Abstract
Background
We examined the relationships between childhood physical activity (PA), dietary calcium intake and bone size and density.
Subjects and methods
Children aged 4 years were recruited from the Southampton Women’s Survey. They underwent measurement of bone mass by DXA (Hologic, QDR 4000). Physical activity was assessed by accelerometry (Actiheart, Cambridge Neurotechnology Ltd, Cambridge, UK) for 7 continuous days.
Results
422 children (212 boys) participated. After adjusting for gender, daily mean time (min/day) spent in moderate to very vigorous activity (MVPA) was positively related to hip bone area (p<0.001), mineral content (p<0.001), mineral density (p=0.001) and estimated volumetric density (p=0.01). Mean daily calcium intake positively predicted bone indices (p=0.002 with BMC) in those with a low calcium intake (<800 mg/day), but there was a much attenuated relationship in those above this threshold (p=0.229 with BMC). The relationships between MVPA and bone indices were stronger in children with higher calcium intakes (> 800mg/day) (For BMC and MVPA, p=0.121 below and p<0.001 above).
Conclusions
These results support the notion that adequate calcium intake may be required for optimal action of physical activity on bone development and that improving levels of physical activity and calcium intake in childhood may help to optimise accrual of bone mass.
Introduction
The marked secular increase in childhood obesity over recent decades (1) has generated much debate about possible reciprocal changes in physical activity. Recent systematic reviews have suggested that levels of physical activity in children have actually been reasonably stable over the last decade or two in the US (2) and Europe (3). Obesity itself is a risk factor for childhood fracture (4), and it is unclear whether current general levels of physical activity provide the optimal stimulus for bone development; public health strategies aimed at increasing physical activity in childhood might potentially help to improve bone health and reduce obesity. Recent work has suggested that factors in early life, starting in utero, influence the accrual of bone mineral in childhood and thus the peak bone mass achieved in the 3rd decade of life (5-7). Peak bone mass is a strong determinant of osteoporosis risk in later life (8), and thus measures to improve childhood bone health to peak represent an important public health agenda. There are few data on the relationship between habitual free-living physical activity and bone mineral in young children (9). The effect of exercise interventions has been examined in several studies, but these have shown only short term results (10,11), and there are few data examining physical activity and dietary factors, such as calcium intake, in free living young children. In this paper, we have used a large free-living population cohort, the Southampton Women’s Survey (SWS), to examine the relationships between objectively measured childhood physical activity, calcium intake and bone size and density measured by DXA.
Methods
Participants
The Southampton Women’s Survey is a unique, prospective cohort study of 12,583 women aged 20-34 years recruited from the general population (12). At enrolment the participants were characterised in detail in terms of diet, lifestyle, health, physical activity and anthropometric measurements. 3156 of these women became pregnant and were studied in similar detail several times during gestation. The subsequent children are being followed and characterised at regular intervals, with the oldest children now reaching 8 years old. 900 children underwent assessment by DXA at 4 years old and of these 422 had their habitual physical activity measured, forming the cohort presented in this paper.
Childhood dietary assessment
At 3 years of age, diet was assessed using an 80-item food frequency questionnaire (FFQ), administered by trained research nurses, to record the average frequency and quantity of the foods consumed over the preceding 3 months. Daily milk consumption was recorded separately at the end of the FFQ. In a validation study of 887 SWS children aged 3 years, in which nutrient intakes estimated from the FFQ were compared with intakes estimated from 2-day prospective food records, the Spearman rank correlation coefficient for calcium intake was 0.55 (data unpublished).
