Abstract
Background
Little is known about whether patterns of growth are associated with altered respiratory and immune development. This study relates prenatal and infant growth patterns to wheeze and atopy at age 3 years
Methods
Birth weight and length were measured in 1548 children born at term. Conditional fetal head and abdominal circumference growth velocities were calculated from antenatal ultrasound measurements. Conditional postnatal growth velocities were calculated from infant weight, length and adiposity data. .Measures of size and conditional growth were related to parentally-reported infant and early childhood wheeze and to atopic status at age 3.
Results
Atopy risk increased by 46% per standard deviation (SD) increase in abdominal circumference growth velocity from 11-19 weeks’ gestation but by 20% per SD decrease in abdominal growth velocity from 19-34 weeks (p=0.007 and p=0.011). Atopic wheeze risk increased by 20% per SD decrease in 19-34 week abdominal growth (p=0.046). Non-atopic wheeze risk increased by 10% per SD decrease in 11-19 week head circumference growth. Greater relative infant weight and adiposity gains were associated with both atopic and non-atopic wheeze.
Conclusions
Rapid growth during 11-19 weeks’ gestation followed by growth faltering is associated with atopy, suggesting that influences affecting fetal growth may also alter immune development. A lower early fetal growth trajectory is associated with non-atopic wheeze, possibly reflecting an association with smaller airways. An association between postnatal adiposity gain and wheeze may partly reflect prenatal influences that cause fetal growth to falter but are then followed by postnatal adiposity gain.
Keywords: asthma, preschool-wheeze, allergic sensitisation, growth, nutrition
INTRODUCTION
Children and adults who were small at birth tend to have reduced lung function and an increased risk of respiratory mortality and morbidity.[1-3 ] Smaller birth size is associated with reduced lung function from early infancy,[1-7] and genetic and environmental influences on early lung development appear to have lasting effects on later respiratory health.[8] It has been proposed that an adverse intrauterine environment might induce fetal adaptations which restrict somatic growth and also have adverse functional consequences for the developing immune system and lungs.[3]
Studies examining the association between birth anthropometry and later asthma have, however, had inconsistent findings [2] and children who had experienced intrauterine growth retardation (IUGR) had decreased lung function but no difference in wheeze compared with children who were appropriate weight for gestational age at birth.[1] The inconsistent findings may partly reflect methodological differences including adequacy of correction for gestation and other confounding factors. In twin studies birth weight exerts a greater influence upon later asthma in monozygotic than in dizygotic twin pairs, suggesting fetal growth and childhood asthma may be associated independently of shared genetic factors.[9;10]
In healthy term infants within the normal birth weight range, we previously found that smaller birth size and rapid postnatal weight gain were associated with reduced lung function at age 5-14 weeks.[4] Subsequent studies showed an inverse relationship between infant lung function and early postnatal weight gain in premature infants,[5] and that lung function at 1 and 12 months is inversely related to infant weight gain.[11] Rapid postnatal weight gain can result from prenatal growth restriction, and we hypothesised that faltering growth in late gestation might be associated with later respiratory ill-health.[4]
Studies to date have generally used birth anthropometry as a proxy for fetal growth and no previous study in an unselected population has utilised detailed characterisation of pre- and postnatal growth patterns from longitudinal anthropometric data collected before and after birth. Here we measured fetal size longitudinally and derived conditional head and abdominal circumference growth velocities to assess both the early trajectory of fetal growth and faltering of abdominal growth in late gestation; the latter is recognised as an important fetal adaptation to an adverse intrauterine environment which acts to protect brain growth.[12,13] We also derived conditional velocities of postnatal weight and adiposity gain, because faltering of prenatal growth may lead to an increased rate of infant weight gain. We then examined the relationships between antenatal and postnatal growth parameters and early childhood wheeze and atopy.
METHODS
Study Population
We studied offspring of Southampton Women’s Survey participants.[14] Between 1998-2002, 12,583 women aged 20-34 years were recruited; those who became pregnant were followed through pregnancy and their children visited at 6, 12, 24 and 36 months. We excluded infants born at <37 weeks’ gestation to avoid confounding effects of abnormal lung development associated with prematurity. By December 2003, 1868 term infants were born; 1548 (83%) were followed up at age 3 years, with 98% seen at all four postnatal visits.
Growth Variables
Gestational age was determined using an algorithm combining the mother’s last menstrual period and early ultrasound data. Using Acuson 128 XP, Aspen & Sequoia ultrasound machines calibrated to 1540 m/s experienced research ultrasonographers used standardised anatomical landmarks to measure fetal head and abdominal circumferences at 11, 19 and 34 weeks’ gestation. Research nurses measured weight and crown-heel length at birth, and weight, length and subscapular skinfold thickness at 6 and 12 months.
Respiratory Symptoms
At 6, 12, 24 and 36 months mothers were asked whether their child had ‘experienced any episodes of chestiness associated with wheezing or whistling in his/her chest since they were last seen’. A positive response on any postnatal visit was considered evidence of early childhood wheeze.
Atopic Status
Atopy at age 3 years was defined as skin prick test reactivity to any allergen (cata, doga, house dust mitea , milka, grass pollensa, and eggb (Hollister-Stier, Spokane, WAa; Alyostal, Antony, Franceb) ≥3mm in diameter in the presence of appropriate positive and negative controls. Maternal atopic status was assessed at the 12-month interview.
