Abstract
People who were small at birth and had poor infant growth have an increased risk of adult cardiovascular disease, osteoporosis and type 2 diabetes, particularly if their restricted early growth is followed by increased childhood weight gain. These relations extend across the normal range of birth size in a graded manner, so reduced size is not a prerequisite. In addition larger birth size is associated with risks of obesity and type 2 diabetes. The associations appear to reflect developmental plastic responses made by the fetus and infant based on cues about the environment, influenced by maternal characteristics including diet, body composition, stress and exercise levels. These responses involve epigenetic processes which modify the offspring’s phenotype. Vulnerability to ill-health results if the environment in infancy, childhood and later life is mismatched to the phenotype induced in development, informed by the developmental cues. This mismatch may arise through unbalanced diet or body composition of the mother, or change in lifestyle factors between generations. These insights offer new possibilities for early diagnosis and prevention of chronic disease.
Keywords: Nutrition, fetal growth, metabolic disease, epigenetics
Epidemiological observations
Non-communicable diseases (NCD), including diabetes, cardiovascular disease and the metabolic syndrome, account for 60% of all deaths globally. In low to middle income countries, NCD are becoming particularly important: a rapid increase in their prevalence has been observed as these countries undergo socio-economic improvement.1 Whilst the increase in NCD arises in part through adopting a western lifestyle, there is growing recognition of the role played by developmental factors. This is in accordance with the fundamental principles of life-course biology, whereby developmental trajectories established in early life influence the response of the individual to later exposures, such as adult lifestyle. It is now being proposed that the temporal trends in NCD may in significant part arise from effects on phenotype established by the interaction between genes and the developmental environment using the processes of developmental plasticity.2
This perspective is underpinned by research examining the developmental origins of health and disease (DOHaD) concept. This originated from studies in Germany and in Scandinavia linking aspects of a poor start to life to risks of chronic disease later.3,4 Subsequent epidemiological studies of cause of death in Britain in babies born in during the early 1900s led to the suggestion that low rates of growth before birth are linked to the development of coronary heart disease in adult life.5 Direct evidence that coronary heart disease and associated disorders have their origins during early development initially came from longitudinal studies of 25,000 UK men and women whose birth records had been preserved. Follow-up of these individuals in late adulthood showed associations between lower birthweight and increased rates of cardiovascular disease, type 2 diabetes and osteoporosis in adulthood. 6,7 People who were small or disproportionate (thin or short) at birth, or whose infant growth faltered, had high rates of coronary heart disease, raised blood pressure and cholesterol levels, and impaired glucose tolerance. Epidemiological studies have also linked impaired early growth with later osteoporosis and obstructive airways disease, including asthma.8,9
In adulthood, individuals who had a lower birthweight have a higher risk of developing the metabolic syndrome, including insulin resistance, hypertension, and raised serum low-density lipoprotein and fibrinogen concentrations. The associations with birthweight are graded, affecting individuals across the normal range of birth-weights, and do not simply reflect effects in severely growth-restricted fetuses.10 Subsequent studies have now shown that risk of metabolic syndrome is also elevated in babies at the higher level of the birthweight distribution. Thus in many populations the relation between the developmental environment and disease risk is U-shaped.10
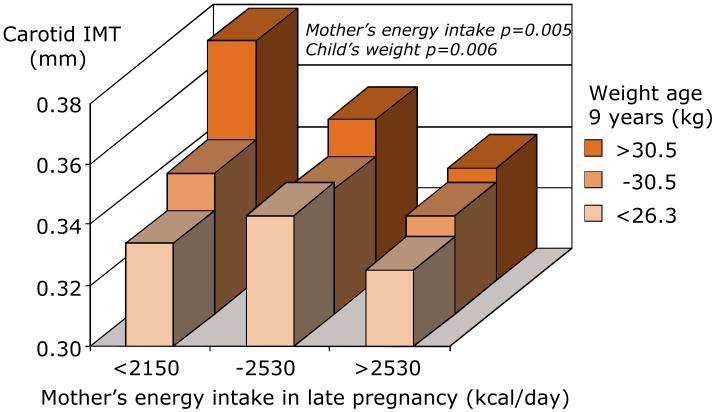
While it was historical cohort studies that first described links between fetal growth and adult cardiovascular and metabolic disease, more recent mother-offspring studies have shown that maternal diet in pregnancy and constrained fetal growth are linked to childhood cardiovascular structure and function. Arterial intima-media thickness (IMT) is a known predictor of vascular events in adults, but few studies have investigated IMT in children. In 9-year old children from a large unselected cohort in Southampton, we have shown that carotid IMT was greater in children who were heavier, had a higher BMI, had higher systolic blood pressure and participated less in sport or exercise. However, independent of these child characteristics and maternal BMI, higher IMT was found in children whose mothers had a lower energy intake in pregnancy (Figure 1).11 Moreover other studies in contemporaneous cohorts have shown that higher mother’s body mass index, gestational diabetes, higher blood glucose and greater pregnancy weight gain are all associated with greater adiposity of the children.12 This provides evidence that developmental influences continue to be important for the cardiovascular health of today’s children in developed countries and suggests that variations in maternal diet can influence susceptibility to atherosclerosis and metabolic disease in the offspring.
