Abstract
Research suggests that lower respiratory sinus arrhythmia (RSA) is associated with greater aversive responding. One physiological indicator of aversive responding is startle potentiation. While a few studies have demonstrated an inverse association between RSA and startle potentiation, no study to date has distinguished whether this relation is similar for predictable versus unpredictable aversive stimuli. This is an important distinction, given that degree of predictability has been shown to be an important determinant of aversive responding. The present study examined whether resting RSA was associated with startle eye blink responding during predictable and unpredictable threat of electric shock. Resting RSA was collected during a 6-minute seated baseline phase at the beginning of the experimental session. Participants then completed a computerized startle task in which predictable and unpredictable shocks were administered. Results indicated that lower resting RSA was associated with greater startle potentiation during unpredictable threat, but not during predictable threat. These findings are consistent with a growing body of literature suggesting that individual differences in RSA are associated with aversive responding, and extend previous work by suggesting that RSA may be more robustly associated with a heightened sensitivity to unpredictable threat. This pattern of results may have implications for the understanding of pathological anxiety given that individuals with anxiety disorders typically exhibit low RSA and heightened responding during unpredictable threat.
Keywords: respiratory sinus arrhythmia, predictability, startle, anxiety
Researchers have long been interested in examining individual differences in affective responding. In particular, there has been considerable emphasis on correlates of aversive emotional responding, including cognitive, behavioral and physiological reactions to threating stimuli (Grillon et al., 2008; Nelson & Shankman, 2011). Importantly, empirical evidence has indicated that specific patterns of aversive responding and enhanced processing of threating information may be underlying mechanisms of dysfunction in those who are vulnerable to anxiety disorders (Davis & Whalen, 2001).
One important physiological factor that may contribute to emotional responding is respiratory sinus arrhythmia (RSA). RSA is the rhythmic fluctuation of heart rate during the respiratory cycle and is considered to be a measure of autonomic vagal control on the heart’s pacemaker (Berntson et al., 1997; Porges, 1995, 2007; Thayer & Lane, 2000). The vagus nerve (i.e., the 10th cranial nerve) regulates the heart through a sophisticated negative feedback loop between afferent and efferent vagal fibers that originate in the brainstem and terminate at the sinoatrial (SA) node of the heart. When there are deficits within this feedback loop, individuals exhibit low RSA or low inhibitory influences on the heart (Porges, 1995, 2003, 2007).
A large body of empirical evidence indicates that low vagal tone is associated with deficits in the regulation of emotional states and increased aversive responding (see Beauchaine, 2001 for a review; Porges, 1995, 2007). For example, lower resting vagal tone has been found in a wide range of psychopathologies, all characterized by affect regulation difficulties, including anxiety disorders, depression, and substance use disorders (Carney et al., 2000; Friedman, 2007; Ingjaldsson, Laberg, & Thayer, 2003). Further, low RSA is associated with an attentional bias toward threat-related stimuli among those with pathological anxiety (Johnsen et al., 2003) and with increased anxiety to phobic stimuli among phobic flyers (Bornas et al., 2005). Moreover, those with lower resting heart rate variability (HRV; an analogous indicator of vagal tone) evidence longer cardiac recovery from an acute psychological stressor relative to those with higher resting HRV (Souza et al., 2007), and have an increased cortisol response to cognitive stressors (Johnsen, Hansen, Sollers, Murison, & Thayer, 2002). Taken together, accumulating evidence suggests that individual differences in resting RSA may index aversive and anxious responding.
Recently, research has begun to explore the relation between resting vagal tone and the human startle response (an index of defensive responding; Bradley, Cuthbert, & Lang, 1999; Lang, 1995). Ruiz-Padial, Sollers, Vila, and Thayer (2003) examined the association between resting vagal tone and startle responses while viewing neutral, pleasant, and unpleasant pictures. Results indicated that low vagal tone was associated with heightened startle potentiation during all three affective foregrounds and during task intertrial intervals (i.e., when no pictures were present). A more recent report by Melzig, Weike, Hamm, and Thayer (2009) expanded on these findings by examining the relation between resting HRV and startle reactivity during threat-of-shock. The authors found that lower baseline HRV was associated with greater startle responding during threat-of-shock, but there were no differences between individuals with high and low HRV during the safe periods. Therefore, in contrast to the results of Ruiz-Padial et al., the results of Melzig et al. suggested that low vagal tone was associated with threat-potentiated startle, rather than with startle responding more broadly.
