Abstract
Levulinyl cellulose esters have been produced as an effective renewable binder for architectural coatings. The title compound, C7H10O4 (systematic name: 2-methyl-5-oxotetrahydrofuran-2-yl acetate), assigned as the esterifying species, was isolated and crystallized to confirm the structure. In the crystal, the molecules pack in layers parallel to (102) utilizing weak C—H⋯O interactions.
Related literature
For related structures, see: Cai et al. (2004 ▶). For hydrogen-bonding motifs, see: Bernstein et al. (1995 ▶). For background information, see: Bredt (1886 ▶); Rasmussen & Brattain (1949 ▶); Suami & Day (1959 ▶); Glenny et al. (2012 ▶). For a previous description of the title compound but without supporting crystal structure data, see: Bell & Covington (1975 ▶).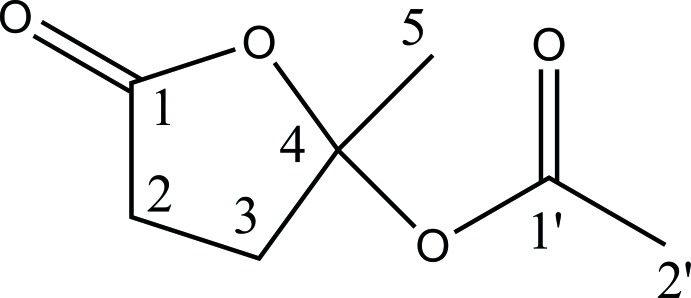
Experimental
Crystal data
C7H10O4
M r = 158.15
Monoclinic,
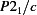
a = 5.86715 (15) Å
b = 12.7280 (3) Å
c = 10.2756 (3) Å
β = 106.020 (3)°
V = 737.55 (3) Å3
Z = 4
Cu Kα radiation
μ = 1.00 mm−1
T = 120 K
0.19 × 0.12 × 0.07 mm
Data collection
Agilent SuperNova (Dual, Cu at zero, Atlas) diffractometer
Absorption correction: gaussian (CrysAlis PRO; Agilent, 2013 ▶) T min = 0.812, T max = 1.000
4959 measured reflections
1469 independent reflections
1350 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.084
S = 1.03
1469 reflections
102 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.22 e Å−3
Data collection: CrysAlis PRO (Agilent, 2013 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL2012 (Sheldrick, 2008 ▶); molecular graphics: ORTEP in WinGX (Farrugia, 2012 ▶); software used to prepare material for publication: SHELXL2012 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813013561/cv5410sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813013561/cv5410Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813013561/cv5410Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3B⋯O2i | 0.99 | 2.68 | 3.5827 (13) | 152 |
| C1—H1B⋯O3ii | 0.98 | 2.63 | 3.4617 (14) | 142 |
Symmetry codes: (i) 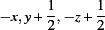 ; (ii)
; (ii) 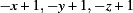 .
.
Acknowledgments
We thank Dr Matthew Polson of the University of Canterbury, New Zealand, for the data collection.
supplementary crystallographic information
Comment
Acetoxy-γ-valerolactone (2-methyl-5-oxotetrahydrofuran-2-yl acetate) was first described by Bredt (1886) and became of interest during our investigation of novel, renewable levulinyl cellulose esters. Esterification of cellulose in the presence of levulinic acid and an aliphatic anhydride affords a mixed cellulose levulinyl ester which we have shown has particular utility in architectural coatings [Glenny et al., 2012]. Levulinyl acetyl cellulose was generated by the sulfuric acid catalysed esterification of cellulose in the presence of acetic anhydride and levulinic acid. Analysis of this reaction mixture indicated that a valero-lactone species predominated rather than the anticipated mixture of anhydrides.
Acetoxy-γ-valerolactone was isolated by flash chromatography and identified as the major species in the reaction solution and has been assigned as the esterifying reagent. The generation of acetoxy-γ-valerolactone from acetic anhydride and levulinic acid had previously been reported (Rasmussen & Brattain, 1949) and also had been shown to be an esterifying agent forming levulinyl and acetyl amides (Suami & Day, 1959). When isolated in our hands, acetoxy-γ-valerolactone remained as a super cooled liquid, much like levulinic acid which exists as a light yellow solid or liquid, but will crystalize and displays a melting point between 30–33°C. Bell and Covington (1975) described the material as a solid with a melting point between 75–76°C but without supporting crystal structure data. The molecule was therefore crystallized from DCM and petroleum ether and the crystal structure elucidated. This confirmed the molecular structure and assisted with investigation into its esterification chemistry.