Figure 1.
Child’s carotid artery intima-media thickness in relation to their weight and the mother’s diet in pregnancy
Evidence from cohorts with information on the duration of gestation suggests that both reduced fetal growth and preterm delivery are associated with an increased risk of cardiovascular and metabolic disease. Moreover, new observations indicate that normal variations in fetal size in mid-gestation are associated with differences in gestational length.13 Experimental studies show that neither a reduction nor an increase in fetal growth above the expected trajectory is an essential step in the causal pathway to disease. Rather the change in trajectory is part of a coordinated ‘strategy’ of responses which confers future adaptive advantage.10
These ideas accord with the concept that all human fetuses are under a degree of maternal constraint of their growth, a process which is dynamic and variable. Maternal constraint is believed to have particularly evolved in H. sapiens as a way of matching the size of the fetus, especially that of the head as greater brain volume evolved, to that of the pelvic canal associated with the changes in pelvic orientation and dimensions associated with bipedalism.14 Maternal constraint is greater in primiparous women, in both younger and older mothers, in those bearing twins, and in mothers of shorter stature. Fetal growth is also constrained in those who smoke, have a low body mass index and gain little weight in pregnancy and in those consuming an unbalanced diet, although these exposures are likely to act through a variety of different mechanisms.
Developmental plasticity and mismatch
Development represents a period of rapid change in the expression of the genome, during which environmental cues may induce persistent changes in the phenotype of an organism. The developmental program tends to follow a path in which the characteristics of the wild type or typical phenotype are buffered against genetic and epigenetic change, termed canalization.15 However, many organisms respond during development to cues about their likely future environment, and this alters the developmental program and generates alternative phenotypes. Such deviation from canalized development allows production of different phenotypes from a single genotype more rapidly than could be achieved by mutation. For example, crowding of adult desert locusts (Scistocerca gregaria) induces gregarious, diurnal and migratory offspring, in contrast to the nocturnal, sedentary forms which are produced under low population density;16 and the duration of day light to which meadow voles (Microtus pennsylvanicus) are exposed before conception determines coat thickness in the offspring in anticipation of winter or spring temperatures.17 Such rapid changes in phenotype may facilitate short-term survival, but may also be genetically assimilated and so produce stable phenotypes on which natural selection can act.18 Increasing evidence suggests that such persistent changes in the expression of the genome involve altered epigenetic regulation of specific genes.