Although Melzig et al. (2009) demonstrated an important relationship between vagal tone and threat-potentiated startle, their threat-of-shock manipulation may not have been able to address the distinction between responses to predictable versus unpredictable threat. There is a growing literature suggesting that predictable and unpredictable threat can elicit qualitatively distinct aversive states. Specifically, predictable aversive stimuli have been shown to elicit a phasic response to an identifiable stimulus (often labeled fear), while unpredictable aversive stimuli elicit a generalized feeling of apprehension not associated with a clearly identifiable stimulus (often labeled anxiety; Davis, 1998; Barlow, 2000). These responses have been shown to be pharmacologically distinct (Grillon et al., 2006; Grillon et al., 2011) and mediated by overlapping, but separable, neural circuits (Alvarez et al., 2011; Davis, 2006). Additionally, several studies have shown that compared to healthy controls, those with panic disorder (Grillon et al., 2008) and posttraumatic stress disorder (PTSD; Grillon et al., 2009) evidence heightened potentiated startle in response to unpredictable, but not predictable, threat. These clinical studies suggest that a heightened sensitivity to unpredictable threat (rather than threat in general) characterizes those with pathological anxiety.
In Melzig et al.’s (2009) task, participants were told that different colored slides represented safe periods where no shocks would be delivered (i.e., blue slides) or threat-of-shock periods in which 1-3 shocks would be delivered (i.e., yellow slides). Therefore, the threat-of-shock slides contained both predictable features (i.e., participants knew they would receive at least 1 shock), but also unpredictable features (i.e., participants did not know how many shocks or at which time point the shocks would be delivered). As such, this design confounds predictable and unpredictable features of aversive stimuli, and leaves the relation between resting vagal tone and startle to predictable versus unpredictable aversive stimuli unexplored.
The current study examined whether resting RSA was associated with startle responding during the anticipation of temporally predictable and unpredictable aversive stimuli. In addition, the current study aimed to expand prior investigations by utilizing a significantly larger sample size (N = 126), and exploring the specificity of any associations by examining the relation between heart period (HP; a measure of cardiac functioning that is regulated by both the mylinated and unmylinated vagus) and startle potentiation. Given that previous research has demonstrated that individuals with pathological anxiety evidence low RSA (Blom, Olsson, Serlachius, Ericson, & Ingvar, 2010; Jovanovic, Norrholm, Sakoman, Esterajher, & Kozarić-Kovačić, 2009) and heightened potentiation to unpredictable threat (Grillon et al., 2009; Grillon et al., 2008), we hypothesized that low RSA would be associated with greater startle potentiation during unpredictable threat. Due to the limited empirical findings regarding the relation between RSA and predictable aversive events, we did not have any specific hypotheses concerning predictable threat and considered this to be an exploratory aim.
Methods
Participants
One hundred and twenty-six introductory to psychology students from a large university participated for course credit. Participants were 61.6% female with an average age of 19.1 years (SD = 1.5). The sample was ethnically diverse with 36.6% Caucasian, 27.7% Latino, 20.5% Asian, 8.0% African-American, and 7.1% “Other.” Participants were excluded from the study if they were unable to read or write English, had a history of head trauma, were left-handed (confirmed by the Edinburgh Handedness Inventory, range of laterality quotient: +20 to +100; Oldfield, 1971), or were currently taking benzodiazepines, which have been shown to dampen startle to unpredictable threat (Grillon et al., 2006). Informed consent was obtained prior to participation and the protocol was approved by the local Institutional Review Board.
Stimuli and Physiological Recordings
All stimuli were administered using PSYLAB (Contact Precision Instruments, London, UK) and electrocardiogram (ECG) and electromyography (EMG) measures were acquired using Neuroscan 4.4 (Compumedics, Charlotte, NC). The acoustic startle probe was a 40-ms duration, 103-dB burst of white noise with near instantaneous rise time presented binaurally through Kensington Hi-Fi headphones (model K33137). Two 4-mm Ag/AgCl electrodes were placed over the orbicularis oculi muscle below the right eye for startle blink measurement. As per published guidelines (Blumenthal et al., 2005), one electrode was placed 1-cm below the pupil and the other electrode was placed 1-cm lateral. ECG electrodes were placed on the participant’s sternum and below the left clavicle to record heart rate. EMG and ECG data were collected using a bandpass filter of DC-200-Hz at a sampling rate of 1000-Hz. The ground electrodes were placed along the midline of the anterior scalp and a noise cancellation reference was located on the scalp between electrodes CZ and CPZ electrodes of an electroencephalography (EEG) cap that participants were wearing as part of the protocol for the larger study (EEG data not presented).
The electric shocks lasted 400-ms and were administered to the wrist of the participants’ (non-dominant) left hand. The maximum shock level a participant could achieve was 5 mA, and within our sample the mean shock level was 2.30 mA (SD = 1.2).