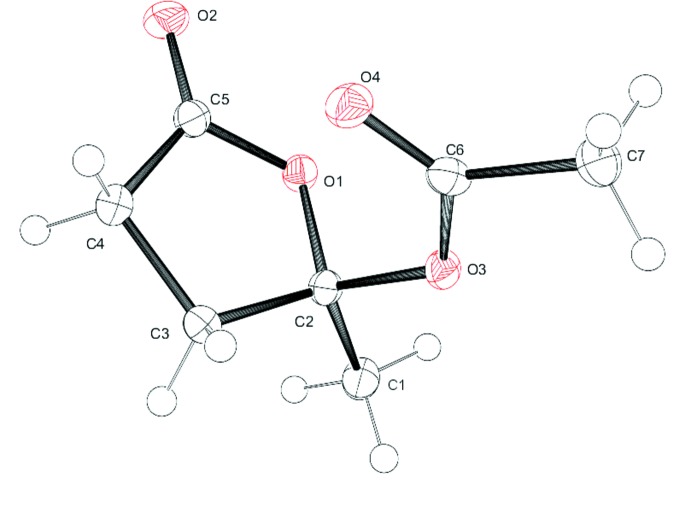
The title compound, C7H10O4, crystallizes with one unique molecule per asymetric unit. The five-membered ring adopts a flattened envelope conformation with O1 atom as a flap which deviates by 0.128 (1) Å from the mean plane P formed by the four C atoms. The acetate fragment is oriented in such a way that its mean plane and plane P are almost perpendicular to each other with an interplanar angle of 83.18 (7)°. There are no closely related structures in the Cambridge Structural Database, the closest being 5-(1-adamantyl)-5-ethoxytetrahydrofuran-2-one, LAGQUG (Cai et al., 2004).
In the crystal, weak Cmethyl—H···Oacetate hydrogen bonds link the molecules into centrosymmetric dimers with the well known R22(8) motif (Bernstein et al., 1995), and weak Cmethylene—H···Oketone interactions (Table 1) link further these dimers into layers parallel to (102). Table 1.
Experimental
Levulinic acid (5.24 g, 45.1 mmol), acetic anhydride (3.46, 33.9 mmol) and concentrated sulfuric acid (12.9 mg, 129µmol) were placed in a 50 ml round bottom flask. The solution was heated to 120°C for 10 min with stirring, then quenched with 8 ml of a 5% Mg(OAc)2 solution in 50/50 acetic acid water. The reaction solution was extracted with DCM recovering an orange brown liquid. A portion of the recovered material was purified with flash chromatography; the column was packed with 40–63µm silica, (Davisil) to the dimensions of 110x35 mm. A gradient solvent system was used (petroleum ether/ethyl acetate 60/40 (400 ml), 50/50 (200 ml), 30/70 (200 ml), 10/90 (200 ml)) to separate and elute the 4-acetoxy-γ-valerolactone (Rf 0.42 in 60/40 petroleum ether/ethyl acetate). Suitable crystals were obtained by recrystallization from DCM and petroleum ether.
4-Acetoxy-γ-valerolactone m.p. 72–74°C (DSC): 1H NMR (500 MHz, CDCl3): δ 1.77 (s, H-3), 2.06 (s, H-1'), 2.31 (ddd, H-2, J 8.8, 10.6, 18.7 Hz), 2.61 (m, H-1 and H-2), 2.87 (ddd, H-1, J 7.75, 9.95, 17.6 Hz); 13C NMR δ 21.6 (C-1',OC(O)CH3), 26.1 (C-5,CCH3), 28.5 (C-2, CH2CH2), 32.7 (C-3, CH2C), 108.4 (C-4, Quaternary), 169.2 (C-1'), 175.4 (C-1); TOF-HRMS found 181.0472; [C7H10NaO4]+ calc. 181.0477.
Refinement
All methyl H atoms were constrained to an ideal geometry (C—H = 0.98 Å) with Uiso(H) = 1.5Ueq(C), but were allowed to rotate freely about the adjacent C—C bond. All other C bound H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances of 0.99 Å and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
ORTEP (Farrugia, 2012) view of the title molecule showing the atomic numbering and 50% probability displacement ellipsoids.
