Abstract
Labdanes, leonurenones A–C, two known labdanes, luteolin 7-O-β-glucoside and luteolin were isolated and characterized from a commercial source of Leonotis leonurus. Genetic methods allowed for identification of the plant material. The leonurenones contain an uncommon α,β-unsaturated enone moiety in ring B, and leonurenones A and B were evaluated in a competitive inhibition assay at the GABA A neuroreceptor site.
Keywords: Labdane, Diterpene, Leonotis leonurus, Leonurenone
1. Introduction
Leonotis leonurus R. Br. (Lamiaceae) is a shrub 2–5 m in height that is native to South Africa. In traditional medicine, decoctions have been used externally for dermatological problems (rashes, boils and eczema) and internally to treat coughs, fever, headaches and hypertension (Scott et al., 2004). Leaves of the plant are smoked for its anti-epileptic effects (Bienvenu et al., 2002). Aqueous leaf preparations have been reported to possess anticonvulsant, antinociceptive, anti-inflammatory and antidiabetic properties in rodents (Bienvenu et al., 2002; Ojewole, 2005; Oyedemi et al., 2011). Additionally, crude aqueous extracts have been shown to possess antihelminthic activity (Maphosa and Masika, 2012; Maphosa et al., 2010).
In addition to folkloric uses mentioned above, L. leonurus reportedly produces marijuana-like effects (Stafford et al., 2008). The plant is commercially available and is marketed largely for its psychoactive effects. Some internet websites claim that this activity is attributable to an alkaloid, leonurine. There is some doubt about the validity of this claim since leonurine has never been reported to occur in the plant (although its presence in related species is documented) (Chen and Kwan, 2001; Hayashi, 1962; Kong et al., 1976; Luo, 1985). Thus the component(s) responsible for the reputed psychoactive effects are scientifically unverified at this time. Prior phytochemical investigations of various extracts of the plant have uncovered a number of labdane diterpenes, an iridoid glycoside and phenolic compounds, primarily of the flavonoid class (Agnihotri et al., 2009; El-Ansari et al., 2009; Kaplan and Rivett, 1968; Kruger and Rivett, 1988; Laonigro et al., 1979; McKenzie et al., 2006; Naidoo et al., 2011; Obikeze et al., 2008; Piozzi et al., 2007; Rivett, 1964).
As part of our program to identify natural products with central nervous system (CNS) activity from Leonotis plants, an investigation of a commercially available source of L. leonurus was carried out. Verification of the taxonomic identity of the plant was achieved via molecular methods. Details of the taxonomic identification as well as our chemical, spectroscopic and biological studies are reported herein.
2. Results and discussion
Since the plant material procured was not of sufficient quality to permit positive morphological identification (i.e. as distinct from other similar Leonotis species), identification of the plant using genetic methods was done.
Molecular markers have proved a powerful tool in diagnostics of species and varieties of various commercial products in order to identify and delimiting closely related species and to ensure quality control. Both DNA fingerprinting techniques and DNA sequence information have been employed, even from degraded and unrecognizable plant material (Martellossi et al., 2005; Zerega et al., 2002). The application, sometimes referred to as “DNA barcoding”, can assist in the process of identifying unknown plant specimens to known species. (Hollingsworth et al., 2011) For example, this method has been suggested as useful for an accurate and rapid authentication of medicinal plant products and their adulterants (Chen et al., 2010).