Procedure
After electrode placement, participants were seated in an electrically-shielded, sound-attenuated booth approximately 3.5-ft from a 19-in computer monitor where visual stimuli were presented. Next, baseline ECG data was collected during alternating 90-second, eyes open (O) and eyes closed (C) recording conditions for a total of 6 minutes (counterbalanced: OCCO vs. COOC). Participants were instructed to alternate between eyes open and eyes closed conditions because resting EEG data was being simultaneously collected as part of the larger protocol. Afterwards, two electrodes were placed on the participants’ wrist of their left hand for administration of the electrical shocks. In order to prevent early exaggerated startle responses, participants completed a 2.5-minute baseline habituation task in which 9 acoustic startle stimuli were presented. Next, a shock work-up procedure, consistent with prior studies (e.g., Grillon et al., 2004), was completed in which participants received increasing levels of shock intensity until they reached a level that they described as feeling “highly annoying but not painful.” Shock level was determined ideographically to be consistent with prior studies (e.g., Grillon et al., 2004), and given the large individuals differences in perceived aversiveness to shocks (Rollman & Harris, 1987), to ensure that subjects experienced the shocks as equally aversive. Following the shock work up, the shock task (described below) was administered for a total of 10 minutes.
The threat-of-shock task was modeled after that used by Grillon and colleagues (i.e., NPU-threat task; see Schmitz & Grillon, 2012) and included three within-subjects conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informed participants of the current threat condition by displaying the following information: “no shock” (N), “shock at 1” (P), or “shock at anytime” (U). Each condition lasted 90-s, during which a 6-s visual countdown (CD) was presented five times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 7 to 17-s (M = 12.4-s) during which only the text describing the condition was on the screen. During the N condition, no shocks were delivered. During the P condition, participants received a shock every time the CD reached 1. During the U condition, shocks were administered at any time (i.e., during the CD or ISI). Startle probes were presented both during the CD (1-5-s following CD onset) and ISI (5-14-s following ISI onset). The time interval between a shock and the following startle probe was always greater than 10-s, which ensured that the subsequent startle response was never affected by an immediately preceding shock.
The task consisted of two presentations of each 90-s condition (N, P, U) during which the CD appeared five times. Conditions were presented in one of two following orders (counterbalanced): PNUPNU or UNPUNP. All participants received 16 electric shocks (8 during P and 8 during U), and 36 startle probes (12 during N, 12 during P, and 12 during U) during the countdown and ISI (with an equal number of startle probes occurring during the CDs and ISIs).
Before and after the task, participants completed the Positive Affect and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988), to assess positive and negative affect pre and post NPU-threat task. In addition, participants rated their level of anxiousness during the countdown and ISI for each condition on a scale ranging from 1 (Not at all) to 7 (Extremely). Participants also rated how intense, annoying, and anxiety provoking they found the shocks to be, on a scale ranging from 1 (Not at all) to 7 (Extremely), and the degree to which they would avoid the shocks, on a scale ranging from 1 (Would definitely not avoid) to 7 (Would definitely avoid).
RSA Data Processing
Data were initially processed using QRSTool (Allen et al., 2007) and all artifacts were identified and corrected by hand. After initial correction, inter-beat-interval (IBI) series were extracted for each 90-s recording block. Each block was then visually inspected using CardioEdit (Brain-Body Center, University of Illinois at Chicago) and any additional artifacts were corrected by hand. After data were processed, average RSA was calculated based on methods developed by Porges and Bohrer (1990), using CardioBatch (Brain-Body Center, University of Illinois at Chicago). Specifically, IBIs were resampled into 500 ms intervals to produce time-based data and a 21-point moving polynomial cubic filter was stepped through the time-based data to produce a smoothed template series. The template was subtracted from the original time series and a digital bandpass filter was applied to this detrended time series to extract variance in the frequency band associated with spontaneous breathing in adults. The amplitude of RSA was then calculated using the natural logarithm (ln) of the bandpassed time series. Recent evidence has demonstrated that short segments of IBI data (i.e., 90-s blocks) are suitable for the calculation of RSA (Lewis, Furman, McCool, & Porges, 2011). Of note, respiration was not controlled or measured in the present study. Neither HP nor RSA were skewed or kurtotic, and thus did not require transformation.
Startle Data Processing
Blinks were scored according to guidelines provided by Blumenthal et al. (2005). Offline, a 28-Hz high-pass filter was applied to the startle data, followed by rectification and smoothing of the signal using a 40-Hz FIR low-pass filter. Peak amplitude of the blink reflex was defined within the 20-150-ms time frame following the startle probe onset relative to baseline (average baseline EMG level for the 50-ms preceding the startle probe onset). Each peak was first identified by software but then hand scored by an experimenter blind to condition and cue to ensure acceptability (e.g., correct peak marked). All experimenters were trained to criterion by the PI of the study (S.A.S.) and reliability checks were done on approximately 20% of all data (corrections were rare). Blinks were scored as non-responses if EMG activity during the 20-150-ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Blink amplitude1 (i.e., average of acceptable blinks only) was used in all analyses. Blinks were standardized within-subjects using a T-score transformation, which reduces the influence of outlier blink responses.