4 year DXA assessment
The mother and child were invited to visit the Osteoporosis Centre at Southampton General Hospital for assessment of bone mass. At this visit written informed consent for the DXA scan was obtained from the mother or father. The child’s height (using a Leicester height measurer) and weight (in underpants only, using calibrated digital scales [Seca Ltd]) were measured. A whole body DXA scan was obtained, using a Hologic Discovery instrument (Hologic Inc., Bedford, MA, USA). To encourage compliance, a sheet with appropriate coloured cartoons was laid on the couch first; to help reduce movement artefact, the children were shown a suitable DVD cartoon. The total radiation dose for the scans was 4.7 microsieverts for whole body measurement (paediatric scan mode). The manufacturer’s coefficient of variation (CV) for the instrument was 0.75% for whole body bone mineral density, and the experimental CV when a spine phantom was repeatedly scanned in the same position 16 times was 0.68%.
4 year physical activity assessment
In a subset of the children who attended for DXA, an Actiheart combined accelerometer and heart rate monitor (Cambridge Neurotechnology Ltd, Cambridge, UK) was fitted to the child. The main component of the Actiheart unit is approximately 1.5 cm across and 3mm thick, which is attached with a 70 to 100 mm wire to a smaller clip. Both of these parts were secured to the skin via standard electrocardiograph electrode pads. The main unit was positioned in the midline just below the xiphisternum and the smaller clip horizontally to the left chest wall. The mother was asked to persuade the child to wear the monitor continuously for 7 days except during bathing and swimming. Monitors were returned by post. The mother was asked to estimate how long the child spent watching television daily (none, < 1 hour, 1-2 hours, 2-3 hours, 3-4 hours, 4-5 hours and > 5 hours). The Actiheart accelerometry data, rather than the heart rate output, was used to assess physical activity as, a priori, one would not expect heart rate to predict bone-forming impact activity. The acceleration output from the Actiheart has previously been validated in children (13,14). The accelerometer in this device has a linear (R2 =0.999) response to acceleration (15) and was oriented to measure acceleration along the body’s longitudinal axis using a 1 minute epoch.
The output was derived as counts per minute (cpm) and thresholds for low (20 cpm), moderate (400 cpm), vigorous (600 cpm) and very vigorous activity (800 cpm) were used to categorise the data into average daily time at each threshold. These intensity thresholds are broadly equal to 100, 2000, 3000 and 4000 counts per minute obtained by the Actigraph GT1M accelerometer (Actigraph, Pensacola, Fl, USA) (Ekelund, personal communication) Moderate, vigorous and very vigorous activity levels were grouped to give the primary exposure measure (MVPA).
Statistical analysis
All variables were checked for normality. Non-normally distributed variables were transformed logarithmically. t-tests and Mann-Whitney U tests were used to test the difference between normally and non-normally distributed variables respectively. Correlation and regression methods were used to explore the relationships between physical activity and bone mass. Bone outcomes used at 4 years include bone area (BA), bone mineral content (BMC), areal bone mineral density (aBMD) and estimated volumetric bone mineral density (vBMD: BMC adjusted for BA, height and weight to minimise the effect of body size), at the whole body minus head, lumbar spine and total hip sites.