Recent molecular work in the Lamiaceae subfamily Lamioideae, to which Leonotis belongs, has produced a large database of chloroplast DNA sequences. (Scheen and Albert, 2009) A diagnostic assay was thus employed based on two chloroplast intron gene sequences (trnL intron and rps16 intron) that provide sufficient sequence variation to distinguish members of the group Leonotis belongs to, including closely related Leonotis and Leucas R.Br. species. After sequencing these gene regions from the procured Leonotis plant material several approaches were taken to ensure a correct identification. First, the sequences were analyzed using the BLAST search tool against sequences in the public database, GenBank, held at the National Center for Biotechnology Information (NCBI). Secondly, the DNA sequences were compared to the large data set of several hundred lamioid sequences, which clearly confirmed a large diagnostic “gap” in the rps16 sequence, which was shared with the L. leonurus sample in the data set. Finally, phylogenetic reconstruction was performed verifying that the obtained plant material grouped with the other L. leonurus in the data set. Together, these results clearly confirm the obtained material to belong to Leonotis leonurus.
The aqueous extracts of the aerial parts of the plant have not been subjected to phytochemical study and given the pharmacological activity attributed to this extract, it was considered worthy of such an investigation.
Repeated purification procedures (HPLC and flash column chromatography) on an aqueous extract of aerial parts of L. leonurus gave compounds 1, 2, 6 and 7 (Fig. 1). Similarly, repeated chromatography of an acetone extract afforded compounds 3–5.
Fig. 1.
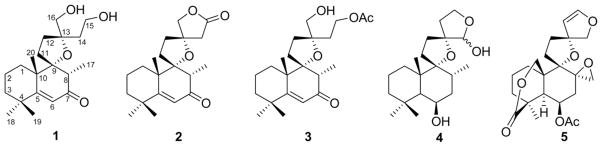
Structures of isolated compounds 1–5.
The initial diterpenoid nature of 1 came from its 13C NMR spectrum where 20 carbons were observed. The HRESIMS spectrum showed a molecular ion peak at m/z 336.2380. This implied a molecular formula of C20H32O4 in agreement with the 13C NMR spectroscopic data. In the 13C NMR spectrum, signals typical of the quaternary C-9 and C-13 positions of a spiroether labdane framework were observed (δC 95.9 and 85.7 ppm, respectively). Other low field quaternary signals were observed at δC 200.0 and 172.4 ppm. The former resonance was placed at position 7 of the labdane core based on HMBC correlations with the C-17 methyl protons (δH 1.21, d). The resonance at δC 172.4 showed HMBC cross-peaks to the C-18 and C-19 methyl protons as well as the C-20 methyl protons. This signal was thus attributed to C-5 of the diterpene skeleton. A vinylic system appeared to be present based on the signal at δC 123.1 (CH). The proton attached to this vinylic carbon (δH 6.06) showed HMBC correlations to C-8 (δC 47.7) and C-10 (δC 45.2). On the basis of the preceding, it became evident that the resonances at δC 200.0, 172.4 and 123.1 were due to the presence of an α,β-unsaturated enone system in ring B. The presence of two oxymethylene carbons was inferred from DEPT-135 and HSQC data (δC 66.9 and 59.2). That at δC 66.9 was attached to mutually coupled doublets at δH 3.50 and 3.56, thereby placing this carbon at position 16. Further HMBC correlations from the proton at δH 3.56 to C-12, C-13 and the C-14 methylene carbon supported this assignment. The oxymethylene carbon at δC 59.2 was assigned to position 15. HMBC cross-peaks were observed for the H-15 protons to C-13 and C-14. The above established the gross structure of compound 1. The relative stereochemical assignments for 1 were made on the basis of NOESY spectroscopic data. Here cross-peaks were seen for H-8 to the C-20 and C-11 protons, thereby establishing that these groups were on the same side of the labdane structure. H-20 in turn showed NOESY cross-peaks to H-16 while H-17 showed cross-peaks to H-14. The α,β-unsaturated ketone system in ring B seen in 1 is very rare in the Leonotis genus having only been reported once in the literature (Kaplan and Rivett, 1968).