To ensure data quality, individuals were excluded from analyses if they were missing startle data (N = 3), produced less than 50% usable blinks in two or more conditions (N = 4), had T-scored startle amplitude values greater than two standard deviations above the mean in any condition (N = 5), or produced unusable or contaminated RSA data (N = 4). The final sample size was 110 participants.
Data Analysis Plan
First, we examined whether there were any differences in resting RSA during the eyes open versus the eyes closed recording blocks using a repeated measures analysis of variance (ANOVA) with the average of the two eyes open blocks and the two eyes closed blocks entered as within-subjects factors. Next, we examined whether there were any differences in RSA across time by conducting a repeated measures ANOVA with Time (90-s blocks 1, 2, 3, and 4) entered as a within-subjects factor. Pearson’s correlations were also used to test the rank-order stability of participants’ RSA across each of the 4 recording blocks. Finally, we examined whether the NPU-threat task had the intended effect on startle amplitude using a Condition (N, P, U) × Cue (ISI vs. CD) general linear model. We expected to find that startle amplitude would be higher during threat-of-shock (i.e., PCD, UISI, UCD) compared with no threat-of-shock (i.e., NCD, NISI, PISI). To determine whether RSA was associated with startle responses during the NPU-threat task, we conducted a Condition (N, P, U) × Cue (ISI vs. CD) × RSA general linear model with Condition and Cue entered as within-subjects factors and RSA entered as a (mean-centered) between subjects variable. We also included pre-task negative affect as a covariate to control for baseline differences in mood. We predicted that those with low RSA would have greater startle responses during the U condition relative to the P condition, and greater startle responses during the P condition relative to the N condition. This would result in a linear effect across the N, P, and U conditions. We also expected this effect to vary as a function of Cue as previous studies using the NPU-threat task have demonstrated that the Cue (i.e., the CD) often becomes paired with the phasic fear response elicited during the P condition (Grillon et al., 2008; Grillon et al., 2004). Thus, because the task is within-subjects, once individuals are exposed to the CD during the first block of the P condition, there would likely be some carry-over effects from the CD phase of the P condition to the CD phase of the other conditions. In contrast, the ISI never reliably signals information during the task and does not become paired with an affective response. Responding during the ISI is therefore more dependent on the condition (N, P, or U) and is less contaminated by the design of the task. Given that we hypothesized that low RSA would be associated with anxious responding, we specifically expected to find this association during the UISI since it is the least confounded elicitor of contextual anxiety. As such, we expected a significant linear Condition × Cue × RSA interaction.
To follow-up a Condition × Cue × RSA interaction, we first determined whether RSA was correlated with responses during the N condition (i.e., whether there were baseline differences in startle between those with high versus low RSA). Results indicated that RSA was not significantly correlated with startle during the NISI (r = .07, ns) or NCD (r = −.03, ns). Therefore, we created ‘potentiation’ scores (see Moberg & Curtin, 2009) in which responses during the NISI were subtracted from the PISI and UISI (i.e., PISI - NISI and UISI - NISI) and responses during the NCD were subtracted from the PCD and UCD (i.e., PCD - NCD and UCD - NCD). Potentiation scores are useful for controlling for baseline differences in startle responding during the task. Finally, we conducted partial Pearson’s correlations between RSA and P and U potentiation, while controlling for pre-task negative affect.
Two additional analyses were conducted to also examine 1) whether RSA was associated with self-reported anxiety and 2) whether HP (i.e., average R-R interval in ms) was associated with startle responding. These secondary analyses were similar to those outlined above except that self-reported anxiety was used as the dependent variable in the first model, and HP (and not RSA) was included as the between subjects variable in the second model. Pre-task negative affect was included as a covariate in both analyses. This allowed us to examine the specificity of our findings to startle responding and/or RSA (i.e., vagal influences on heart rate).
Results
Baseline RSA
There were no significant differences in RSA when eyes were open compared to when eyes were closed, F(1, 109) = 0.07, ns. Therefore, RSA scores were collapsed across eyes open and closed phases. Results indicated that RSA significantly decreased across the four recording periods, F(3, 327) = 16.79, p < .001, ηp2 = .13. This natural decrease in RSA over the experimental session may be attributed to sustained attention or anticipation of the NPU-threat task (Hofmann et al., 2005; Suess, Porges, & Plude, 1994). Notably, Pearson’s correlations among the 4 blocks revealed high rank-order stability of participants’ RSA (all r’s between .77 and .91, all p’s < .001). As such, all four blocks were averaged to create a single composite RSA variable for subsequent analyses.