Statistical Analysis
The method of Royston was used to calculate conditional measures of fetal size and infant length and weight, correcting for the exact age at measurement and regression to the mean.[15] Velocities of prenatal and infant growth were calculated from change in size adjusted for gestation or age, as appropriate. For subscapular skinfold thickness, the method of Royston proved unsuitable as adiposity does not increase monotonically with age; subscapular skinfold growth velocity conditional upon initial size was calculated using regression. Anthropometric and growth velocity variables were logarithmically transformed to achieve a normal distribution, then standardised to z-scores. Outcomes in these cases were expressed in units of change in outcome per SD change in predictor. All outcome variables were binary but common; therefore Poisson regression with robust variance was used to derive relative risks. Logistic regression was not used as odds ratios relating to common outcomes are hard to interpret.[16]
Relative risks were determined for two primary outcomes: ‘early childhood wheeze’ and ‘atopy’, comparing children with and without each condition. Children who were reported to wheeze were divided according to atopic status to form two secondary outcomes ‘atopic wheeze’ and ‘non-atopic wheeze’; children in these groups were compared with non-atopic children who had never wheezed. Online Table 1 shows potential confounders examined. Regression models were built including confounders significantly associated with each outcome in a mutually-adjusted model (p<0.05) and key factors thought essential to adjust for because of potential biological significance (maternal education, maternal atopy and child’s birth order for atopy at 3 years and atopic wheeze; maternal education, smoking in pregnancy, maternal asthma, paternal asthma and child’s birth order for early childhood wheeze and non-atopic wheeze). Adjusted and unadjusted relative risks are presented for birth anthropometry and pre and postnatal growth velocities; relative risks for static measures of fetal size are presented in the online supplement. Analyses were performed using Stata™ 8.2 (StataCorp, Texas).
RESULTS
Table 1 shows the wide variation in the characteristics of the children seen at 3 years. Cohort members not seen had lower mean birth weight, and their mothers were younger, more likely to smoke, less likely to have tried breastfeeding and had lower educational attainment.
Table 1.
Characteristics of children who were and were not seen at age 3 years
| Parental characteristics | Children seen at 3 years (n=1548) |
Children not seen at 3 years (n=320) |
P-value | ||
|---|---|---|---|---|---|
| Mother’s age at child’s birth (years), mean (SD) | 30.2 | (3.8) | 29.4 | (3.8) | 0.001 |
| Mother’s educational A Level or above, n (%) | 900 | (57%) | 150 | (47%) | <0.001 |
| Maternal smoking during pregnancy, n (%) | 245 | (16%) | 80 | (27%) | <0.001 |
| Maternal asthma, n (%) | 344 | (22%) | 74 | (24%) | 0.599 |
| Maternal eczema in childhood, n (%) | 275 | (18%) | 50 | (16%) | 0.432 |
| Maternal rhinitis, n (%) | 639 | (42%) | 118 | (38%) | 0.225 |
| Paternal asthma, n (%) | 264 | (17%) | 60 | (20%) | 0.567 |
| Paternal eczema in childhood, n (%) | 156 | (11%) | 31 | (10%) | 0.897 |
| Paternal rhinitis, n (%) | 506 | (34%) | 102 | (34%) | 0.883 |
| Birth characteristics | |||||
| Birth weight (g), mean (SD) | 3525 | (475) | 3456 | (467) | 0.020 |
| Gestational age (weeks), mean (SD) | 40.1 | (1.2) | 40.1 | (1.2) | 0.909 |
| Primiparous, n (%) | 704 | (46%) | 114 | (36%) | 0.001 |
| Attempted breastfeeding, n (%)* | 1269 | (83%) | 162 | (70%) | 0.000 |
| Characteristics at 6 month follow-up * | |||||
| Maternal smoking, n (%) | 284 | (18%) | 73 | (30%) | <0.001 |
| Other smokers in the home, n (%) | 461 | (31%) | 86 | (35%) | 0.145 |
| Ever wheezed, n (%) | 402 | (26%) | 83 | (34%) | 0.013 |
| Cat or dog in home, n (%) | 699 | (45%) | 105 | (43%) | 0.384 |
| Characteristics at 1 year follow-up * | |||||
| Wheezed in past 6 months, n (%) | 464 | (30%) | 80 | (41%) | 0.002 |
| Cat or dog in home, n (%) | 675 | (44%) | 82 | (42%) | 0.575 |
| Characteristics at 2 year follow-up * | |||||
| Wheezed in past year, n (%) | 414 | (27%) | 28 | (27%) | 0.954 |
Of the 320 children not seen at 3 years, 247 were seen at 6 months, 196 at 1 year and 104 at 2 years
Wheeze status to age 3 years was known for 1522 children (98%); 890 children (58%) had ever experienced wheeze. Atopic status was known for 1342 mothers (87%) and 1184 children (76%); 199 children (17%) were atopic. Both wheeze and atopy data were available for 1164 children; of these 127 (11%) had wheezed and were atopic, 555 (48%) had wheezed but were not atopic, 67 (6%) had never wheezed but were atopic and 415 (36%) had never wheezed and were not atopic (Figure 1).
Figure 1.
