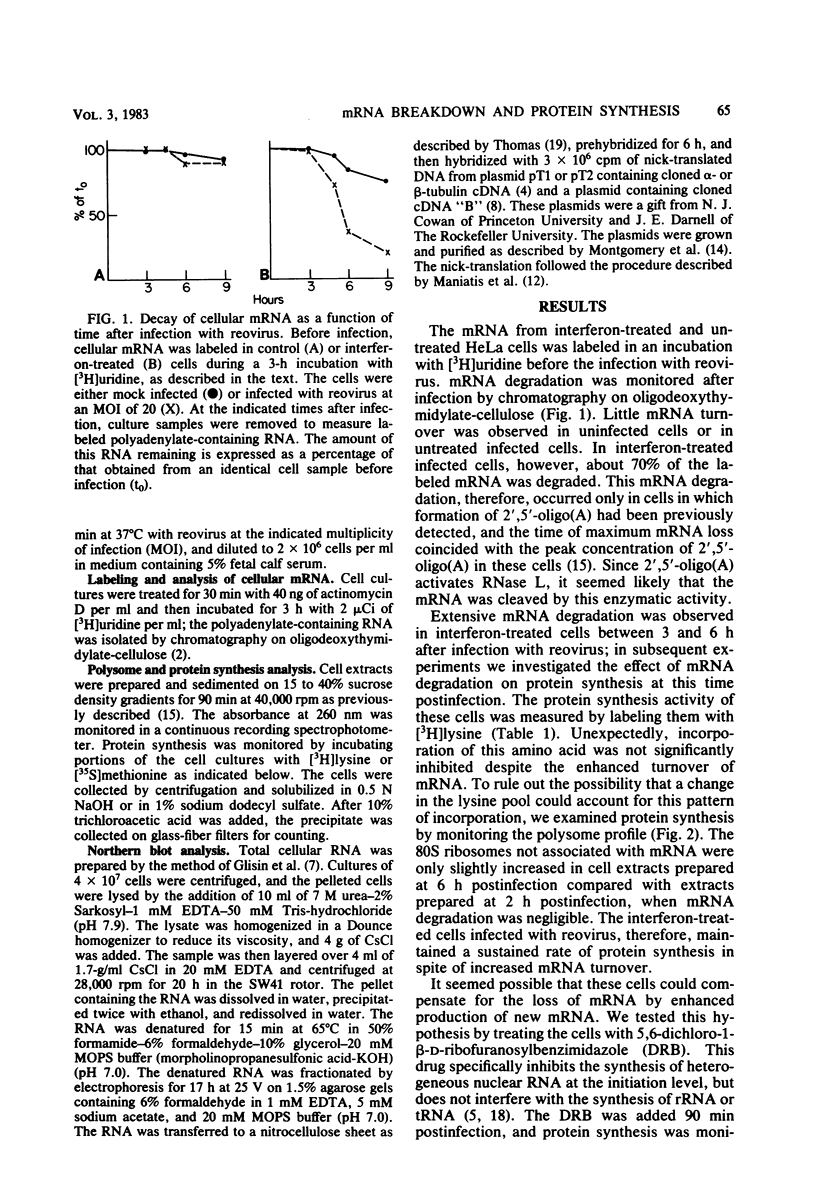
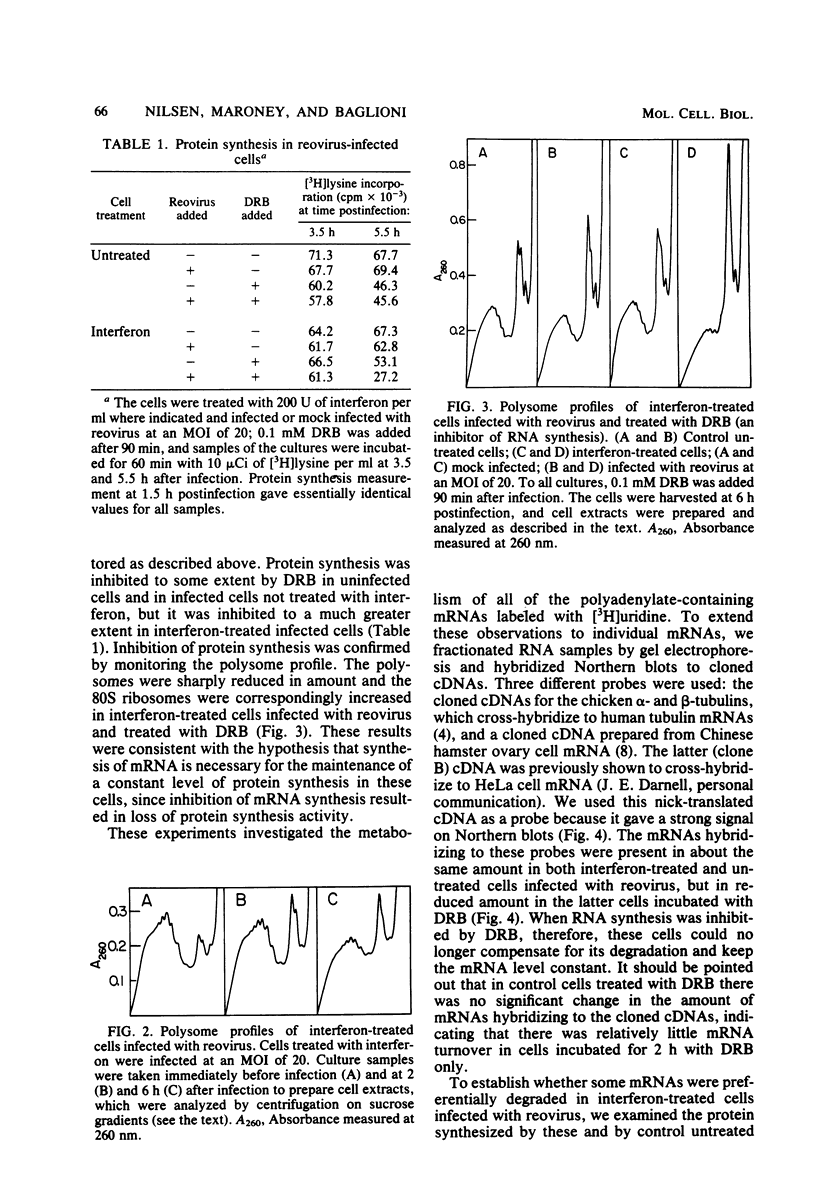
Interferon induces the synthesis of an enzyme which synthesizes 2',5'-oligoadenylate [2',5'-oligo(A)] when activated by double-stranded RNA. The 2',5'-oligo(A) in turn activates an endonuclease (RNase L). Concentrations of 2',5'-oligo(A) sufficient to activate RNase L are formed in interferon-treated HeLa cells infected with reovirus, and a large fraction of cellular mRNA is degraded (T. W. Nilsen, P. A. Maroney, and C. Baglioni, J. Virol. 42:1039-1045, 1982). We report here that in spite of this mRNA degradation, protein synthesis was not significantly inhibited in these cells. When mRNA synthesis was inhibited with 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole, protein synthesis was markedly decreased, as shown by reduced incorporation of labeled amino acids and a decrease in polyribosomes. This suggested that the turnover of mRNA could be compensated for by increased production of mRNA. The relative concentration of specific mRNAs was measured with cloned cDNA probes. The amount of these mRNAs present in control cells was comparable to that in interferon-treated cells infected with reovirus, whereas it was decreased in the latter cells treated with 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Egyházi E. Inhibition of Balbiani ring RNA synthesis at the initiation level. Proc Natl Acad Sci U S A. 1975 Mar;72(3):947–950. doi: 10.1073/pnas.72.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J., Meurs E., Montagnier L. Increased nuclease activity in cells treated with pppA2'p5'A2'p5' A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3261–3265. doi: 10.1073/pnas.76.7.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Synthesis of 2'5'-oligo(A) in extracts of interferon-treated HeLa cells. J Biol Chem. 1979 Jun 25;254(12):5058–5064. [PubMed] [Google Scholar]
- Montgomery D. L., Boss J. M., McAndrew S. J., Marr L., Walthall D. A., Zitomer R. S. The molecular characterization of three transcriptional mutations in the yeast iso-2-cytochrome c gene. J Biol Chem. 1982 Jul 10;257(13):7756–7761. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Darnell J. E., Jr, Tamm I. The inhibition by DRB (5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976 Nov;9(3):473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- West D. K., Baglioni C. Induction of interferon in HeLa cells of a protein kinase activated by double-stranded RNA. Eur J Biochem. 1979 Nov;101(2):461–468. doi: 10.1111/j.1432-1033.1979.tb19740.x. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Brown R. E., Gilbert C. S., Kerr I. M. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature. 1979 Dec 6;282(5739):582–586. doi: 10.1038/282582a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]