Results
Characteristics of the mothers and children
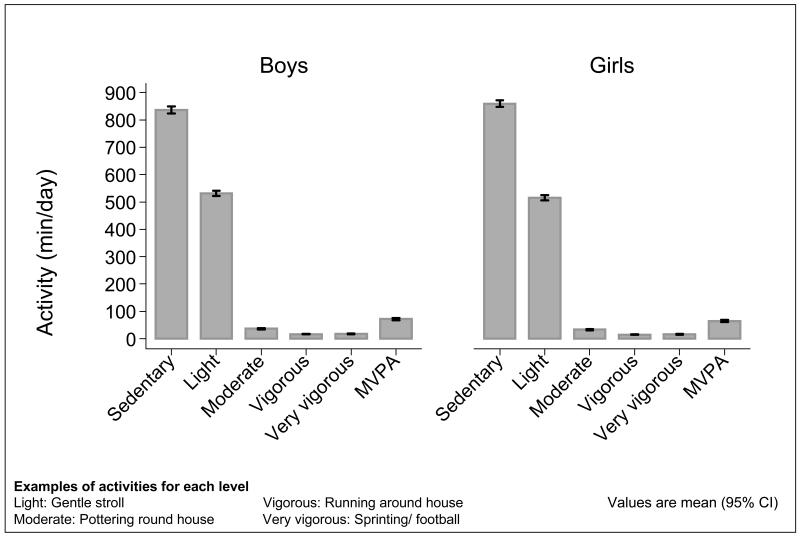
422 children (212 boys) took part. Table 1 summarises the characteristics of the mothers and the children. Boys spent more time in moderate to very vigorous physical activity (MVPA) than girls (70 minutes/day vs 61 minutes per day, p=0.037). Figure 1 shows the distribution of time spent in each level of physical activity in boys and girls. On average, children wore the Actiheart monitors for 19.5 hours per day for 6.9 days. The boys tended to have greater bone indices at the hip and so the outcomes were adjusted for gender as well as the age of the child. Comparison of mothers of children who underwent DXA assessment at 4 years with those of children who did not revealed that the former were, on average, slightly older at the birth of their child (mean age 31.2 years vs 30.6 years respectively, p=0.007), better educated (24.8% with higher degree vs 20.6% respectively, p=0.002) and smoked less (8.5% smoked before pregnancy vs 17.4% respectively, p<0.001).
Table 1. Characteristics of mothers and children.
| Mothers (n=422) |
Mean (sd) or median (IQR) |
||
|---|---|---|---|
| Age (years) | 35.4 (3.6) | ||
| Height (cm) | 164.0 (6.2) | ||
| Weight (kg) | 65.4 (59.4-73.2) | ||
| BMI (kg/m2) | 24.0 (22.1-27.4) | ||
| Children |
Boys (n=212) Mean (sd) or median (IQR) |
Girls (n=210) Mean (sd) or median (IQR) |
P difference |
|
| |||
| Age (years) | 4.1 (4.07-4.16) | 4.1 (4.06-4.14) | 0.07 |
| Height (cm) | 104.3 (3.8) | 103.9 (4.2) | 0.2 |
| Weight (kg) | 17.4 (16.4-19.0) | 17.5 (16.0-18.8) | 0.5 |
| BMI (kg/m2) | 16.1 (15.3-16.9) | 16.0 (15.3-16.9) | 0.9 |
| Calcium intake at 3 yrs (g/day)* | 985 (796-1251) | 935 (752-1208) | 0.1 |
| Sedentary (min/day) | 837 (93) | 860 (84) | 0.009 |
| Active (min/day) | 603 (93) | 580 (84) | 0.009 |
| Lightly active (min/day) | 532 (73) | 516 (72) | 0.026 |
| Moderately active (min/day) | 34 (26-46) | 31 (24-39) | 0.008 |
| Vigorously active (min/day) | 16 (10-21) | 14 (10-19) | 0.049 |
| Very vigorously active | 15 (10-24) | 15 (8-23) | 0.3 |
| (min/day) | |||
| MVPA (min/day) | 70 (47-90) | 61 (45-83) | 0.037 |
| Hip BA (cm2) | 12.8 (1.9) | 12.6 (2.0) | 0.6 |
| Hip BMC (g) | 7.5 (1.6) | 7.0 (1.7) | 0.003 |
| Hip aBMD (g/cm2) | 0.59 (0.06) | 0.55 (0.06) | <0.001 |
| Hip vBMD (~units) | 7.3 (0.8) | 6.9 (0.05) | <0.001 |
n=188 (boys) and 187 (girls)
Figure 1.