Shock Aversiveness and Affect Ratings
Participants rated the shocks as moderate to extremely intense (range = 2-7, M = 4.57, SD = 1.1), annoying (range = 2-7, M = 5.88, SD = 1.2), and anxiety provoking (range = 3-7, M = 4.95, SD = 1.4). Most participants also indicated that they would avoid receiving the shocks again to a moderate degree (range = 1-7, M = 5.25, SD = 1.6). These results indicate that the shocks were generally perceived as aversive. Paired sample t-tests indicated that negative affect significantly increased from pre-task (range = 10-30, M = 14.33, SD = 4.5) to post-task (range = 10-40, M = 16.44, SD = 5.9), t(111) = -3.94, p < .001. However, there was no change in positive affect pre-task (range = 10-44, M = 25.51, SD = 7.8) to post task (range = 11-46, M = 25.00, SD = 8.4), t(111) = 0.80, ns. RSA was not associated with any of the self-report shock ratings or affect ratings (all p’s > .24).
NPU-Threat Task Manipulation Check
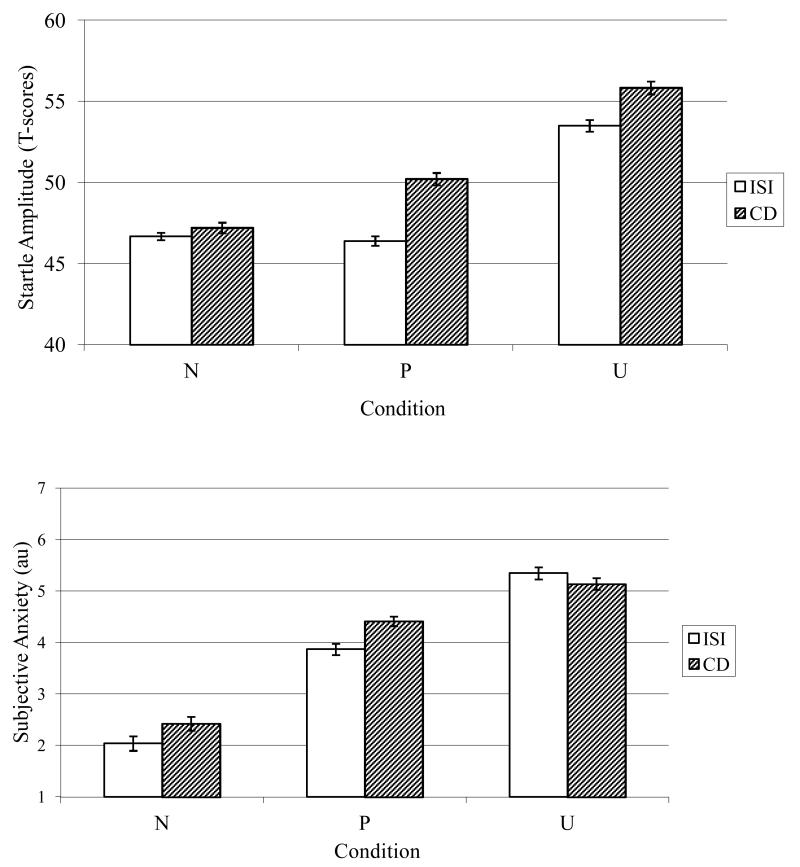
Figure 1 displays means (and standard errors) for startle amplitude across conditions and cues. Results indicated main effects for Condition, F(1, 109) = 102.19, p < .001, ηp2 = .48, and Cue, F(1, 109) = 46.71, p < .001, ηp2 = .30, that were qualified by a Condition × Cue linear interaction, F(1, 109) = 11.48, p < .01, ηp2 = .10. The Condition × Cue interaction was followed-up by conducting separate repeated measures ANOVAs for each level of Cue (i.e., CD and ISI; see Table 1). During the ISI, startle amplitude differed among the conditions such that startle was significantly greater during UISI relative to PISI and NISI. However, startle during PISI did not significantly differ from startle during NISI. Startle amplitude during the CD also differed among the conditions, due to greater startle during PCD and UCD relative to the NCD, and greater startle during UCD relative to PCD. Taken together, this pattern of results suggests that startle was greater during threat-of-shock and that the task had the intended effect on startle amplitude.
Figure 1.
Mean startle amplitude (top) and subjective anxiety (bottom) at different levels of condition and cue; Error bars represent standard error; N = Neutral, P = Predictable, U = Unpredictable; ISI = Interstimulus Interval; CD = Countdown.
Table 1.
Results from analyses examining differences in startle amplitude T-scores during each level of Condition by Cue
| Comparison | F | df | P | η p 2 |
|---|---|---|---|---|
| NISI vs. PISI | 0.08 | 1, 109 | .78 | < .01 |
| NISI vs. UISI* | 267.91 | 1, 109 | < .001 | .71 |
| PISI vs. UISI* | 191.34 | 1, 109 | < .001 | .64 |
| NCD vs. PCD* | 32.90 | 1, 109 | < .001 | .32 |
| NCD vs. UCD* | 231.92 | 1, 109 | < .001 | .68 |
| PCD vs. UCD* | 79.39 | 1, 109 | < .001 | .42 |
Note.
p < .001;
N = Neutral condition, P = Predictable condition, U = Unpredictable condition; ISI= Interstimulus Interval; CD = Countdown.