Compound 2 was isolated as colorless oil and showed a molecular ion peak in its HRESIMS spectrum (m/z 332.1992) corresponding to the molecular formula C20H28O4. The structural similarity to compound 1 was evident upon inspection of the 13C NMR and 1H NMR spectroscopic data. The 1H NMR spectrum showed 3 methyl singlets and a methyl doublet as seen in 1. Typical 13C NMR signals for the α,β-unsaturated enone in ring B (δC 174.6, C-5; 122.3, C-6; 201.1, C-7) and the quaternary C-9 and C-13 positions (δC 96.5 and 86.6, respectively) were also observed. The main difference in the 1H and 13C NMR spectra of the 2, as compared to 1, was the absence of additional signals corresponding to an oxymethylene group. This oxymethylene group was apparently replaced by an ester-like carbonyl functionality (δC 175.8). A pair of coupled oxymethylene doublets (δH 4.30 and 4.23; J = 9.0 Hz) was reminiscent of similar signals for C-16 of 1. The resonance at δH 4.23 correlated to C-13 as well as δC 175.8. These data support the presence of a γ-butyrolactone moiety comprising C-13 to C-16. In the NOESY spectrum, correlations from H-8 to H-20 and H-11 were seen, placing these groups on the same face of the diterpene scaffold. Further NOESY cross-peaks between H-14 and H-17 and H-16 and H-12 established the 13S relative stereochemistry depicted. It is interesting that 2 being relatively non-polar was isolated from the aqueous extract; we cannot exclude the possibility that 2 is an artifact being formed via lactonization of a more polar C15 hydroxyl, C16 carboxylate intermediate during the isolation process.
The HRESIMS of compound 3 indicated a molecular formula of C22H34O5 (m/z 378.2407). The previously described compounds 1 and 2 were obviously similar to 3 based on the NMR spectroscopic data accumulated. Assignments for C-1 to C-13 (particularly the now familiar α,β-unsaturated enone moiety) could readily be made by comparison of their 1H NMR and 13C NMR spectra in tandem with HMBC analysis. Protons at δH 3.52 and 3.40 (both doublets, J = 11.3 Hz) were correlated to C-13 (δC 85.2) and C-12 in the HMBC spectrum. These protons were assigned to the oxymethylene group at C-16. HMBC cross-peaks were observed from protons at δH 1.91, 2.15, 4.06 and 4.16 to C-13. Signals at δH 4.06 and 4.16 were attached to δC 61.5. Their downfield chemical shift suggested attachment to an acyloxy group. Indeed, presence of an acetoxyl group was established via resonances at δH 2.05 (3H, s); δC 21.0 (CH3) and δC 171.1 (C). Thus the carbon at δC 61.5 was assigned to C-15 enabling complete assignments for the molecule and elucidation of the structure proposed. The NOESY data acquired suggested that 3 had the same relative configuration as seen in 1 and 2. In that regard, key NOESY correlations were seen for H-8 to H-20 and H-11 and for H-17 to H-14.
In addition to the three new compounds above, the known labdane 4 was also isolated as an epimeric mixture, (Naidoo et al., 2011) nepetaefolin (5), (Hussein et al., 2003; Ohsaki et al., 2005; Von Dreele et al., 1975; White and Manchand, 1973) luteolin glycoside (6) (El-Ansari et al., 2009) and its aglycone (luteolin, 7) (El-Ansari et al., 2009). The epimeric ratio of 4 was approximately 3:1 based on integration of the H-16 signals of the major and minor isomers – δH 4.75 and 5.32, respectively. NMR and physicochemical data for the known compounds are in close agreement with those previously reported (El-Ansari et al., 2009; Hussein et al., 2003; Naidoo et al., 2011). The presence of 4, 6 and 7 in this species is known (El-Ansari et al., 2009; Naidoo et al., 2011). However, this is the first report of the presence of nepetaefolin (5) in L. leonurus. It is proposed that the absolute configurations of the new compounds 1–4 are in agreement with the absolute configurations of other known labdanes given the co-occurrence of 1–4 and 5 (for which the crystal structure has been reported) (Von Dreele et al., 1975).