Gluckman and Hanson19,20 and Uller21 have argued that the developmental environment can produce a range of effects, from overt disruption of development (i.e. teratogenesis), through altered fetal growth, with both its immediate and later consequences, to a range of phenotypes which become manifest only well after birth. This latter class can be induced by maternally transduced cues operating even within the normal range of developmental environments but nonetheless affecting several components of the trajectory of phenotypic development. The responses do not necessarily confer any immediate advantage for the fetus but give a Darwinian fitness advantage in later environments, the nature of which is predicted on the basis of the developmental experience. As the phenotype develops, the nature of this advantage may change at different points across the life course. Thus increased insulin sensitivity may promote adipogenesis, providing nutritional reserves to protect the brain after weaning;22 earlier puberty enhances fitness in a predicted adverse environment;23,24 and the development of later insulin resistance confers a degree of “thrift” in a predicted adverse environment, as may reduction in numbers of energy-consuming skeletal and cardiac muscle cells or renal nephrons. This type of response has been termed a predictive adaptive response (PAR),25 and experimental and clinical studies supporting the concept have now been reported.26,27
According to the PARs model, the accuracy of the responses is dependent on the environment remaining relatively constant throughout the lifecourse. Although environments can fluctuate, modelling studies have shown that induced phenotypes can persist for several generations and provide an adaptive advantage in environments considered stochastically.27 Thus, the fidelity of the predictions made during early life need not be high for PARs to confer a fitness advantage and thus be selected through evolution. When the anticipated environment is effectively constant over many generations, the predictive trait/response may become fixed, or genetically encoded in a process known as genetic assimilation.28 This process may include selection of advantageous mutations.
PARs thus act as an integrated regulator in early life to establish a life-course strategy for meeting the demands of the predicted later environment29 and are only adaptive when the post-developmental environment is in the predicted range.29 If the later environment lies outside the anticipated range, the individual is “mismatched”, having a phenotype which is not appropriate for that environment. This can affect a range of traits including abdominal fat deposition, reduced skeletal muscle deposition, reduced endothelial function, fewer cardiomyocytes, fewer nephrons, earlier puberty (at least in females), alterations in Th1 to Th2 cell balance associated with atopic/allergic reactions, reduced DNA repair leading to earlier ageing, and a range of effects on behaviour, including affective disorders and stress responses which are sex specific.30,31 It is important to note that neither the developmental nor the later environment need to provide extreme challenges, only that the phenotype induced by the former is not optimal for responding on a long-term basis to the latter.
Mismatch can arise through a range of circumstances. It can result from exposure to an environment which is evolutionarily novel and thus beyond the predictive capacity of the fetus. Indeed, the contemporary diets and lifestyles of developed societies constitute such a novel environment for Homo sapiens. The risk of NCD is then related to the degree of mismatch rather than to the absolute level of the adult environment per se. This is demonstrated in a number of experimental studies in which pre- and postnatal diets were manipulated.32-34 For example, male sheep exposed to prenatal undernutrition but normal postnatal diet, and vice versa, showed altered cardiovascular function which was absent in animals subjected solely to undernutrition.35 In rats exposed to a high-fat diet in utero, endothelial dysfunction was observed in offspring fed a normal post-weaning diet, but not in those fed a high-fat post-weaning diet.36
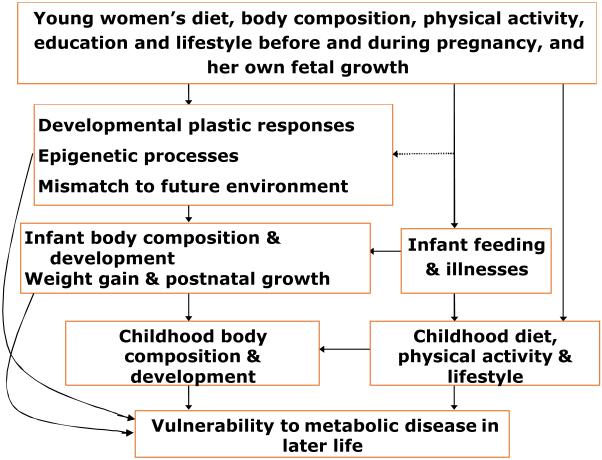
The degree of mismatch can by definition be increased by either poorer environmental conditions during development, or richer conditions later, or both.37 Unbalanced maternal diet, body composition or disease can perturb the former; a rapid increase in energy-dense foods and reduced physical activity levels associated with a western lifestyle will increase the degree of mismatch via the latter (Figure 2). Such changes are of considerable importance in developing societies going through rapid socio-economic transitions.
Figure 2.