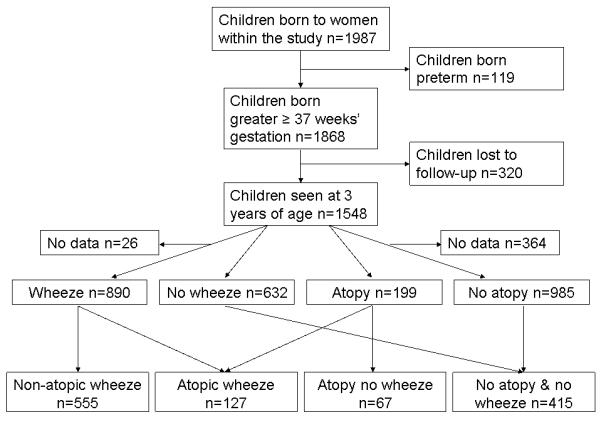
Study outline and numbers included in outcome groups
Early childhood wheeze
Early childhood wheeze was not significantly associated with either fetal measurements (Online Table 2) or birth weight or length (Table 2), but was associated with greater weight and adiposity gains both between birth and 6 months (5% per SD increase, p= 0.02 for both) and from 6-12 months (6% per SD increase in weight, p=0.04 and 7% per SD increase in adiposity gain, p=0.001). There was no association between early childhood wheeze and postnatal length gain.
Table 2.
Relative risks (RR) for the associations between pre- and postnatal growth and whether the child had ever wheezed by age 3 years (unadjusted and adjusted)
| Unadjusted analyses | Adjusted* analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | n | RR | (95% CI) | P-value | n | |
| Birth size variables | ||||||||
| Crown-heel length | 0.96 | 0.92-1.00 | 0.061 | 1479 | 0.96 | 0.92-1.01 | 0.088 | 1479 |
| Weight | 0.97 | 0.93-1.01 | 0.197 | 1506 | 0.97 | 0.93-1.02 | 0.206 | 1506 |
| Conditional fetal growth | ||||||||
| 11-19 weeks | ||||||||
| Head circumference | 0.98 | 0.91-1.06 | 0.574 | 597 | 0.94 | 0.87-1.02 | 0.155 | 597 |
| Abdominal circumference | 0.98 | 0.89-1.07 | 0.611 | 562 | 0.95 | 0.87-1.04 | 0.285 | 562 |
| 19-34 weeks | ||||||||
| Head circumference | 0.98 | 0.93-1.04 | 0.598 | 877 | 0.98 | 0.93-1.04 | 0.572 | 877 |
| Abdominal circumference | 1.04 | 0.99-1.10 | 0.129 | 911 | 1.04 | 0.99-1.10 | 0.092 | 911 |
| Conditional Infant growth | ||||||||
| 0 – 6 months | ||||||||
| Length | 1.01 | 0.97-1.05 | 0.574 | 1450 | 0.98 | 0.94-1.03 | 0.409 | 1450 |
| Weight | 1.08 | 1.03-1.12 | 0.000 | 1485 | 1.05 | 1.01-1.09 | 0.020 | 1485 |
| Subscapular skinfolds | 1.06 | 1.02-1.10 | 0.003 | 1480 | 1.05 | 1.01-1.10 | 0.017 | 1480 |
| 6 – 12 months | ||||||||
| Length | 0.97 | 0.93-1.02 | 0.307 | 1346 | 0.98 | 0.93-1.04 | 0.579 | 1346 |
| Weight | 1.04 | 0.98-1.09 | 0.190 | 1372 | 1.06 | 1.00-1.12 | 0.041 | 1372 |
| Subscapular skinfolds | 1.06 | 1.01-1.10 | 0.010 | 1368 | 1.07 | 1.03-1.12 | 0.001 | 1368 |
N = 1522
Adjusted for gender, smoking during pregnancy, age last breastfed, maternal asthma, maternal rhinitis, paternal asthma, maternal education and birth order.
Atopy at age 3 years
The relative risk of atopy at 3 years increased by 46% per SD increase in abdominal circumference growth velocity from 11-19 weeks’ gestation (p=0.007) (Table 3), and was higher in children who had a larger abdominal circumference at 19 weeks’ gestation (RR=1.24 per SD increase in abdominal circumference, p=0.02) (Online Table 3). In contrast, each SD increase in abdominal growth velocity from 19-34 weeks’ gestation decreased atopy risk by 20% (p=0.01) (Table 3). Atopy risk was associated with greater crown-heel length at birth (RR=1.17 per SD, p=0.03) but not with prenatal head circumference growth, birth weight or measures of postnatal growth velocity. Results were similar when sensitisation to food allergens was excluded from the definition of atopy.
Table 3.