Distribution of time spent in different levels of physical activity in boys and girls. Figure shows mean and 95% CI
Relationships between calcium intake and bone size and density
Mean daily calcium intake, as a continuous variable, assessed at 3 years old, positively predicted bone indices at the hip, and was more weakly associated with whole body minus head BMC (R2=1.2%, p=0.04). There appeared to be a threshold in the relationship with the hip indices such that the associations were statistically significant for calcium intakes below, but not above, 800mg/day. Thus, for daily calcium intakes below 800mg/day the variance explained and p values were: BA: 3.2%, p=0.064; BMC: 9%, p=0.002; aBMD: 13.7%, p<0.001; vBMD: 8.2%, p=0.003. For daily calcium intakes above 800mg/day, the corresponding values were: BA: 0.2%, p=0.479; BMC: 0.6%, p=0.229; aBMD: 1%, p=0.113; vBMD: 0.6%, p=0.197. Table 2 summarises the mean bone indices by calcium intake above and below this threshold.
Table 2. Daily calcium intake at 3 years and hip bone size and density at 4 years.
| Calcium (mg/day) | |||||
|---|---|---|---|---|---|
| <=800 (n=107) | >800(n=264) | ||||
| Mean | SD | Mean | SD | P diff | |
| Hip BA (cm2) | 12.433 | 1.951 | 12.755 | 2.033 | 0.162 |
| Hip BMC (g) | 6.984 | 1.579 | 7.401 | 1.673 | 0.028 |
| Hip aBMD (g/cm2) | 0.558 | 0.063 | 0.576 | 0.06 | 0.01 |
| Hip vBMD (units) | 6.984 | 0.694 | 7.165 | 0.697 | 0.024 |
Relationships between physical activity and bone size and density
Mean minutes per day in MVPA was positively related to hip BA (R2=3%, p<0.001), BMC (R2=4%, p<0.001), aBMD (R2=3%, p=0.001) and vBMD (R2=2%, p=0.01). MVPA was not statistically significantly related to bone indices at whole body and lumbar spine sites. The magnitude of associations between time spent in MVPA and bone indices was similar in both boys and girls. There was a negative association between hours of television watched per day and hip BA and BMC. Thus for each category increase in television watching, BA decreased by 0.2 g (p=0.041) and BMC by 0.2 cm2 (p=0.036). Table 3 shows the mean bone indices by mean daily time spent in MVPA (dichotomised for illustrative purposes into those with less or more than 1 hour of MVPA per day).
Table 3. Minutes per day of childhood moderate to vigorous physical activity and hip bone size and density.
| MVPA (mins/day) | |||||
|---|---|---|---|---|---|
| <=60 (n=187) | >60(n=230) | ||||
| Mean | SD | Mean | SD | P diff | |
| Hip BA (cm2) | 12.377 | 2.094 | 12.966 | 1.81 | 0.002 |
| Hip BMC (g) | 6.981 | 1.656 | 7.516 | 1.551 | 0.001 |
| Hip aBMD (g/cm2) | 0.56 | 0.061 | 0.577 | 0.062 | 0.006 |
| Hip vBMD (units) | 6.988 | 0.681 | 7.152 | 0.738 | 0.019 |
MVPA and DXA outcomes by calcium intake
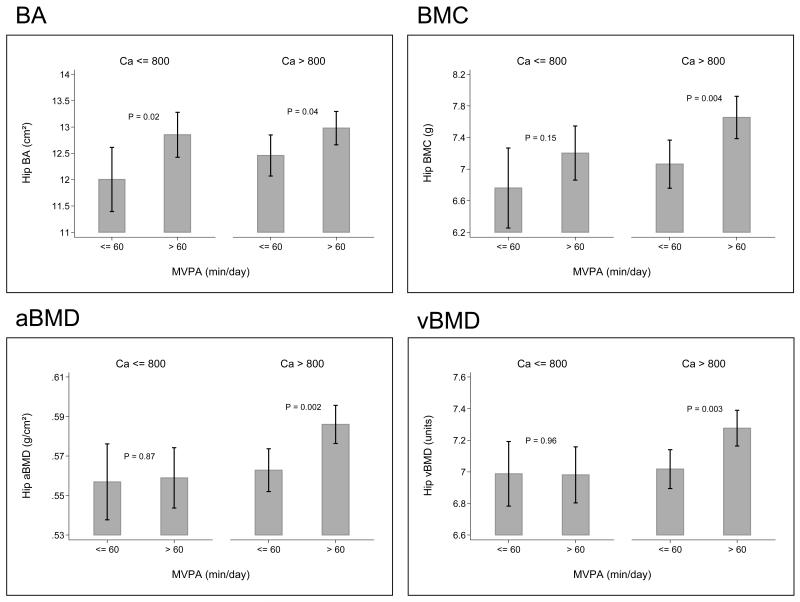
There was no association between time spent in MVPA and calcium intake (p=0.27). However, the relationships between mean daily MVPA and bone indices were stronger when calcium intake was above compared with below 800mg/day. Thus for BMC, the variance explained by MVPA when daily calcium intake was below 800mg/day was 2.3% (p=0.121) and above 800mg/day was 5.4% (p<0.001). The respective values for the remaining bone indices at the hip were: BA: R2=3%, p=0.073 below and R2=3.4%, p=0.003 above 800mg/day; aBMD: R2=1.3%, p=0.242 below and R2=4.9%, p<0.001 above; vBMD: R2=0.6%, p=0.423 below and R2=3.5%, p=0.002 above). Thus the difference was more marked for measures of mineralisation (BMC, aBMD, vBMD) than bone size (BA). However the formal interaction terms testing these relationships (whether the association between MVPA and bone indices varied by calcium intake) did not achieve statistical significance (BA: p=0.906, BMC: p=0.445, aBMD: p=0.397, vBMD: p=0.361). Figure 2 shows the relationships between time spent in MVPA and bone size and density by calcium intake.