Developmental influences on vulnerability to metabolic disease
Timing and nature of the effects
Detailed information on the aetiology and timing of the prenatal effects in humans is, of course, not available for adults born 50 or more years ago. However, studies of individuals whose birth proportions were recorded have provided some insights. For example, follow-up of a large cohort of births in Uppsala, Sweden, suggested that late-onset fetal growth restriction (asymmetrical growth restriction) is particularly associated with the risk of raised blood pressure and type 2 diabetes in later adult life.38,39
A particular insight from animal studies is that the effects of poor maternal nutrition confined to the periconceptional period can have long-term effects on cardiovascular and metabolic function in the offspring.40,41 In rodents the effects may involve embryonic stem cell differentiation42 and yolk sac function.43 Whereas initial work concentrated on fetal life, further studies demonstrated that the sensitive period during which the early environment can have long-lasting effects on the offspring encompasses the time from conception, through gestation and into postnatal life, hence the terminology of the ‘developmental origins of health and disease’.
Some studies with follow-up into adolescence or early adulthood have concluded that greater infant weight gain increases levels of cardiovascular risk factors. An increased risk of obesity is even reported in infants gaining weight in the first few weeks after birth.44 People at particular risk of cardiovascular and metabolic disease in adult life are those in whom restricted fetal and infant growth was followed by increased childhood weight gain. For example, in a cohort of men born in Helsinki, Finland, the highest risks of coronary heart disease and type 2 diabetes were associated with small body size at birth, low weight gain during infancy and rapid weight gain in childhood. This path of growth is associated with the later development of hypertension, insulin resistance and a body composition characterised by low muscle mass, but high fat mass, which is known to be associated with the metabolic syndrome.45,46 In contrast, children who later develop thrombotic or haemorrhagic stroke are small at birth, have low weight gain between birth and two years but remain thin through childhood.47 This path of growth is associated with later hypertension and an atherogenic lipid profile.48
Recent animal studies and epidemiological data indicate that although maternal thinness and an unbalanced diet during pregnancy may have modest effects on size at birth, they are nonetheless associated with raised blood pressure and altered glucose-insulin metabolism and stress responsiveness in the adult offspring.49 Moreover, studies in Europe and India have shown that high maternal weight and adiposity are associated with coronary heart disease, insulin deficiency and type-2 diabetes in the offspring. 49 The data suggest that fetal development can be affected by nutritional variation even within the normal range of Western diets. Although nutrition has received the most focus, other early environmental factors such as infection, season of birth and smoking or exposure to toxins may also have long-term effects.
Mechanisms underlying long-term consequences of impaired early development
A variety of species from small to large animals have been used for experimental studies of the mechanisms underlying DOHaD. They have the advantage that a defined prenatal challenge can be administered and the offspring can be studied in utero or at various postnatal ages. The challenges used have largely involved unbalanced maternal nutrition or glucocorticoid administration. The phenotypic outcomes resemble those reported in humans from epidemiological studies, including impaired glucose homoeostasis, insulin secretion and action, cardiovascular, renal and endothelial function and effects on bone. There is also now evidence for effects on neural and endocrine function, including the regulation of appetite and control of the hypothalamic-pituitary-adrenal axis.50 The experimental studies reveal that the changes involved in animals constitute a change in life course strategy, perhaps akin to the induction of alternative phenotypes in invertebrates.51 Thus the effects are not necessarily disruptive, e.g. they may involve effects on reproductive function. Indeed Sloboda et al reported that pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat,24 paralleling their observation that the combination of small size at birth and relatively high weight at age 8 yrs is associated with an advancement in human menarche by about 2 years.26
Experimental studies have given insights in four broad areas: (1) epigenetic processes, such as DNA methylation, histone structure and small non-coding RNAs, (2) mitochondrial function, (3) irreversible changes in the developmental trajectory of specific organs and tissues, and (4) effects on homoeostatic control systems. They are not mutually exclusive because, for example, epigenetic processes and altered mitochondrial function may also become manifest as alterations in organ/tissue differentiation or in homoeostatic control systems.