Resting RSA and Startle during NPU-Threat Task
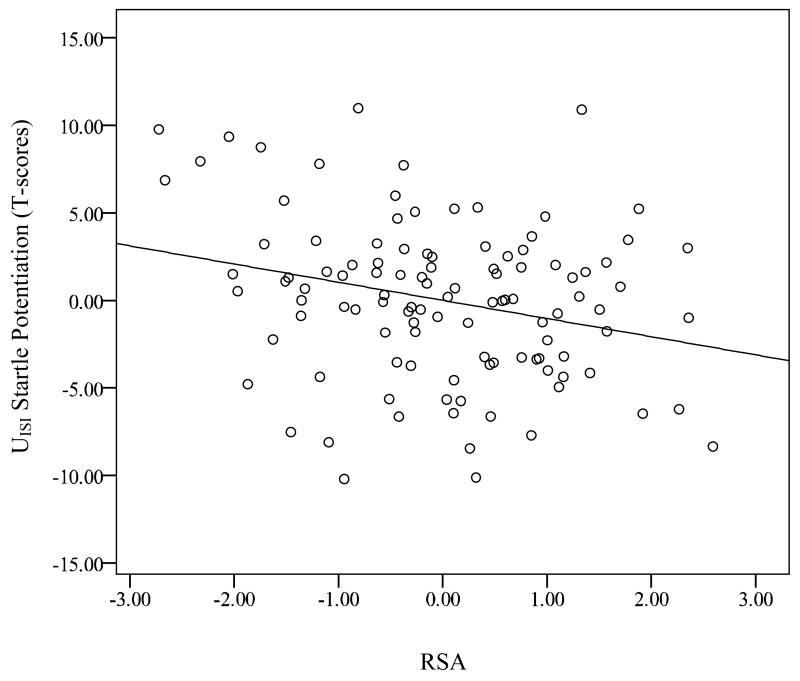
Results indicated no main effect of RSA, F(1, 107) = 0.01, ns. The main effect of pre-task negative affect was a trend, such that individuals with greater negative affect exhibited a trend for greater overall startle responding during the task, F(1, 107) = 3.53, p = .06, ηp2 = .04. There were no significant Condition × RSA, F(1, 107) = 2.17, ns, or Cue × RSA interactions, F(1, 107) = 0.91, ns. Consistent with the aforementioned analyses, there was a significant Condition × Cue interaction, F(1, 107) = 9.13, p < .01, ηp2 = .08. Importantly, there was also a Condition × Cue × RSA linear interaction2-4, F(1, 107) = 4.12, p = .04, ηp2 = .04. Follow-up partial correlation analyses, controlling for pre-task negative affect, with startle potentiation scores (i.e., PCD - NCD, UCD - NCD, PISI - NISI, and UISI - NISI) indicated that resting RSA was negatively associated with startle potentiation during the UISI (r = −.27, p = .01; see Figure 2). In contrast, RSA was not associated with startle potentiation during the UCD (r = −.01, p = .93), PCD (r = .14, p = .16), or PISI (r = .08, p = .44).
Figure 2.
Partial regression plot displaying the correlation between resting RSA and startle potentiation during UISI, while controlling for pre-task negative affect; UISI potentiation scores were created by subtracting startle amplitude T-scores during NISI from UISI; RSA = Respiratory sinus arrythymia; N = Neutral condition; U = Unpredictable condition; ISI = Interstimulus Interval.
Resting RSA and Self-Reported Anxiety during NPU-Threat Task
There was no main effect of RSA, Condition × RSA, Cue × RSA, or Condition × Cue × RSA interaction for self-reported anxiety responses (all p’s > .43).
Resting HP and Startle during NPU-Threat Task
There was no main effect of HP, Condition × HP, Cue × HP, or Condition × Cue × HP interaction (all p’s > .18).
Discussion
Prior investigations have demonstrated that individual differences in RSA are associated with aversive responding (Bornas et al., 2005; Johnsen et al., 2001; Souza et al., 2007). However, to date research has not attempted to distinguish between responses to predictable versus unpredictable threat-a noteworthy omission given that separable aversive states are elicited by each type of threat (Davis, 1998, 2006). The aim of the current study was therefore to examine the relation between resting RSA and startle responses to predictable and unpredictable threat-of-shock.