Figure 2.
Childhood MVPA and hip bone size and estimated volumetric density by daily mean calcium intake below or above 800mg/day. Values are mean (95%CI)
Maternal factors
We explored the effect of adding maternal social class, education, smoking, body mass index, height, parity as covariates in the regression models relating MVPA to offspring bone indices and found that the relationships remained robust.
Discussion
We have shown, in a free living population cohort, that levels of daily moderate to vigorous physical activity were positively associated with hip size and density at 4 years old. These associations were more marked in those children consuming more than 800mg of calcium per day, consistent with the notion that adequate calcium intake may be necessary for optimal action of PA on bone development.
However, there are several limitations that should be taken into account when interpreting our results. Firstly, measurement of physical activity in free-living children presents some difficulties. Children may remove monitors and we did not ask the parents to keep a diary of this. However, children were asked to wear the monitor for 24 hours per day except during water activities. On average, children wore the monitors for 19.5 hours per day for 6.9 days, indicating excellent compliance. Secondly, measurement of bone mineral in young children is hampered by their tendency to move and also by their low absolute BMC. However, we used specific paediatric software, and movement artefact was modest and uniform across the cohort; those few children with excessive movement were excluded from the analysis. The accuracy of DXA for the assessment of bone mineral in small animals has been demonstrated in piglets (16,17). Thirdly we assessed diet using a FFQ. Although there is concern that FFQs are prone to measurement error calcium intakes assessed by this FFQ were highly correlated with intakes determined using detailed prospective food records, and it is unlikely that measurement error could explain the differences in relationships with bone status that we observed. Detailed assessment of calcium intake was not performed when the child was 4 years old, but there was a good correlation (r=0.44, p<0.0001) between calcium intake at three years and milk intake at 4 years (the predominant determinant of dietary calcium intake). Fourthly, the study cohort was a subset of the SWS, but mothers whose children underwent DXA scanning and those whose babies did not were broadly similar: The former were on average slightly older and smoked slightly less. There is no reason to suppose, however, that relationships between MVPA, calcium intake and bone mineral would differ between these two groups. Finally, the use of DXA does not allow measurement of true volumetric bone density, thus making it difficult to be certain about differential determinants of skeletal size and volumetric density.