Epigenetic processes
An epigenetic modification is one that does not alter the heritable DNA sequence but does affect gene expression. DNA methylation is the best understood epigenetic modification. During gametogenesis, and in the preimplantation embryo, there is considerable de-methylation and re-methylation, and these may be critical windows for the establishment of epigenetic modification.52 Furthermore, there are graded changes in the epigenetic control of some genes during development, providing the opportunity for environmental influences to act via them.53 The DNA methylation and histone structure processes underlying the epigenetic control of gene expression depend on the supply of one-carbon groups, predominantly from glycine, a non-essential amino acid but one for which the fetal requirements are very large in late gestation. In pregnant rats fed a low protein diet, supplementation of the dam with glycine prevents hypertension and endothelial dysfunction in the offspring.54
In the rat maternal protein restriction alters DNA methylation of the glucocorticoid receptor and peroxisomal proliferator-activated receptor alpha (PPARα) genes in the offspring, changing their expression, and altering the expression of other genes controlled by these transcription factors.55 These genes are of particular interest because alteration of their expression is associated with perturbation of cardiovascular and metabolic control.55,56 The methylation changes are accompanied by alterations in histone methylation and acetylation, which similarly change gene expression. Maternal dietary folate supplementation prevents the epigenetic modification associated with maternal protein restriction in these rats.55
One of the most striking phenomena in this field is that phenotypic effects can be induced by nutritional and other environmental challenges in early gestation in a range of species.42,57-59 Such effects underline the possible influence of epigenetic processes in the embryo, and also raise issues about the long-term consequences of assisted reproductive therapies in which a period of embryo culture in exogenous media occurs.
Altered mitochondrial function
Mitochondria are central to metabolic control and mitochondrial DNA is susceptible to environmental effects, which could produce changes in mitochondrial copy number. Epigenetic effects may operate by changes in mitochondrial DNA methylation, by the effects of pro-inflammatory cytokines, and via the effects of reactive oxygen species. Changes in mitochondrial DNA are passed via the female line to future generations, so they offer the possibility of a trans-generational process for induction of phenotype. Support for this has recently been gained via studies in which animals were bred over 11 generations to select for reduced exercise tolerance. The animals then showed all the components of the human metabolic syndrome and underlying defects in mitochondrial function.60 Mitochondria are a feasible target in the aetiology of the metabolic syndrome because mitochondrial damage builds up over the life course. Impaired mitochondrial function in the offspring of rats fed a high fat diet during pregnancy is coupled with insulin and leptin resistance and relative insulin depletion of the pancreatic islets.61 There are also effects on fat deposition in the liver, which resembles the non-alcoholic fatty liver disease associated with the metabolic syndrome.62
Organ structure and composition
A range of experimental studies and human observations has shown that a severe reduction in nutrient and oxygen supply differentially affects the growth and development of organs and tissues. This may occur because those not essential to fetal survival are sacrificed. Organs affected include the lungs, kidney, gut, pancreas, vasculature and liver.63 However, in the face of a milder challenge, changes in fetal tissue or organ development may occur as part of a strategy to tune phenotype to the predicted post-natal environment, based on nutritional and endocrine cues from the mother. Examples for which there is strong experimental and preliminary human evidence include reductions in capillary density, skeletal muscle growth and nephron number which would reduce nutrient demands postnatally.37 The fetal strategy may include promoting the growth of other tissues, such as adipose tissue, to buffer anticipated nutrient scarcity.22
The vascular endothelium is a target tissue that is particularly affected by the developmental environment. Vascular endothelial cells play an important role in normal life regulating vessel calibre, remodelling, tissue and organ growth and metabolism, immune responses, blood fluidity, platelet and white cell stickiness and vascular permeability. Endothelial dysfunction occurs in hypertension, atherogenesis, type 2 diabetes, coronary heart disease, in the metabolic syndrome and obesity, and in diseases of pregnancy, including pre-eclampsia. Studies of the vascular reactivity of low birthweight children have revealed altered responsiveness.64
Resetting of homeostatic control
Clinical and experimental studies provide evidence for developmental changes in the homeostatic set-points for many hormones and for alterations in tissue sensitivity to these hormones. An example of resetting of homeostatic control with direct relevance to the developmental origins of cardiovascular disease is the influence of nutrition and stress on placental 11-hydroxysteroid dehydrogenase type 2 (11β-HSD2) activity. This enzyme plays an important role in protecting the fetus from high levels of circulating glucocorticoids in the mother. Mothers who report dieting before pregnancy have decreased placental 11β-HSD2 activity at term.65 In rats, reduced placental 11β-HSD2 activity is associated with increased blood pressure in the offspring during adult life.66 In the rat, low placental 11β-HSD2 activity may lead to premature activation of the fetal hypothalamic-pituitary-adrenal (HPA) axis. If a similar mechanism operates in human pregnancy, this could explain the relationship between maternal influences and alterations of adrenocortical function in the offspring.