Although it has been suggested that lower resting RSA may be associated with overall heightened threat sensitivity (Melzig et al., 2009), our findings suggest that resting RSA may differentially relate to predictable and unpredictable threat. More specifically, our results indicated that lower resting RSA was significantly associated with greater startle potentiation during unpredictable threat, but was not associated with startle potentiation during predictable threat. Our results also suggest that the current findings are specific to the mylineated vagal influences on the heart’s pacemaker given that there was no relation between HP and startle responding. This pattern of results suggests that low RSA may be associated with greater sensitivity to unpredictable threat.
In one of the few other studies that have examined the relation between startle and RSA, Melzig et al. (2009) found that lower resting RSA was associated with potentiated startle during a threat-of-shock task which contained predictable and unpredictable features. Specifically, the threat-of-shock condition within that study was designed such that participants knew they would receive at least 1 shock during a conditioned stimulus (CS), but did not know how many shocks or at which time point the shocks would be delivered during the CS. Markedly, their threat condition was similar to the current study’s unpredictable condition given that, across both studies, participants were told they could receive shocks, but the timing of the shocks were unknown. Thus, our finding that low RSA was associated with heightened startle potentiation during the unpredictable condition is consistent with their results. The Melzig et al. (2009) study, however, did not have a completely predictable condition and within the extant literature, it was unclear whether RSA would be associated with startle responding to predictable aversive stimuli. Our results are the first to address this question and suggest that RSA is more robustly associated with sensitivity to unpredictable threat and not predictable threat.
There are several potential mechanisms through which low RSA may influence aversive responding to unpredictable threat. RSA is often considered an index of self-regulation, with lower levels of vagal tone indicating more difficulties regulating stress, emotional states, and attention (Friedman, 2007; Friedman & Thayer, 1998; Porges et al., 1996; Porges et al., 1994), and a vulnerability to sustained and prolonged dysregulation (Berntson, Sarter, & Cacioppo, 1998). Importantly, several theoretical models (e.g., polyvagal theory, neurovisceral integration model) suggest that the vagus, in coordination with neural circuits including the prefrontal cortex and anterior cingulate cortex, plays a key role in the modulation of emotion (Porges, 2007; Thayer & Lane, 2009). Given that unpredictable threat elicits heightened contextual anxiety (i.e., sustained apprehension in response to the experimental context; Grillon et al., 2004), it is possible that individuals with low RSA have difficulty regulating their anxiety, and thus evidence heightened aversive responding.
Given the specificity of the current findings, it is important to consider the role of unpredictability and RSA in human anxiety disorders. Although anxiety disorders are heterogeneous, unpredictability is considered an important component of the development and maintenance of several disorders (Craske, Glover, & DeCola, 1995; Foa, Zinbarg, & Rothbaum, 1992). For example, a hallmark feature of panic disorder is the experience of recurrent, unpredictable panic attacks and persistent apprehension of future attacks (American Psychiatric Association [APA], 1994). Similarly, PTSD is thought to be the result of unpredictable traumatic events and is associated with sustained heightened arousal (APA, 1994; Foa et al., 1992; Mineka & Zinbarg, 2006). Several studies have also shown that panic disorder and PTSD are associated with heightened startle potentiation during unpredictable, but not predictable, threat (Grillon et al., 2009; Grillon et al., 2008). As such, some have suggested that contextual anxiety, not explicit fear, is what differentiates clinically anxious from non-anxious individuals (Grillon et al., 2008; Pole, Neylan, Best, Orr, & Marmar, 2003). Given that the current study demonstrated an association between low RSA and heightened startle during the unpredictable condition, our findings suggest that deficits in vagal functioning may relate to anxious responding and could potentially contribute to the onset or maintenance of anxiety. However, future research is needed to replicate the present findings and explore the directionality between low RSA and anxious states.
In the present study, although both the CD and ISI during the unpredictable condition had the same meaning (i.e., participants could be shocked at any time), our results indicated that RSA was associated with startle during the UISI but not the UCD. There are several reasons for this finding. First, as was previously discussed, it is possible that the CD became at least partially paired with the phasic fear response elicited during the predictable condition which resulted in carry-over effects into the other conditions (Macfie, Bratchell, Greenhoff, & Vallis, 1989). Indeed, the results yielded a significant main effect of Cue, F (1, 109) = 46.71, p < .001. Notably, other studies utilizing a similar design have also reported carry-over effects during the startle task (Grillon et al., 2008; Grillon et al., 2004). Because the ISI never reliably signaled information during the task, responding during the ISI was more dependent on the condition (N, P, or U), and the UISI was likely a more potent elicitor of contextual anxiety than the UCD. This pattern of results further suggests that RSA may be more robustly associated with response to contextual anxiety.