Several reports in children and adolescents involved in competitive sport or ballet indicate that intense exercise is associated with an increase in bone mineral accrual at weight-bearing skeletal sites (18-20). Prospective studies of moderate exercise programmes undertaken in schools have demonstrated a positive effect on bone mineral (10,11), but the long term benefit of such interventions remains unclear. Much less is known about the influence of physical activity on bone mass in younger children, and in particular, whether bone mineral accrual varies significantly within the normal range of habitual activity for a healthy population.
Our results indicate that up to 5.4% of variance in hip BMC might be accounted for by MVPA, given a calcium intake of > 800mg/day, and up to 4% across all calcium intake, and suggest that an increase in MVPA of 10 minutes per day might be associated with a 100mg increase in hip BMC at 4 years old. These figures are comparable to those found in other observational studies, in particular the one other study to examine the relationship between PA and bone indices in a similar free-living paediatric population. In this study of 368 children aged 4 to 6 years, statistically significant positive associations were found between physical activity and BMD at the hip; hours of television viewing per day inversely predicted hip BMD in girls (r=−0.15, p<0.01) (9). Additionally femoral neck cross sectional area was positively associated with physical activity in this US population and relationships were similar in boys and girls (21). In this study there was a weak association with BMC at the whole body site in boys in only and stronger associations with BMC at the lumbar spine in both genders. In contrast we only found associations between MVPA and bone indices at the total hip site. Indeed in many of the studies linking PA to bone mineral have found associations primarily at weight bearing sites such as the hip; the children in the Iowa study were on average a year older than in our study and thus it is possible that the ability to detect a relationship between PA and spinal bone indices may have been limited by the smaller size and lower mineral content at this younger age. Our study has replicated the finding that increasing hours of daily television watching are associated negatively with BMC at the hip.
Several, but not all, studies have indicated a positive relationship between dietary calcium intake and bone mineral (22-25). Intervention studies have suggested at least short term benefits from additional calcium supplementation, with some evidence of more persisting (3 years) improvements for those supplements derived from milk (26,27). We demonstrated differences in the relationship between PA and bone mineral according to mean daily calcium intake. Consistent with this finding, Specker et al randomised young children (age 3 to 5 years) to either gross or fine motor exercises and either low or high calcium intake, measuring tibial cortical bone thickness and area by PQCT at 12 months. They found that children in the high exercise, high calcium group had an increase in these parameters, but children on no calcium supplement in the high activity group had lower cortical thickness and area than children in the low activity group (28). The same group demonstrated similar interactions between calcium intake and bone response to physical activity in toddlers aged 6 to 18 months (29), and a review of the studies available at the time supported the notion that a daily calcium intake in the region of 1000mg is necessary for optimal bone mineral accrual in response to physical activity (30). The effect of calcium in our study seemed most marked on measures of bone that included mineral, ie BMC, aBMD and vBMD, rather than bone area, suggesting that childhood dietary calcium intake is important for mineralisation of the skeleton rather than influencing is its overall size.
The significance of physical activity for bone health can be considered in the context of childhood and the longer term. Work from New Zealand has demonstrated that children who fracture have lower bone density at the radius and are more likely to be obese than those who do not (4), and a study from the UK yielded similar results (31). Given the positive associations between bone density and physical activity in childhood, it might be expected that increased physical activity would be associated with decreased risk of childhood fracture. However, this ignores one of the other risk factors for fracture, which is physical activity itself. Indeed examination of the children in the ALSPAC (UK) cohort showed that although increased physical activity (measured by questionnaire) was associated with increased bone mineral density, it also predicted an increased risk of childhood fracture (32). Thus the children who are most active have the strongest bones, but are also putting greatest forces through them and so at greatest risk of traumatic fracture.