Adjusted relative risks (RR) for the associations between fetal and infant growth and atopy at age 3 years
| Unadjusted analyses | Adjusted* analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | n | RR | (95% CI) | P-value | n | |
| Birth size variables | ||||||||
| Crown-heel length | 1.20 | 1.06-1.36 | 0.005 | 1149 | 1.17 | 1.01-1.34 | 0.032 | 1004 |
| Weight | 1.10 | 0.97-1.25 | 0.137 | 1171 | 1.08 | 0.95-1.24 | 0.241 | 1023 |
| Conditional fetal growth | ||||||||
| 11-19 weeks | ||||||||
| Head circumference | 0.93 | 0.72-1.18 | 0.534 | 464 | 0.80 | 0.60-1.06 | 0.123 | 405 |
| Abdominal circumference | 1.44 | 1.20-1.86 | 0.005 | 431 | 1.46 | 1.11-1.93 | 0.007 | 378 |
| 19-34 weeks | ||||||||
| Head circumference | 0.96 | 0.81-1.13 | 0.608 | 682 | 0.98 | 0.82-1.17 | 0.817 | 601 |
| Abdominal circumference | 0.80 | 0.69-0.94 | 0.006 | 707 | 0.80 | 0.68-0.95 | 0.011 | 625 |
| Conditional Infant growth | ||||||||
| 0 – 6 months | ||||||||
| Length | 0.93 | 0.83-1.05 | 0.244 | 1131 | 0.89 | 0.78-1.02 | 0.104 | 991 |
| Weight | 1.09 | 0.97-1.22 | 0.131 | 1157 | 1.06 | 0.92-1.21 | 0.416 | 1013 |
| Subscapular skinfolds | 1.09 | 0.97-1.23 | 0.157 | 1153 | 1.09 | 0.96-1.24 | 0.185 | 1011 |
| 6 – 12 months | ||||||||
| Length | 1.08 | 0.93-1.26 | 0.326 | 1069 | 1.02 | 0.86-1.21 | 0.843 | 941 |
| Weight | 1.00 | 0.83-1.19 | 0.961 | 1086 | 1.03 | 0.84-1.26 | 0.778 | 956 |
| Subscapular skinfolds | 1.05 | 0.92-1.20 | 0.485 | 1078 | 1.11 | 0.97-1.27 | 0.142 | 950 |
N = 1184;
Adjusted for gender, maternal eczema, maternal atopy, maternal education and birth order
Atopic wheeze
The pattern of risk for atopic wheeze was similar to that for atopy (Tables 3 and 4). Relative risk increased with higher 11-19 week abdominal growth velocity (32% per SD, p=0.1), larger 19 week fetal abdominal circumference (34% increase per SD, p=0.02) (Online Table 4), and lower 19-34 week abdominal growth velocity (20% per SD, p=0.046). Atopic wheeze risk was not associated with prenatal head circumference growth, weight or crown heel length at birth (p=0.3) (Online Table 4), but was associated with greater weight and adiposity gain in infancy (Table 4). SD increases in subscapular skinfold gain and weight gain between birth and 6 months were associated with 27% and 22% increases in atopic wheeze risk (p=0.002 and p=0.02, respectively); each SD increase in subscapular skinfold gain between 6 and 12 months was associated with a 20% increase in atopic wheeze risk (p=0.02). In contrast, postnatal length gain was not associated with atopic wheeze risk.
Table 4.
Relative risks (RR) for the associations between pre- and postnatal growth and whether the child had ever wheezed by age 3 years and was atopic, compared with children who had never wheezed and were not atopic (unadjusted and adjusted)
| Unadjusted analyses | Adjusted* analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Birth size variables | ||||||||
| Crown-heel length | 1.09 | 0.94-1.26 | 0.247 | 527 | 1.08 | 0.93-1.26 | 0.313 | 456 |
| Weight | 1.06 | 0.91-1.23 | 0.461 | 537 | 1.02 | 0.87-1.19 | 0.810 | 465 |
| Conditional fetal growth | ||||||||
| 11-19 weeks | ||||||||
| Head circumference | 0.96 | 0.69-1.33 | 0.818 | 207 | 0.79 | 0.54-1.15 | 0.216 | 183 |
| Abdominal circumference | 1.42 | 1.05-1.92 | 0.024 | 197 | 1.32 | 0.94-1.85 | 0.114 | 172 |
| 19-34 weeks | ||||||||
| Head circumference | 0.98 | 0.80-1.21 | 0.866 | 308 | 0.88 | 0.69-1.12 | 0.294 | 276 |
| Abdominal circumference | 0.82 | 0.66-1.01 | 0.066 | 321 | 0.80 | 0.65-1.00 | 0.046 | 288 |
| Conditional Infant growth | ||||||||
| 0 – 6 months | ||||||||
| Length | 1.00 | 0.86-1.16 | 0.987 | 518 | 0.96 | 0.82-1.12 | 0.602 | 499 |
| Weight | 1.25 | 1.09-1.43 | 0.001 | 530 | 1.22 | 1.03-1.43 | 0.020 | 460 |
| Subscapular skinfolds | 1.22 | 1.07-1.40 | 0.004 | 533 | 1.27 | 1.09-1.49 | 0.002 | 463 |
| 6 – 12 months | ||||||||
| Length | 1.02 | 0.85-1.22 | 0.829 | 497 | 0.98 | 0.80-1.20 | 0.842 | 432 |
| Weight | 1.14 | 0.92-1.41 | 0.234 | 504 | 1.19 | 0.94-1.31 | 0.147 | 438 |
| Subscapular skinfolds | 1.14 | 0.99-1.32 | 0.073 | 502 | 1.20 | 1.03-1.39 | 0.018 | 436 |
N = 542;
Adjusted for gender, smoking during pregnancy, maternal asthma and maternal rhinitis, maternal atopy, paternal asthma, maternal education and birth order.