Alterations of the fetal HPA axis and sympathoadrenal responses are likely to be an important mechanism by which developmental exposures affect the subsequent responses of the offspring to stressful challenges. Lower birthweight has been linked with increased fasting cortisol concentrations in later adult life.67 Moreover, studies of children whose antenatal growth was restricted demonstrate alteration of adrenocortical responses to stress in boys and basal adrenocortical activity in girls.68 Similar gender differences in HPA responses have been reported in animals.50 Given the known associations between small alterations in adrenocortical activity and features of the metabolic syndrome, these effects may have important health implications. The maternal influences underlying developmental effects on HPA and sympathoadrenal responsiveness remained to be defined, but there is evidence that both maternal diet and stress in pregnancy may be important.69,70
Sympathetic neural responses
Elevated sympathetic efferent activity has long been known to play a role in the aetiology of hypertension, whether through effects on the arterial resistance vessels, cardiac output, the kidney or via changes in central nervous function. The latter is closely linked with the changes in hypothalamic function. Patients with high blood pressure tend to have features of increased sympathetic nervous system activity, including a high resting pulse rate and a hyperdynamic circulation. Among men and women in Preston, UK, those who had low birthweight had a higher resting pulse rate.71 This suggests that slower growth in utero establishes increased sympathetic nervous activity and contributes to raised blood pressure in later life. There is also now much interest in the ways in which perturbations in sympathetic activity interact with obesity and appetite control via leptin and fat metabolism. Increasing evidence from animal experiments suggests that altered sympathetic activity is an important link between growth restriction in utero and subsequent increased appetite and food intake, and later obesity.72
Conclusion
There is now considerable evidence that variations in the quality of the early life environment induce differential risk of metabolic disease in later life. Studies in animal models show that mechanisms underlying long term effects of the developmental environment include altered epigenetic regulation of DNA methylation and covalent modifications of histones. Such non-genomic tuning of phenotype through developmental plasticity has adaptive value because it attempts to match an individual’s responses to the environment predicted to be experienced, hence such processes have been selected during evolution as conferring fitness advantage. When the responses are mismatched, disease risk increases. Examples of such mismatch are those arising either from inaccurate nutritional cues from the mother or placenta before birth, or from rapid environmental change through improved socio-economic conditions. It is now thought that these contribute substantially to the increasing prevalence of type-2 diabetes, obesity, and cardiovascular disease.
Acknowledgments
MAH is supported by the British Heart Foundation; KMG is supported by the National Institute for Health Research through the Southampton Nutrition, Diet & Lifestyle Biomedical Research Unit; HMI is supported by the UK Medical Research Council.