If an individual can sustain increased physical activity appropriate to bone growth throughout childhood, adolescence and young adulthood to peak bone mass, then peak bone mass should be improved. Recent work has suggested that peak bone mass is a critically important determinant of osteoporosis risk in later life (8). Indeed if the difference in hip BMD between bottom and top quartiles of MVPA (0.4SD) were to be sustained into adulthood, this could equate to an 30 to 50% reduction in risk of fracture (33). Although current clinical practice focuses mainly on identification of those individuals most at risk of fracture, we have previously demonstrated factors in early life, such as maternal lifestyle, body build and 25(OH)-vitamin D status (5,6) which influence intrauterine bone mineral accrual and skeletal geometry in the offspring. Taken together, these data suggest that attention should be given to optimising bone health throughout the life course to reduce the risk of osteoporotic fracture in old age.
In conclusion, we have demonstrated, in a free-living population cohort, that levels of habitual daily physical activity are positively associated with total hip bone size and density at 4 years old. Additionally the effects were greatest in those with a mean daily calcium intake above 800 mg, suggesting that adequate dietary calcium intake is required for the optimal influence of physical activity on bone formation. These results support the notion that increasing habitual childhood physical activity and calcium intake is likely to be a sensible public health strategy to improve child bone health and potentially reduce the burden of osteoporotic fracture in future generations.
Acknowledgements
We thank the mothers who gave us their time; and a team of dedicated research nurses and ancillary staff for their assistance. This work was supported by grants from the Medical Research Council, British Heart Foundation, Arthritis Research Campaign, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, NIHR Biomedical Research Unit, University of Southampton, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. Participants were drawn from a cohort study funded by the Medical Research Council and the Dunhill Medical Trust. We thank Mrs G Strange for helping prepare the manuscript.
Funding sources: Medical Research Council; Arthritis Research Campaign; National Osteoporosis Society; Wellbeing; Cohen Trust; NE (now North) Thames NHS R&D Directorate.
References
- 1.Jackson-Leach R, Lobstein T. Estimated burden of paediatric obesity and comorbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. Int J Pediatr Obes. 2006;1:26–32. doi: 10.1080/17477160600586614. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Treuth MS, Wang Y. How active are American adolescents and have they become less active? Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00685.x. Epub Oct 27. [DOI] [PubMed] [Google Scholar]
- 3.Samdal O, Tynjala J, Roberts C, Sallis JF, Villberg J, Wold B. Trends in vigorous physical activity and TV watching of adolescents from 1986 to 2002 in seven European Countries. Eur J Public Health. 2007;17:242–248. doi: 10.1093/eurpub/ckl245. [DOI] [PubMed] [Google Scholar]
- 4.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 6.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 9.Janz KF, Burns TL, Torner JC, Levy SM, Paulos R, Willing MC, Warren JJ. Physical activity and bone measures in young children: the Iowa bone development study. Pediatrics. 2001;107:1387–1393. doi: 10.1542/peds.107.6.1387. [DOI] [PubMed] [Google Scholar]
- 10.Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res. 1997;12:1453–1462. doi: 10.1359/jbmr.1997.12.9.1453. [DOI] [PubMed] [Google Scholar]
- 11.Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, Carlson J, Seeman E. Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J Bone Miner Res. 1998;13:1814–1821. doi: 10.1359/jbmr.1998.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 12.Inskip H, Godfrey KM, Robinson SM, Law CM, Barker DJP, Cooper C, the SWS Study Group Cohort Profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corder K, Brage S, Mattocks C, Ness A, Riddoch C, Wareham NJ, Ekelund U. Comparison of two methods to assess PAEE during six activities in children. Med Sci Sports Exerc. 2007;39:2180–2188. doi: 10.1249/mss.0b013e318150dff8. [DOI] [PubMed] [Google Scholar]