Given the known involvement of the GABAA system in sedation, the aqueous extract of L. leonurus (1 g/mL) was screened in a binding assay at the GABAA site using the services of Caliper Life Sciences (www.caliperls.com), and showed an 81% inhibition at this concentration. Compounds 1 and 2 (10 μM) were then submitted for CNS receptor binding assays at the GABAA site. However, the compounds did not show any activity (<50% inhibition) in this assay. The paucity of the other compounds isolated did not permit these evaluations. Further studies are required to identify the GABAA- active metabolites present in the crude aqueous extract.
3. Conclusions
There have been a number of phytochemical investigations on L. leonurus and interest in this plant continues to be high because of the reported biological activities. Previous phytochemical work on the leaves of L. leonurus have concentrated on organic extracts. However, the biological activities ascribed to the plant are attributable to consumption of aqueous preparations. An investigation of the aqueous extract was thus prudent at this time.
Our investigation has uncovered three new labdane diterpenes from an aqueous extract of a commercial source of the plant. Thus this work highlights the utility of commercially available plant sources as a viable option for phytochemical work. Clearly however, as carried out in this paper, the appropriate validation of the plant material must accompany any such investigation.
Results from our study, as well as a previous investigation on the anticonvulsant effects of L. leonurus (Bienvenu et al., 2002), suggest that the aqueous extract has GABAA activity. However, compounds 1 and 2 isolated herein did not show any activity at this receptor, indicating that these compounds are not by themselves responsible for the activity.
4. Experimental
4.1. General experimental procedures
Optical rotations were performed on a Rudolph Autopol IV polarimeter with the Na 589 line. IR spectra were recorded on a Thermo Nicolet IR 100 spectrophotometer as thin films. UV spectra were recorded on a Varian Cary 1 Bio UV–Visible spectrophotometer in CH2Cl2 solution. NMR spectroscopic data were obtained with on a Bruker 500 MHz spectrometer in CDCl3 as solvent unless otherwise stated and with TMS as internal standard. Chemical shift (δ) values are reported in ppm and coupling constants in Hertz (Hz). High Resolution Electrospray Ionization Mass Spectra (HRESIMS) were obtained using an Agilent 6520 Q-TOF instrument. TLC analysis was performed with Analtech Uniplate silica gel G/UV 254 pre-coated plates (0.2 mm). TLC plates were visualized by UV (254 and 360 nm), and by staining with 10% sulfuric acid/vanillin reagent followed by heating. Flash column chromatography (FCC) was performed with Silica gel 60 (EMD Chemicals, 230–400 mesh, 0.04–0.063 μm particle size) and Diaion HP-20 (Supelco/1-3607/176255E). HPLC purifications were performed on an Agilent 1200 system equipped with variable wavelength detector and fraction collector with Partisil 10 (Whatman) or ZORBAX SB-C18, 21.2 × 150 mm, 7 μm (Agilent). Lyophilization was performed with a Labconco Freezone 2.5 system.
4.2. 2. Plant material
Plant material (crushed leaves) was purchased from Bouncing Bear Botanicals in September 2009. A voucher specimen (voucher number BKL00104130) is deposited in the Herbarium at the Brooklyn Botanic Gardens, Brooklyn NY 11225. Authentication of the material was performed via genetic analysis as described below. Genetic sequencing data is accessible at www.ncbi.nlm.nih.gov (GenBank accessions JX073278 and JX073279).
4.3. Genetic analysis
Total DNA was extracted using the DNeasy Plant Mini Kit (Qiagen) following the manufacturer’s instructions. Amplification of the chloroplast trnL and rps16 introns was performed following the method outlined in (Lindqvist et al., 2010). Since the DNA was potentially of low quality, the internal primers rpsLR and rpsLF were used to amplify the rps16 intron as two separate, shorter fragments (Lindqvist et al., 2010). The DNA was sequenced at the University of Washington High-Throughput Genomics Unit. The sequences were assembled and edited using Sequencher 4.1.4 (Gene Codes) before being aligned manually to an existing database of lamioid mint DNA sequences using BioEdit (Hall, 1999). Phylogenetic reconstruction was performed using the programs TNT and SplitsTree4. (Huson and Bryant, 2006). Accession numbers for GenBank accessions are JX073278 and JX073279.