Non-atopic wheeze
The associations between fetal growth and risk of non-atopic wheeze differed from those for atopic wheeze. Non-atopic wheeze was not associated with higher 11-19 week abdominal growth velocity followed by abdominal growth faltering, but instead increases in risk were seen for lower head circumference growth velocity between 11 and 19 weeks (RR=0.90 per SD increase, p=0.04) (Table 5) and smaller 34-week head circumference (RR=0.91 per SD increase, p=0.02) (Online Table 5) .Similar to the associations for atopic wheeze, non-atopic wheeze risk increased by 6% per SD increase in adiposity gain from birth to 6 months (p=0.02) and by 8% per SD increase in weight gain between 6 and 12 months (p=0.04) (Tables 5). Similarly to atopic wheeze, postnatal length gain was not associated with non-atopic wheeze risk.
Table 5.
Relative risks (RR) for the associations between pre- and postnatal growth and whether the child had ever wheezed by age 3 years but was not atopic, compared with children who had never wheezed and were not atopic (unadjusted and adjusted)
| Unadjusted analyses | Adjusted* analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | n | RR | (95% CI) | P-value | n | |
| Birth size variables | ||||||||
| Crown-heel length | 0.97 | 0.92-1.03 | 0.282 | 944 | 0.97 | 0.92-1.03 | 0.317 | 926 |
| Weight | 0.98 | 0.93-1.04 | 0.486 | 960 | 0.97 | 0.92-1.03 | 0.356 | 942 |
| Conditional fetal growth | ||||||||
| 11-19 weeks | ||||||||
| Head circumference | 0.92 | 0.83-1.01 | 0.090 | 383 | 0.90 | 0.81-1.00 | 0.041 | 373 |
| Abdominal circumference | 0.98 | 0.88-1.09 | 0.744 | 361 | 0.97 | 0.87-1.09 | 0.637 | 352 |
| 19-34 weeks | ||||||||
| Head circumference | 0.93 | 0.87-1.00 | 0.058 | 564 | 0.94 | 0.88-1.01 | 0.096 | 552 |
| Abdominal circumference | 1.06 | 0.99-1.13 | 0.117 | 584 | 1.05 | 0.98-1.13 | 0.136 | 572 |
| Conditional Infant growth | ||||||||
| 0 – 6 months | ||||||||
| Length | 1.01 | 0.96-1.06 | 0.825 | 933 | 0.99 | 0.94-1.05 | 0.766 | 915 |
| Weight | 1.05 | 1.00-1.10 | 0.044 | 952 | 1.04 | 0.99-1.10 | 0.093 | 934 |
| Subscapular skinfolds | 1.07 | 1.02-1.12 | 0.009 | 950 | 1.06 | 1.00-1.11 | 0.024 | 932 |
| 6 – 12 months | ||||||||
| Length | 0.97 | 0.91-1.03 | 0.320 | 878 | 0.98 | 0.92-1.05 | 0.636 | 862 |
| Weight | 1.04 | 0.97-1.11 | 0.289 | 891 | 1.08 | 1.00-1.15 | 0.036 | 876 |
| Subscapular skinfolds | 1.00 | 0.95-1.06 | 0.929 | 887 | 1.02 | 0.96-1.09 | 0.465 | 871 |
N = 970
Adjusted for gender, maternal age, smoking during pregnancy, maternal asthma, maternal rhinitis, paternal asthma, maternal education and birth order.
Simultaneous analyses
The conditional measures of growth velocity were calculated to ensure that they were independent of initial size. Perhaps as a result, the relative risk ratios for atopy, atopic wheeze and non-atopic wheeze associated with measures of conditional prenatal abdominal growth and postnatal adiposity gain changed little after simultaneous inclusion of these growth measures in a multivariate analysis.
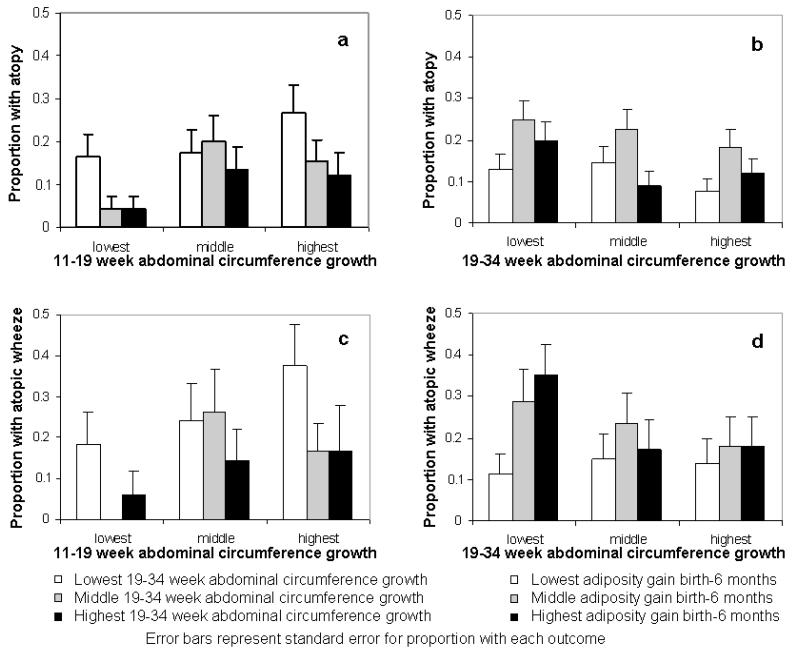
Formal testing showed no significant linear interactions between conditional 11-19 and 19-34 week abdominal growth velocities, or between 19-34 week abdominal growth and birth to 6 months adiposity gain in their predictions of outcomes. Subjects were grouped into thirds of growth velocity over the different time periods. The prevalences of childhood atopy and atopic wheeze were 27% and 38%, respectively, in those in the top third of abdominal growth velocity from 11-19 weeks gestation and the bottom third of growth velocity in late pregnancy, indicating growth faltering in late pregnancy; comparable prevalences in those in the bottom third of abdominal growth velocity from 11-19 weeks gestation and the top third of growth velocity in late pregnancy were 4% and 6% for childhood atopy and atopic wheeze respectively (Figure 2). The association of postnatal adiposity gain with atopic wheeze was strongest in those with below average late pregnancy abdominal growth (Online Table 6); the prevalence was 35% in those in the bottom third of late pregnancy abdominal growth velocity and the top third of adiposity gain from birth to 6 months, as compared with 14% in those in the top third of late pregnancy abdominal growth velocity and the bottom third of early infancy adiposity gain. For non-atopic wheeze the prevalence was 68% in those in the bottom third of 19-34 week head circumference growth velocity and the top third of adiposity gain from birth to 6 months, compared with 41% in those in the top third of 19-34 week head circumference growth velocity and the bottom third of early infancy adiposity gain (Figure 3), with a similar pattern for adiposity gain from age 6-12 months (Figure 3).