- 14.Corder K, Brage S, Wareham NJ, Ekelund U. Comparison of PAEE from combined and separate heart rate and movement models in children. Med Sci Sports Exerc. 2005;37:1761, 1767. doi: 10.1249/01.mss.0000176466.78408.cc. [DOI] [PubMed] [Google Scholar]
- 15.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005 doi: 10.1038/sj.ejcn.1602118. In press. [DOI] [PubMed] [Google Scholar]
- 16.Abrams SA, Schanler RJ, Sheng HP, Evans HJ, Leblanc AD, Garza C. Bone mineral content reflects total body calcium in neonatal miniature piglets. Pediatr Res. 1988;24:693–695. doi: 10.1203/00006450-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Brunton JA, Weiler HA, Atkinson SA. Improvement in the accuracy of dual energy x-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res. 1997;41:590–596. doi: 10.1203/00006450-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–507. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson MK, Johnell O, Obrant KJ. Is bone mineral density advantage maintained long-term in previous weight lifters? Calcif Tissue Int. 1995;57:325–328. doi: 10.1007/BF00302066. [DOI] [PubMed] [Google Scholar]
- 20.Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Janz KF, Burns TL, Levy SM, Torner JC, Willing MC, Beck TJ, Gilmore JM, Marshall TA. Everyday activity predicts bone geometry in children: the iowa bone development study. Med Sci Sports Exerc. 2004;36:1124–1131. doi: 10.1249/01.mss.0000132275.65378.9d. [DOI] [PubMed] [Google Scholar]
- 22.Du XQ, Greenfield H, Fraser DR, Ge KY, Liu ZH, He W. Milk consumption and bone mineral content in Chinese adolescent girls. Bone. 2002;30:521–528. doi: 10.1016/s8756-3282(01)00698-6. [DOI] [PubMed] [Google Scholar]
- 23.Rozen GS, Rennert G, Rennert HS, Diab G, Daud D, Ish-Shalom S. Calcium intake and bone mass development among Israeli adolescent girls. J Am Coll Nutr. 2001;20:219–224. doi: 10.1080/07315724.2001.10719035. [DOI] [PubMed] [Google Scholar]
- 24.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675–680. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 25.Barr SI, Petit MA, Vigna YM, Prior JC. Eating attitudes and habitual calcium intake in peripubertal girls are associated with initial bone mineral content and its change over 2 years. J Bone Miner Res. 2001;16:940–947. doi: 10.1359/jbmr.2001.16.5.940. [DOI] [PubMed] [Google Scholar]
- 26.Bonjour JP, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–1294. doi: 10.1172/JCI119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonjour JP, Chevalley T, Ammann P, Slosman D, Rizzoli R. Gain in bone mineral mass in prepubertal girls 3.5 years after discontinuation of calcium supplementation: a follow-up study. Lancet. 2001;358:1208–1212. doi: 10.1016/S0140-6736(01)06342-5. [DOI] [PubMed] [Google Scholar]
- 28.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res. 2003;18:885–892. doi: 10.1359/jbmr.2003.18.5.885. [DOI] [PubMed] [Google Scholar]
- 29.Specker BL, Mulligan L, Ho M. Longitudinal study of calcium intake, physical activity, and bone mineral content in infants 6-18 months of age. J Bone Miner Res. 1999;14:569–576. doi: 10.1359/jbmr.1999.14.4.569. [DOI] [PubMed] [Google Scholar]
- 30.Specker BL. Evidence for an interaction between calcium intake and physical activity on changes in bone mineral density. J Bone Miner Res. 1996;11:1539–1544. doi: 10.1002/jbmr.5650111022. [DOI] [PubMed] [Google Scholar]
- 31.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark EM, Ness AR, Tobias JH. Vigorous physical activity increases fracture risk in children irrespective of bone mass: a prospective study of the independent risk factors for fractures in healthy children. J Bone Miner Res. 2008;23:1012–1022. doi: 10.1359/jbmr.080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]