4.4. Extraction and isolation
Ground leaves of L. leonurus (50.2 g) were extracted three times with H2O (3 × 1 L) by percolating in distilled H2O at room temperature overnight. The extracts were combined and dried by lyophilization to yield (4.5 g) a dried extract. A portion of the aqueous extract (3.3 g) was applied to a Diaion HP-20 (Supelco/1–3607/176255E) column (19.0 × 4.5 cm) and fractionated using a H2O:MeOH gradient solvent system (100:0, 30:70, 0:100). Fractions were collected and pooled by TLC analysis to afford 5 combined fractions. From these combined fractions, the fraction eluted with 100% MeOH (0.12 g) was subjected to a silica gel CC (15.0 × 1.2 cm) using an EtOAc:hexanes gradient solvent elution (20:80, 40:60, 60:40, 80:20, 100:0) to give 12 combined fractions. The fraction eluted in EtOAc:hexanes (80:20) was purified by HPLC [Partisil 10 column; flow rate: 2.00 mL/min; injection volume: 15 μL; UV detector wavelength: 254 nm] to provide 1 (4 mg).
Compound 2 (3 mg) was isolated following HPLC of the fraction eluted in EtOAc:hexanes (40:60) using the following chromatographic parameters. Column: Partisil 10; flow rate: 2.00 mL/min; injection volume: 20 μL; UV detector wavelength: 254 nm.
Ground L. leonurus leaves (180.1 g) were defatted with hexanes (3 × 1 L) by percolation at room temperature overnight. Extraction of the marc of the hexanes extract with acetone provided an acetone extract (2.1 g) after removal of solvent. A portion of the acetone extract was subjected to repeated HPLC purification in MeOH:CH2Cl2 (1:99, v/v) to afford compound 3 (1 mg). The following parameters were used. Column: Partisil 10; flow rate: 5.00 mL/min; injection volume: 10 μL; UV detector wavelength: 254 nm.
The fraction eluted in EtOAc:hexanes (20:80, v/v) was applied to on a silica gel column using an EtOAc:hexanes gradient solvent system (15:85, 20:80), to give compound 4 (1 mg).
A portion of the acetone extract prepared as described above, was purified by FCC on a silica gel column using an EtOAc:hexanes gradient solvent system (10:90, 20:80, 30:70, 40:60, 50:50). Repeated FCC of the fraction eluted in EtOAc:hexanes (50:50, v/v) gave compound 5 (1 mg).
A separate portion of L. leonurus aqueous extract (1.0 g) was subjected to HPLC with a ZORBAX SB-C18 column (eluted in H2O and MeOH, respectively) yielding three fractions. Compounds 6 (1 mg) and 7 (1 mg) were obtained by repeated HPLC of the latter two fractions (eluted in 100% MeOH). The following parameters were used. Column: ZORBAX SB-C18, 21.2 × 150 mm, 7 μm; flow rate: 4.00 mL/min; injection volume: 55 μL; UV detector wavelength: 254 nm.
4.4.1. Leonurenone A (1)
Colorless oil; ; UV (MeOH) λmax (log ε) 236 (3.11), 269 (2.52) nm; IR (film) νmax 2933, 1664, 1464, 1360, 1170, 1043 cm−1; for 1H NMR (500 MHz) and 13C NMR (125 MHz) spectroscopic data, see Table 1. HRESIMS [M]+ m/z: 336.2308 (calculated for C20H32O4 336.2380).
Table 1.