Figure 2.
Atopy and atopic wheeze prevalence at age 3 years according to groupings of 11-19 week and 19-34 week abdominal circumference growth velocity, and according to 19-34 week abdominal circumference growth and adiposity gain between birth and 6 months.
Figure 3.
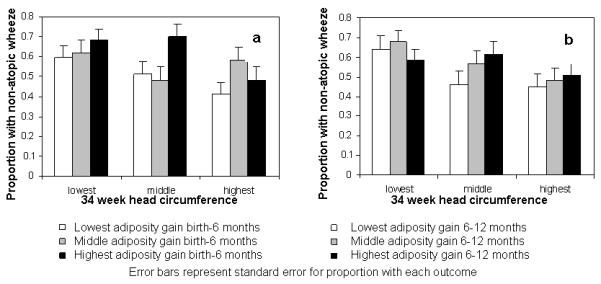
Non-atopic wheeze prevalence according to groupings of 19-34 week head circumference growth velocity and adiposity gain from birth - 6 months and 6 - 12 months.
Infant feeding
As in other studies, formula-fed SWS infants had greater infant weight and adiposity gains than those breast-fed.[17] Stratified analyses showed, however, that the associations of infant weight and adiposity gains with early childhood wheeze, atopic and non-atopic wheeze, were similar in breast and formula-fed infants.
DISCUSSION
Using conditional pre and postnatal growth velocities calculated from serial measurements of fetal and infant anthropometry we examined the influences of pre- and postnatal growth on wheeze and atopy at age 3 years. Rapid 11-19 week fetal abdominal growth followed by faltering of abdominal circumference growth was associated with later atopy, and late gestation abdominal growth faltering associated with atopic wheeze. A lower early trajectory of prenatal head circumference growth was associated with non-atopic wheeze. Postnatal adiposity gain, but not linear growth, was associated with both atopic and non-atopic wheeze. The associations were independent of birth order, gestation, maternal atopy and smoking. These findings support our previous hypothesis that trajectories of growth before and after birth are associated with childhood respiratory health.[4] Although wheezing and atopic disorders have overlapping and mixed phenotypes, our analyses suggest that factors which promote adaptive change in the relative growth of body tissues during fetal life and infancy can have later functional respiratory and immune consequences.
Prenatal growth and wheeze and atopic outcomes
In our analyses, faster abdominal growth velocity from 11-19 weeks gestation followed by growth faltering in late pregnancy was associated with later atopy. This suggests that the development of atopy is influenced by factors causing growth restriction in late gestation. Under conditions of intrauterine stress or restricted placental nutrient transfer in late gestation, brain growth is generally preserved at the expense of reduced accretion of abdominal soft tissue and fat.[18] This results in asymmetrical growth restriction, which is particularly marked in fetuses that initially follow a rapid growth trajectory.[12] Atopy is believed to result from a predominant Th2 lymphocyte response to common antigens.[19] Animal studies demonstrate that poor fetal growth results in impaired thymic development[20] and a Th1/Th2 imbalance.[21] Correlations between seasonal patterns of food availability and infant thymus size,[22] cord blood lymphocyte count[23] and infectious deaths in young adulthood[24] provide evidence for prenatal influences on human immune function.
Whereas atopic wheeze was also associated with faltering of abdominal circumference, non-atopic wheeze was associated with slower head circumference growth from both 11-19 and 19-34 weeks’ gestation; children that developed non-atopic wheeze were not however smaller at birth .This suggests slowed growth in early gestation may be mechanistically linked to later wheeze susceptibility. Early or extreme adversity might be associated with both slowed head growth and altered airway growth or alterations in respiratory mechanics that predispose to airway narrowing and wheeze during viral respiratory infections.