Crystal data
| C7H10O4 | F(000) = 336 |
| Mr = 158.15 | Dx = 1.424 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 5.86715 (15) Å | Cell parameters from 3083 reflections |
| b = 12.7280 (3) Å | θ = 5.7–73.5° |
| c = 10.2756 (3) Å | µ = 1.00 mm−1 |
| β = 106.020 (3)° | T = 120 K |
| V = 737.55 (3) Å3 | Block, colourless |
| Z = 4 | 0.19 × 0.12 × 0.07 mm |
Data collection
| Agilent SuperNova (Dual, Cu at zero, Atlas) diffractometer | 1469 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 1350 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.031 |
| Detector resolution: 5.3250 pixels mm-1 | θmax = 73.8°, θmin = 5.7° |
| ω scans | h = −7→7 |
| Absorption correction: gaussian (CrysAlis PRO; Agilent, 2013) | k = −13→15 |
| Tmin = 0.812, Tmax = 1.000 | l = −12→12 |
| 4959 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.084 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0434P)2 + 0.1941P] where P = (Fo2 + 2Fc2)/3 |
| 1469 reflections | (Δ/σ)max < 0.001 |
| 102 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Experimental. Absorption correction: CrysAlis PRO (Agilent, 2013); numerical absorption correction based on gaussian integration over a multifaceted crystal model |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.25087 (13) | 0.23353 (6) | 0.37683 (8) | 0.01787 (19) | |
| O2 | 0.01399 (17) | 0.10362 (7) | 0.27499 (9) | 0.0268 (2) | |
| O3 | 0.32805 (14) | 0.40425 (6) | 0.32322 (8) | 0.0179 (2) | |
| O4 | 0.04509 (15) | 0.35237 (6) | 0.13677 (8) | 0.0226 (2) | |
| C1 | 0.4004 (2) | 0.36108 (9) | 0.55163 (11) | 0.0217 (3) | |
| H1A | 0.3518 | 0.3185 | 0.6188 | 0.033* | |
| H1B | 0.3959 | 0.4356 | 0.5747 | 0.033* | |
| H1C | 0.5620 | 0.3418 | 0.5514 | 0.033* | |
| C2 | 0.23376 (19) | 0.34162 (8) | 0.41354 (11) | 0.0166 (2) | |
| C3 | −0.0297 (2) | 0.36272 (9) | 0.40270 (11) | 0.0187 (2) | |
| H3A | −0.0526 | 0.3758 | 0.4932 | 0.022* | |
| H3B | −0.0882 | 0.4243 | 0.3442 | 0.022* | |
| C4 | −0.1589 (2) | 0.26306 (9) | 0.34032 (12) | 0.0206 (2) | |
| H4A | −0.2462 | 0.2319 | 0.4007 | 0.025* | |
| H4B | −0.2728 | 0.2785 | 0.2516 | 0.025* | |
| C5 | 0.0322 (2) | 0.18993 (9) | 0.32378 (11) | 0.0187 (2) | |
| C6 | 0.2205 (2) | 0.40306 (8) | 0.18841 (11) | 0.0185 (2) | |
| C7 | 0.3555 (2) | 0.46800 (9) | 0.11343 (12) | 0.0236 (3) | |
| H7A | 0.4708 | 0.4236 | 0.0862 | 0.035* | |
| H7B | 0.4391 | 0.5245 | 0.1723 | 0.035* | |
| H7C | 0.2451 | 0.4984 | 0.0327 | 0.035* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0182 (4) | 0.0148 (4) | 0.0212 (4) | 0.0003 (3) | 0.0065 (3) | 0.0008 (3) |
| O2 | 0.0346 (5) | 0.0185 (4) | 0.0294 (5) | −0.0052 (3) | 0.0121 (4) | −0.0058 (3) |
| O3 | 0.0204 (4) | 0.0174 (4) | 0.0163 (4) | −0.0029 (3) | 0.0059 (3) | 0.0013 (3) |
| O4 | 0.0262 (4) | 0.0233 (4) | 0.0172 (4) | −0.0041 (3) | 0.0043 (3) | −0.0009 (3) |
| C1 | 0.0218 (6) | 0.0257 (6) | 0.0163 (5) | −0.0040 (4) | 0.0032 (4) | 0.0019 (4) |
| C2 | 0.0191 (5) | 0.0141 (5) | 0.0173 (5) | −0.0015 (4) | 0.0061 (4) | 0.0007 (4) |
| C3 | 0.0189 (5) | 0.0184 (5) | 0.0197 (5) | 0.0003 (4) | 0.0069 (4) | −0.0021 (4) |
| C4 | 0.0182 (5) | 0.0211 (5) | 0.0223 (5) | −0.0024 (4) | 0.0054 (4) | −0.0022 (4) |
| C5 | 0.0219 (5) | 0.0185 (5) | 0.0163 (5) | −0.0031 (4) | 0.0065 (4) | 0.0011 (4) |