13C NMR and 1H NMR Spectroscopic Data for Compounds 1–3.
| Position | Leonurenone A (1)a
|
Leonurenone B (2)b
|
Leonurenone C (3)a
|
|||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 29.4 | 1.92 (m) 2.18 (m) |
30.4 | 1.62 (m) 1.73 (m) |
30.7 | 1.66 (m) |
| 2 | 17.9 | 1.72 (m) 1.85 (m) |
17.2 | 1.72 (m) 1.93 (m) |
17.4 | 1.74 (m) 1.87 (m) |
| 3 | 39.2 | 1.44 (m) 1.60 (m) |
38.8 | 1.47 (m) 1.61 (m) |
39.3 | 1.58 (m) |
| 4 | 37.1 | 36.6 | 37.1 | |||
| 5 | 172.4 | 174.6 | 172.0 | |||
| 6 | 123.1 | 6.06 (s) | 122.3 | 6.00 (s) | 123.2 | 6.05 (s) |
| 7 | 200.0 | 201.1 | 199.8 | |||
| 8 | 47.7 | 3.01 (q, 6.8) | 47.1 | 3.05 (q, 6.8) | 47.7 | 2.98 (q, 6.9) |
| 9 | 95.9 | 96.5 | 95.7 | |||
| 10 | 45.2 | 44.9 | 45.0 | |||
| 11 | 30.8 | 1.67 (m) 1.71 (m) |
29.3 | 2.10 (m) 2.40 (m) |
29.7 | 1.91 (m) 2.18 (m) |
| 12 | 35.4 | 1.87 (m) 1.88 (m) |
36.0 | 2.21 (m) | 34.3 | 1.78 (m) 1.91 (m) |
| 13 | 85.7 | 86.6 | 85.2 | |||
| 14 | 40.8 | 1.94 (m) | 40.9 | 2.63 (d, 17.2) 2.80 (d, 17.2) |
35.7 | 1.93 (m) 2.15 (m) |
| 15 | 59.2 | 3.70 (m) 3.74 (m) |
175.8 | 61.5 | 4.06 (m) 4.16 (m) |
|
| 16 | 66.9 | 3.50 (d, 11.2) 3.56 (d, 11.2) |
77.9 | 4.23 (d, 9.0) 4.30 (d, 9.0) |
65.4 | 3.40 (d, 11.3) 3.52 (d, 11.3) |
| 17 | 9.9 | 1.21 (d, 6.8) | 8.6 | 1.17 (d, 7.8) | 9.9 | 1.24 (d, 6.8) |
| 18 | 31.7 | 1.20 (s) | 30.6 | 1.19 (s) | 31.7 | 1.20 (s) |
| 19 | 30.6 | 1.21 (s) | 29.8 | 1.26 (s) | 30.6 | 1.21(s) |
| 20 | 24.0 | 1.38 (s) | 23.0 | 1.43 (s) | 24.1 | 1.38 (s) |
| CH3COO | 21.0 | 2.05 (s) | ||||
| CH3COO | 171.1 | |||||
Measured in CDCl3.
Measured in CD3OD.
4.4.2. Leonurenone B (2)
Colorless oil; ; UV (MeOH) λmax (log ε) 243 (2.90) nm; IR (film) νmax 2932, 1781, 1662, 1464, 1372, 1171 cm−1; for 1H NMR (500 MHz) and 13C NMR (125 MHz) spectroscopic data, see Table 1. HRESIMS [M]+ m/z: 332.1988 (calculated for C20H28O4 332.1976).
4.4.3. Leonurenone C (3)
Colorless oil; ; UV (MeOH) λmax (log ε) 231 (2.51) nm; IR (film) νmax 3449, 2929, 1737, 1663, 1466, 1365, 1242, 1042 cm−1; for 1H NMR (500 MHz) and 13C NMR (125 MHz), see Table 1. HRESIMS [M]+ m/z: 378.2407, (calculated for C22H34O5 378.2406).