Experimental data support the central hypothesis that lung development and later function is sensitive to factors associated with fetal growth restriction, notably fetal hypoxaemia, reduced nutrient supply and hypercortisolaemia. For example, prenatal growth restriction in sheep is associated with a reduced lung weight to bodyweight ratio,[25] reduced alveoliarisation[26] and reduced airway luminal area and airway wall cartilage.[27] Growth restricted fetal lambs have altered lung structure[26] and decreased respiratory and increased chest wall compliance[28] which persist into postnatal life.
Postnatal growth and wheeze and atopic outcomes
The relative risk of atopy was not related to postnatal growth and adiposity gain. In contrast, independently of infant feeding, increased weight and adiposity gain during infancy were associated with an increased risk of wheezing before age 3 years. Similar associations were seen for both non-atopic and atopic wheeze, although the effect size was greatest for atopic wheeze. The associations with weight and adiposity gain contrasted with the absence of associations with length gain.
Above average postnatal weight gain may be associated with atopic wheeze risk because late pregnancy growth faltering (associated with atopy in our study) tends to be followed by “compensatory” increased postnatal weight gain unless the prenatal nutrient restriction has been severe or prolonged.[29] Alternatively, rather than serving as a marker of intrauterine growth restriction, above average postnatal weight gain may itself impair lung development. This is supported by the association between non-atopic wheeze and increased postnatal adiposity gain. Other studies have shown links between asthma and both high BMI [30] and rapid increases in weight [31]; whilst this may reflect common risk factors, increased adiposity in infancy may be mechanistically important in the development of asthma. Potential mechanisms include genetic polymorphisms,[32] sex-specific hormonal changes,[30] direct mechanical effects on lung function,[33] altered immune response[34] and increased susceptibility to gastric reflux.[35]
Strengths and limitations
The concept that birth anthropometry might predict future risk of wheezing and allergic disorders has been the subject of much investigation but little consensus. This is unsurprising as birth measurements only provide indirect evidence of the intrauterine environment. A strength of this study is the detailed assessment of prenatal growth afforded by serial ultrasound scans in a substantial sample of children broadly representative of the general population.
The principal limitations of this analysis arise from its observational nature and from the likely non-homogeneity of the outcome groups; ‘early childhood wheeze’, for example, is likely to include children who wheeze only with viral respiratory infections and a smaller subgroup who may later become persistent asthmatics. The strength of associations between risk factors and outcomes are likely to be lessened by such non-homogeneity. Our study was not equally powered in relation to all outcomes or predictors; for example, there were <150 children in the ‘atopic wheeze’ group and only 542 children contributed data to analyses of this outcome. Fewer children had complete data for conditional measures of prenatal growth than for birth anthropometry due the need for two measurements to calculate the former. Power was also reduced by missing data for some confounding influences, notably maternal atopic status.
In conclusion, we describe a pattern of rapid 11-19 week fetal abdominal growth velocity followed by growth faltering which is associated with later atopy, and demonstrate that late gestation growth faltering is associated with atopic wheeze. This suggests immune development is sensitive to programming by the prenatal environment. Moreover, we describe an association between postnatal adiposity gain and wheezing disorders; this may partly arise from impaired fetal growth which predisposes to ‘compensatory’ postnatal weight and adiposity gains, or may reflect mechanisms linked with adiposity per se. Slower prenatal growth was associated with non-atopic wheeze and we speculate this may reflect smaller airway size.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the help of the parents and infants who participated in this study. They are grateful to the staff of the Southampton Women’s Survey for the collection of the birth and infant anthropometric measurements and to Dr Pam Mahon, Miss Jane Anderson and Mrs Corinne Nisbet for collection of the antenatal ultrasound data.
FUNDING: Follow-up of children in the Southampton Women’s Survey has been funded by the Medical Research Council, University of Southampton, British Heart Foundation, and the Food Standards Agency (contract no N05071). Dr Katharine Pike was supported by a grant from the British Lung Foundation.
Footnotes
COMPETING INTERESTS: None
ETHICS APPROVAL: The Southampton and South West Hampshire Local Research Ethics Committee approved the protocol and written consent was obtained.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and it Licensees to permit this article (if accepted) to be published in [THORAX} editions and any other BMJPG Ltd products to exploit all subsidiary rights, as set out in our licence http://thorax.bmj.com/site/about/licence.pdf
REFERENCES
- 1.Kotecha S, Watkins W, Heron J, Henderson J, Dunstan F, Kotecha S. Spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. American Journal of Respiratory and Critical Care Medicine. 2010;181:969–974. doi: 10.1164/rccm.200906-0897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawlor D, Ebrahim S, Davey Smith G. Association of birth weight and adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;62:396–402. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Godfrey KM, Fall C, et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–5. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas JS, Inskip HM, Godfrey KM, et al. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Resp Crit Care Med. 2004;170:534–40. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich L, Stein RT, Pitrez PM, et al. Reduced lung function in healthy preterm infants in the first months of life. Am J Resp Crit Care Med. 2006;173:442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FD, Morgan WJ, Wright AL, et al. Initial airway function is a risk factor for recurrent wheezing respiratory illnesses during the first three years of life. Group Health Medical Associates. American Review of Respiratory Disease. 1991;143:312–316. doi: 10.1164/ajrccm/143.2.312. [DOI] [PubMed] [Google Scholar]