References
- 1.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Gluckman PD, Hanson MA. Developmental Origins of Metabolic Disease: Life course and intergenerational perspectives. Trends Endocrin Metabolism. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Dörner G. Die mögliche Bedeutung der prä- und/oder perinatalen Ernährung für die Pathogenese der Obesitas. Acta Biologica Medica Germanica. 1973;30:19–22. [PubMed] [Google Scholar]
- 4.Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974-75. J Epidemiol Comm Health. 1978;32:34–7. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 6.Osmond C, Barker DJP, Winter PD, Fall CHD, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–24. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey KM, Barker DJP. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(suppl.):1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 8.Pike K, Hanson MA, Godfrey KM. Developmental mismatch – consequences for later cardio-respiratory health. BJOG. 2008;115:149–157. doi: 10.1111/j.1471-0528.2007.01603.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Javaid MK, Taylor P, Walker-Bone K, Dennison E, Arden N. The fetal origins of osteoporotic fracture. Calcif Tissue Int. 2002;70:391–394. doi: 10.1007/s00223-001-0044-z. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM, Martyn CN. Maternal diet during pregnancy and carotid intima-media thickness in children. Arterioscler Thromb Vasc Biol. 2006;26:1877–82. doi: 10.1161/01.ATV.0000228819.13039.b8. [DOI] [PubMed] [Google Scholar]
- 12.Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutrition. 2010;91:1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnsen SL, Wilsgaard T, Rasmussen S, Hanson MA, Godfrey KM, Kiserud T. Fetal size in the second trimester is associated with the duration of pregnancy, small fetuses having longer pregnancies. BMC Pregnancy and Childbirth. 2008;8:25. doi: 10.1186/1471-2393-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson MA, Godfrey KM. Maternal constraint is a pre-eminent regulator of fetal growth. Int J Epidemiol. 2008;37:252–4. doi: 10.1093/ije/dyn015. [DOI] [PubMed] [Google Scholar]
- 15.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–5. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 16.Pener MP, Yerushalmi Y. The physiology of locust phase polymorphism: an update. J Insect Physiol. 1998;44:365–77. doi: 10.1016/s0022-1910(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee TM, Zucker I. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- 18.Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183:1654–5. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 19.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 20.Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc Roy Soc London. Series B, Containing papers of a Biological character. Royal Society (Great Britain) 2005;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Kuzawa CW. Developmental perspectives on the origin of obesity. In: Fantuzzi G, Mazzone T, editors. Adipose tissue and adipokines in health and disease. Humana Press; Tokowa, NJ: 2007. pp. 207–219. [Google Scholar]
- 23.Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends Endocrinol Metab. 2006;17:7–12. doi: 10.1016/j.tem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. 2009;4:e6744. doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: influences of prenatal and postnatal growth. J Clin Endocrinol Metabolism. 2007;92:46–50. doi: 10.1210/jc.2006-1378. [DOI] [PubMed] [Google Scholar]
- 27.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Quarterly Review Biology. 2009;84:131–76. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 28.West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; Oxford: 2003. [Google Scholar]
- 29.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease; a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 30.Gluckman PD, Hanson MA. The fetal matrix: evolution, development, and disease. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- 31.Hollingsworth JW, Maruoka S, Boon K, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin. Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Hoppe CC, Evans RG, Moritz KM, et al. Combined prenatal and postnatal protein restriction influences adult kidney structure, function and arterial pressure. Am J Physiol. 2007;292:R462–R469. doi: 10.1152/ajpregu.00079.2006. [DOI] [PubMed] [Google Scholar]
- 33.Sellayah D, Sek K, Anthony FW, et al. Appetite regulatory mechanisms and food intake in mice are sensitive to mismatch in diets between pregnancy and postnatal periods. Brain Research. 2008;1237:146–152. doi: 10.1016/j.brainres.2008.07.126. [DOI] [PubMed] [Google Scholar]
- 34.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 35.Cleal JK, Poore KR, Boullin JP, et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation. 2004;110:1097–1102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman PD, Hanson MA. Mismatch; how our world no longer fits our bodies. Oxford University Press; Oxford: 2006. [Google Scholar]
- 38.Leon DA, Lithell HO, Vâgerö D, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915-29. BMJ. 1998;317:241–5. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKeigue PM, Lithell HO, Leon DA. Glucose tolerance and resistance to insulin-stimulated glucose uptake in men aged 70 years in relation to size at birth. Diabetologia. 1998;41:1133–8. doi: 10.1007/s001250051042. [DOI] [PubMed] [Google Scholar]