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Clifford Soll and Dr. Matthew Devany of the Hunter College Mass Spectroscopy and NMR Facilities, respectively, for assistance with data acquisition and to Maha Gaballa and Tilottama Roy of the State University of New York at Buffalo for assistance with molecular analyses.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.phytochem.2012.07.014.
References
- Agnihotri VK, ElSohly HN, Smillie TJ, Khan IA, Walker LA. Constituents of Leonotis leonurus flowering tops. Phytochem Lett. 2009;2:103–105. doi: 10.1016/j.phytol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu E, Amabeoku GJ, Eagles PK, Scott G, Springfield EP. Anticonvulsant activity of aqueous extract of Leonotis leonurus. Phytomedicine. 2002;9:217–223. doi: 10.1078/0944-7113-00103. [DOI] [PubMed] [Google Scholar]
- Chen CX, Kwan CY. Endothelium-independent vasorelaxation by leonurine, a plant alkaloid purified from Chinese motherwort. Life Sci. 2001;68:953–960. doi: 10.1016/s0024-3205(00)00987-5. [DOI] [PubMed] [Google Scholar]
- Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K, Li Y, Li X, Jia X, Lin Y, Leon C. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One. 2010;5:e8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansari MA, Aboutabl EA, Farrag ARH, Sharaf M, Hawas UW, Soliman GM, El-Seed GS. Phytochemical and pharmacological studies on Leonotis leonurus. Pharm Biol (London, U K) 2009;47:894–902. [Google Scholar]
- Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hayashi Y. Studies on the ingredients of Leonurus sibiricus L. II. Structure of leonurine (2) Yakugaku Zasshi. 1962;82:1025–1027. [PubMed] [Google Scholar]
- Hollingsworth PM, Graham SW, Little DP. Choosing and using a plant DNA barcode. PLoS One. 2011;6:e19254. doi: 10.1371/journal.pone.0019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Hussein AA, Meyer MJJ, Rodriguez B. Complete 1H and 13C NMR assignments of three labdane diterpenoids isolated from Leonotis ocymifolia and six other related compounds. Magn Reson Chem. 2003;41:147–151. [Google Scholar]
- Kaplan ER, Rivett DEA. Structures of compounds X and Y, two labdane diterpenoids, from Leonotis leonurus. J Chem Soc C. 1968:262–266. [Google Scholar]
- Kong YC, Yeung HW, Cheung YM, Hwang JC, Chan YW, Law YP, Ng KH, Yeung CH. Isolation of the uterotonic principle from Leonurus artemisia, the Chinese motherwort. Am J Chin Med (Gard City, N Y) 1976;4:373–382. doi: 10.1142/s0192415x76000470. [DOI] [PubMed] [Google Scholar]
- Kruger GJ, Rivett DEA. Diterpenoids of leonotis species. Part 7 The crystal and molecular structure of compound X, a labdane from L leonurus. S Afr J Chem. 1988;41:124–125. [Google Scholar]
- Laonigro G, Lanzetta R, Parrilli M, Adinolfi M, Mangoni L. The configuration of the diterpene spiro ethers from Marrubium vulgare and from Leonotis leonurus. Gazz Chim Ital. 1979;109:145–150. [Google Scholar]
- Lindqvist C, Scheen AC, Bendiksby M, Ryding O, Mathiesen C, Albert VA. Molecular phylogenetics, character evolution, and suprageneric classification of Lamioideae (Lamiaceae) Ann Mo Bot Gard. 2010;97:191–217. [Google Scholar]
- Luo SR. Separation and determination of alkaloids of Leonurus sibiricus. Zhong Yao Tong Bao. 1985;10:32–35. [PubMed] [Google Scholar]