- 7.Dezateux C, Stocks J, Dundas I, et al. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Resp Crit Care Med. 1999;159:403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 8.Hancox R, Poulton R, Greene J, McLachlan C, Pearce M, Sears M. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009;64:226–232. doi: 10.1136/thx.2008.103978. [DOI] [PubMed] [Google Scholar]
- 9.Ortqvist A, Lundholm C, Carlstrom E, Lichtenstein P, Cnattingius S, Almqvist C. Familial factors do not confound the association between birth weight and childhood asthma. Pediatrics. 2009;124(4):e737–e747. doi: 10.1542/peds.2009-0305. [DOI] [PubMed] [Google Scholar]
- 10.Kindlund K, Thomsen S, Stensballe L. Birth weight and risk of asthma in 3-9 year old twins: exploring the fetal origins hypothesis. Thorax. 2010;65(2):146–9. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 11.Turner S, Zhang G, Young S, Cox M, Goldblatt J, Landau L, Le Souëf P. Associations between postnatal weight gain, change in postnatal pulmonary function, formula feeding and early asthma. Thorax. 2008;63:234–239. doi: 10.1136/thx.2006.064642. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S, Thoms A. Ultrasound measurement of the fetal head to abdomen circumference ratio in the assessment of growth retardation. Br J Obstet Gynaecol. 1977;84:165–174. doi: 10.1111/j.1471-0528.1977.tb12550.x. [DOI] [PubMed] [Google Scholar]
- 13.Baker PN, Johnson IR, Gowland PA, et al. Measurement of fetal liver, brain and placental volumes with echo-planar magnetic resonance imaging. Br J Obstet Gynaecol. 1995;102:35–39. doi: 10.1111/j.1471-0528.1995.tb09023.x. [DOI] [PubMed] [Google Scholar]
- 14.Inskip HM, Godfrey KM, Robinson, et al. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417–36. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- 16.Barros A, Hirakata V. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate theprevalence ratio. BMC Medical Research Methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baird J, Poole J, Robinson S, et al. Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatr Perinat Epidemiol. 2008;22:575–86. doi: 10.1111/j.1365-3016.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph AM. The fetal circulation and its response to stress. J Dev Physiol. 1984;6:11–19. [PubMed] [Google Scholar]
- 19.Prescott SL, Macaubas C, Smallacombe T, et al. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 20.Lang U, Baker RS, Khoury J, et al. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol - Regul Integr Comp Physiol. 2000;279:R53–R59. doi: 10.1152/ajpregu.2000.279.1.R53. [DOI] [PubMed] [Google Scholar]
- 21.Bass H, Adkins B, Strober S. Thymic irradiation inhibits the rapid recovery of TH1 but not TH2-like functions of CD4+ T cells after total lymphoid irradiation. Cell Immunol. 1991;137:316–328. doi: 10.1016/0008-8749(91)90082-m. [DOI] [PubMed] [Google Scholar]
- 22.Collinson AC, Moore SE, Cole TJ, et al. Birth season and environmental influences on patterns of thymic growth in rural Gambian infants. Acta Paediatr. 2003;92:1014–1020. [PubMed] [Google Scholar]
- 23.Collinson AC, Ngom PT, Moore SE, et al. Birth season and environmental influences on blood leucocyte and lymphocyte subpopulations in rural Gambian infants. BMC Immunol. 2008;9:18. doi: 10.1186/1471-2172-9-18. 2008.:18, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore SE, Cole TJ, Poskitt EME, et al. Season of birth predicts mortality in rural Gambia. Nature. 1997;388:434. doi: 10.1038/41245. [DOI] [PubMed] [Google Scholar]
- 25.Maloney JE, Bowes G, Brodecky V, et al. Function of the future respiratory system in the growth retarded fetal sheep. J Del Physiol. 1982;4:279–297. [PubMed] [Google Scholar]
- 26.Maritz GS, Cock ML, Louey S, et al. Effects of fetal growth restriction on lung development before and after birth: a morphometric analysis. Pediatr Pulmonol. 2001;32:201–210. doi: 10.1002/ppul.1109. [DOI] [PubMed] [Google Scholar]
- 27.Wignarajah D, Cock ML, Pinkerton KE, et al. Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Pediatr Res. 2002;51:681–688. doi: 10.1203/00006450-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Joyce BJ, Louey S, Davey MG, et al. Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction. Pediatr Res. 2001;50:641–649. doi: 10.1203/00006450-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Tanner JM. Catch-up growth in man. Br Med Bull. 1981;37:233–238. doi: 10.1093/oxfordjournals.bmb.a071708. [DOI] [PubMed] [Google Scholar]
- 30.Camera P, Zeiger L, Guilbert R, Bacharier T, Taussig L, Morgan L, Covar R, Krawiec M, Bloomberg G, Mauger D. Relationship between infant weight gain and later asthma. Pediatric allergy and immunology. 2009;21:82–89. doi: 10.1111/j.1399-3038.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, et al. Increased incidence of asthma like symptoms in girls who become overweight or obese during the school years. Am J Resp Crit Care Med. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 32.Hallstrand TS, Fischer ME, Wurfel MM, et al. Genetic pleiotropy between asthma and obesity in a community-based sample of twins. J Allergy Clin Immunol. 2005;116:1235–1241. doi: 10.1016/j.jaci.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zerah F, Harf A, Perlemuter L, et al. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 34.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunnbjornsdottir MI, Omenaas E, Gislason T, et al. Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Respir J. 2004;24:116–121. doi: 10.1183/09031936.04.00042603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.