| C6 | 0.0229 (6) | 0.0160 (5) | 0.0170 (5) | 0.0022 (4) | 0.0064 (4) | 0.0004 (4) |
| C7 | 0.0293 (6) | 0.0226 (6) | 0.0207 (5) | −0.0026 (5) | 0.0099 (5) | 0.0023 (4) |
Geometric parameters (Å, º)
| O1—C5 | 1.3660 (14) | C3—C4 | 1.5253 (15) |
| O1—C2 | 1.4373 (13) | C3—H3A | 0.9900 |
| O2—C5 | 1.2000 (15) | C3—H3B | 0.9900 |
| O3—C6 | 1.3546 (14) | C4—C5 | 1.5025 (16) |
| O3—C2 | 1.4443 (13) | C4—H4A | 0.9900 |
| O4—C6 | 1.2063 (15) | C4—H4B | 0.9900 |
| C1—C2 | 1.5049 (15) | C6—C7 | 1.4972 (15) |
| C1—H1A | 0.9800 | C7—H7A | 0.9800 |
| C1—H1B | 0.9800 | C7—H7B | 0.9800 |
| C1—H1C | 0.9800 | C7—H7C | 0.9800 |
| C2—C3 | 1.5424 (15) | ||
| C5—O1—C2 | 111.57 (8) | H3A—C3—H3B | 108.8 |
| C6—O3—C2 | 119.86 (8) | C5—C4—C3 | 105.24 (9) |
| C2—C1—H1A | 109.5 | C5—C4—H4A | 110.7 |
| C2—C1—H1B | 109.5 | C3—C4—H4A | 110.7 |
| H1A—C1—H1B | 109.5 | C5—C4—H4B | 110.7 |
| C2—C1—H1C | 109.5 | C3—C4—H4B | 110.7 |
| H1A—C1—H1C | 109.5 | H4A—C4—H4B | 108.8 |
| H1B—C1—H1C | 109.5 | O2—C5—O1 | 120.27 (11) |
| O1—C2—O3 | 107.02 (8) | O2—C5—C4 | 129.16 (11) |
| O1—C2—C1 | 109.32 (9) | O1—C5—C4 | 110.56 (9) |
| O3—C2—C1 | 104.52 (9) | O4—C6—O3 | 123.78 (10) |
| O1—C2—C3 | 106.79 (8) | O4—C6—C7 | 125.25 (10) |
| O3—C2—C3 | 114.29 (9) | O3—C6—C7 | 110.90 (10) |
| C1—C2—C3 | 114.62 (9) | C6—C7—H7A | 109.5 |
| C4—C3—C2 | 104.93 (9) | C6—C7—H7B | 109.5 |
| C4—C3—H3A | 110.8 | H7A—C7—H7B | 109.5 |
| C2—C3—H3A | 110.8 | C6—C7—H7C | 109.5 |
| C4—C3—H3B | 110.8 | H7A—C7—H7C | 109.5 |
| C2—C3—H3B | 110.8 | H7B—C7—H7C | 109.5 |
| C5—O1—C2—O3 | −112.79 (9) | C1—C2—C3—C4 | −128.27 (10) |
| C5—O1—C2—C1 | 134.56 (9) | C2—C3—C4—C5 | 2.08 (11) |
| C5—O1—C2—C3 | 10.02 (11) | C2—O1—C5—O2 | 172.31 (10) |
| C6—O3—C2—O1 | 62.87 (11) | C2—O1—C5—C4 | −8.92 (12) |
| C6—O3—C2—C1 | 178.76 (9) | C3—C4—C5—O2 | −177.45 (11) |
| C6—O3—C2—C3 | −55.15 (12) | C3—C4—C5—O1 | 3.91 (12) |
| O1—C2—C3—C4 | −7.04 (11) | C2—O3—C6—O4 | 0.50 (16) |
| O3—C2—C3—C4 | 111.11 (10) | C2—O3—C6—C7 | −176.52 (9) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3B···O2i | 0.99 | 2.68 | 3.5827 (13) | 152 |
| C1—H1B···O3ii | 0.98 | 2.63 | 3.4617 (14) | 142 |
Symmetry codes: (i) −x, y+1/2, −z+1/2; (ii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CV5410).
References
- Agilent (2013). CrysAlis PRO Agilent Technologies Ltd, Yarnton, England.
- Bell, R. P. & Covington, A. D. (1975). J. Chem. Soc. Perkin Trans. 2, pp. 1343–1348.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bredt, J. (1886). Ann. Chem. 236, 225–232.
- Cai, Y., Roberts, B. P., Tocher, D. A. & Barnett, S. A. (2004). Org. Biomol. Chem. 2, 2517–2529. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Glenny, M., Gooch, C., Hinkley, S., Mason, J., Tristram, C. & Williams, D. (2012). NZ Patent No. PCT/NZ2012/000728.
- Rasmussen, R. S. & Brattain, R. R. (1949). J. Am. Chem. Soc. 71, 1073–1079.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suami, T. & Day, A. R. (1959). J. Org. Chem. 24, 1340–1343.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813013561/cv5410sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813013561/cv5410Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813013561/cv5410Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report