- Maphosa V, Masika PJ. In vivo validation of Aloe ferox (Mill). Elephantorrhiza elephantinaBruch Skeels and Leonotis leonurus (L) R BR as potential anthelminthics and antiprotozoals against mixed infections of gastrointestinal nematodes in goats. Parasitol Res. 2012;110:103–108. doi: 10.1007/s00436-011-2455-8. [DOI] [PubMed] [Google Scholar]
- Maphosa V, Masika PJ, Bizimenyera ES, Eloff JN. In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop Anim Health Prod. 2010;42:301–307. doi: 10.1007/s11250-009-9421-9. [DOI] [PubMed] [Google Scholar]
- Martellossi C, Taylor EJ, Lee D, Graziosi G, Donini P. DNA extraction and analysis from processed coffee beans. J Agric Food Chem. 2005;53:8432– 8436. doi: 10.1021/jf050776p. [DOI] [PubMed] [Google Scholar]
- McKenzie JM, Green IR, Mugabo P. Leonurun, a novel labdane diterpenoid from Leonotis leonurus. S Afr J Chem. 2006;59:114–116. [Google Scholar]
- Naidoo D, Maharaj V, Crouch NR, Ngwane A. New labdane-type diterpenoids from Leonotis leonurus support circumscription of Lamiaceae sl. Biochem Syst Ecol. 2011;39:216–219. [Google Scholar]
- Obikeze KC, McKenzie JM, Green IR, Mugabo P. Characterization and cardiovascular effects of (13S)-9α,13α-epoxylabda-6β(19),15(14)diol dilactone, a diterpenoid isolated from Leonotis leonurus. S Afr J Chem. 2008;61:119–122. [Google Scholar]
- Ohsaki A, Kishimoto Y, Isobe T, Fukuyama Y. New labdane diterpenoids from Hyptis fasciculata. Chem Pharm Bull (Tokyo) 2005;53:1577–1579. doi: 10.1248/cpb.53.1577. [DOI] [PubMed] [Google Scholar]
- Ojewole JA. Antinociceptive, antiinflammatory and antidiabetic effects of Leonotis leonurus (L.) R. BR [Lamiaceae] leaf aqueous extract in mice and rats. Methods Find Exp Clin Pharmacol. 2005;27:257–264. doi: 10.1358/mf.2005.27.4.893583. [DOI] [PubMed] [Google Scholar]
- Oyedemi SO, Yakubu MT, Afolayan AJ. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J Med Plants Res. 2011;5:119–125. [Google Scholar]
- Piozzi F, Bruno M, Rosselli S, Maggio A. Structure and biological activity of the furan-diterpenoids from the genera Leonotis and Leonurus. Heterocycles. 2007;74:31–52. [Google Scholar]
- Rivett DEA. Isolation of marrubiin from Leonotis leonurus. J Chem Soc. 1964:1857–1858. [Google Scholar]
- Scheen AC, Albert VA. Molecular Phylogenetics of the Leucas Group (Lamioideae; Lamiaceae) Syst Bot. 2009;34:173–181. [Google Scholar]
- Scott G, Springfield EP, Coldrey N. A pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm Biol (Lisse, Neth) 2004;42:186–213. [Google Scholar]
- Stafford GI, Pedersen ME, van SJ, Jager AK. Review on plants with CNSeffects used in traditional South African medicine against mental diseases. J Ethnopharmacol. 2008;119:513–537. doi: 10.1016/j.jep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Von Dreele RB, Pettit GR, Ode RH, Perdue RE, Jr, White JD, Manchand PS. The crystal and molecular structure of the unusual spiro dihydrofuran diterpene nepetaefolin. J Am Chem Soc. 1975;97:6236–6240. doi: 10.1021/ja00854a049. [DOI] [PubMed] [Google Scholar]
- White JD, Manchand PS. Structures of nepetaefolin, nepetaefuran, and nepetaefuranol. J Org Chem. 1973;38:720–728. [Google Scholar]
- Zerega NJC, Mori S, Lindqvist C, Zheng Q, Motley TJ. Using amplified fragment length polymorphisms (AFLP) to identify black cohosh (Actaea racemosa) Econ Bot. 2002;56:154–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