- 40.Torrens C, Snelling TH, Chau R, et al. Effects of pre- and periconceptional undernutrition on arterial function in adult female sheep are vascular bed dependent. Exp Physiol. 2009;94:1024–33. doi: 10.1113/expphysiol.2009.047340. [DOI] [PubMed] [Google Scholar]
- 41.Gardner DS, Van Bon BW, Dandrea J, et al. Effect of periconceptional undernutrition and gender on hypothalamic-pituitary-adrenal axis function in young adult sheep. J Endocrinol. 2006;190:203–12. doi: 10.1677/joe.1.06751. [DOI] [PubMed] [Google Scholar]
- 42.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 43.Watkins AJ, Ursell E, Panton R, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008;78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- 44.Stettler N, Stallings VA, Troxel AB, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–21. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- 46.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56-70 y. Am J Clin Nutr. 2008;87:1769, 75. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 47.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke. 2007;38:264–70. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- 48.Kajantie E, Barker DJ, Osmond C, Forsen T, Eriksson JG. Growth before 2 years of age and serum lipids 60 years later: the Helsinki Birth Cohort study. Int J Epidemiol. 2008;37:280–9. doi: 10.1093/ije/dyn012. [DOI] [PubMed] [Google Scholar]
- 49.Godfrey KM. The “Developmental Origins” hypothesis: epidemiology. In: Hanson MA, Gluckman PD, editors. Developmental Origins of Health and Disease – a Biomedical Perspective. Cambridge University Press; 2006. pp. 6–32. [Google Scholar]
- 50.Hawkins P, Hanson MA, Matthews SG. Maternal undernutrition in early gestation alters molecular regulation of the hypothalamic-pituitary-adrenal axis in the ovine fetus. J Neuroendocrinol. 2001;13:855–61. doi: 10.1046/j.1365-2826.2001.00709.x. [DOI] [PubMed] [Google Scholar]
- 51.Gluckman PD, Lillycrop KA, Vickers MH, et al. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA. 2007;104:12796–800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 53.Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–46. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brawley L, Torrens C, Anthony FW, et al. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554(Pt 2):497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 56.Burdge GC, Phillips ES, Dunn RL, Jackson AA, Lillycrop KA. Effect of reduced maternal protein consumption during pregnancy in the rat on plasma lipid concentrations and expression of peroxisomal proliferator-activated receptors in the liver and adipose tissue of the offspring. Nutr Res. 2004;24:639–646. [Google Scholar]
- 57.Watkins AJ, Platt D, Papenbrock T, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA. 2007;104:5449–54. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gopalakrishnan GS, Gardner DS, Rhind SM, et al. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R12–20. doi: 10.1152/ajpregu.00687.2003. [DOI] [PubMed] [Google Scholar]
- 59.Edwards LJ, McMillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R669–79. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- 60.Wisloff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 61.Taylor PD, McConnell J, Khan IY, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 62.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 63.Jensen A, Roman C, Rudolph AM. Effects of reducing uterine blood flow on fetal blood flow distribution and oxygen delivery. J Dev Physiol. 1991;15:309–23. [PubMed] [Google Scholar]
- 64.Jones A, Beda A, Osmond C, Godfrey KM, Simpson DM, Phillips DIW. Prenatal growth is associated with sex-specific differences in cardiovascular physiology in children. Eur Heart J. 2008;29:2164–2170. doi: 10.1093/eurheartj/ehn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnstone JF, Zelsman M, Crozier S, et al. The relationship between placental 11ßHSD-2 and maternal body composition, age and dieting status. J Soc Gynecol Invest. 2005;12:453. [Google Scholar]
- 66.Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Physiol. 2001;28:938–41. doi: 10.1046/j.1440-1681.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 67.Phillips DI, Walker BR, Reynolds RM, et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–6. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- 68.Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DIW. Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab. 2006;91:1868–1871. doi: 10.1210/jc.2005-2077. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds RM, Godfrey KM, Barker M, Osmond C, Phillips DI. Stress responsiveness in adult life: influence of mother’s diet in late pregnancy. J Clin Endocrinol Metab. 2007;92:2208–10. doi: 10.1210/jc.2007-0071. [DOI] [PubMed] [Google Scholar]
- 70.Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–8. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 71.Phillips DI, Barker DJ. Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med. 1997;14:673–7. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 72.McMillen IC, MacLaughlin SM, Muhlhausler BS, Gentili S, Duffield JL, Morrison JL. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxicol. 2008;102:82–9. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]