A second (and related) reason for the different results for CD and ISI may relate to the difference in ‘situational strength’ between the CD and ISI. Situational strength has long been identified as an important factor that moderates the relationship between individual differences and behavior (Caspi & Moffitt, 1993; Cooper & Withey, 2009; Mischel, 1977). Strong situations provide unambiguous stimuli that generally yield uniform reactions across individuals. Weak situations are more ambiguous events that attenuate the influence of the situation and increase the contribution of individual differences (such as RSA) on subsequent responses. Within the present study, the CD is considered a strong situation since an unambiguous cue (i.e., when the CD reaches “1”) was reliably associated with receiving a shock in the predictable condition (Cooper & Withey, 2009; Lissek Pine, & Grillon, 2006; Mischel, 1977). However, the ISI did not signal reliable information and was relatively ambiguous in all three conditions, resulting in a weaker situation. Given this distinction, RSA may have played more of a role in responding during the ISI (a weak situation), relative to the CD (a strong situation).
Even though the task was effective in inducing changes in self-reported anxiety, our results indicated that there was no significant relation between RSA and self-reported anxiety. This was somewhat unexpected given that previous studies have reported an association between RSA and self-reported emotional states during laboratory tasks (Butler, Wihelm, & Gross, 2006; Bornas et al., 2005; Kettunen, Ravaja, Näätänen, & Keltikangas-Järvinen, 2000). One possible explanation for this discrepancy is that the wording of the self-report measure of ‘anxiety/nervousness’ may have assessed participants’ level of arousal and not valence. This distinction is important given that startle responding has been shown to be sensitive to valence and not arousal (Lang, Greenwald, Bradley, & Hamm, 1993). Startle and self-report may have therefore indicated slightly different constructs. Another possibility is that RSA and startle responding are drawn from the same response system (i.e., physiology), while RSA and self-reported anxiety are drawn from different response systems (i.e., self-report vs. physiology). Several studies have demonstrated that the convergence between self-report and physiological measures of emotion are often weak (see Mauss & Robinson, 2009), and this may have limited our ability to detect a significant association between RSA and self-report. A third possibility is that the high ratings of self-reported anxiety elicited by NPU-threat paradigm induced somewhat of a ceiling effect. When participants were asked to report how anxiety provoking they found the shocks on a scale of 1 (Not at all) to 7 (Extremely), 64.5% indicated scores greater than or equal to 5 during the threat-of-shock conditions. Therefore, within the current study, there was a tendency for the majority of individuals to report high levels of anxiety during the predictable and unpredictable threat conditions.
Although these findings address important gaps within the literature, the present study had several limitations. First, the correlation between RSA and the UISI condition was not robust, and would not withstand correction for multiple comparisons. The present findings should therefore be interpreted with caution. It is possible that our use of a non-clinical sample may have restricted the variance of psychophysiological responding and thus, prevented the detection of large effects. Nonetheless, the current findings are informative and provide important information to aid in the future examination of these processes. Second, we did not assess lifetime psychopathology and the potential impact of psychiatric symptoms is unknown. Third, our sample consisted of undergraduate college students and findings may not be generalizable across all populations. However, because RSA has been shown to decrease with age (Yeragani et al., 1994), it was important to examine the effects of RSA in a more homogenous sample. Fourth, although RSA has demonstrated moderate to high test-retest reliability (.59-.92) ranging from 3 days to 3 years (Boomswa, van Baal, & Orlebeke, 1990; Goedhart, Van Der Sluis, Houtveen, Wilemsen, & Geus, 2007), it has been shown to be influenced by several state factors (Casadei, Moon, Johnston, Caiazza, & Sleight, 1996; Porges, 2001, 2007). Therefore, RSA is not necessarily a trait-like factor, which may have contributed to our inability to detect large effects. Lastly, within the extant literature, there is considerable debate about the influence of respiration on the quantification of RSA (see Denver, Reed, & Porges, 2007; Porges, 2007; but also see Grossman, Karemaker, & Wieling, 1990; Grossman & Taylor, 2007; Houtveen et al., 2002). Several studies have demonstrated that ignoring indices of respiration leads to biased or inaccurate measures of cardiac vagal tone (e.g., Grossman & Taylor, 2007), while others studies have presented data refuting these conclusions (e.g., Denver et al., 2007). Within the present study, we did not collect respiration data and thus, we are unable to assess the potential impact of respiration on the present indices of RSA.
There are several important implications of these findings. First, our results add to a growing body of literature noting that individual differences in resting RSA are associated with aversive responding (Bornas et al., 2005; Melzig et al., 2009; Souza et al., 2007). Further, these findings extend prior research by suggesting that low RSA may be more robustly associated with heightened responding during contextual anxiety/unpredictable threat. This effect is noteworthy given that it has been suggested that those with anxiety disorders have a heightened response to unpredictability and low vagal tone is thought to characterize those with anxiety disorders (Cohen et al., 2000; Friedman, 2007). Future research is needed to explore the potential role of RSA in the relation between anxiety disorders and sensitivity to unpredictable threat.
Acknowledgement
This work was supported by National Institute of Mental Health R21 MH080689, K08MH083888, and UIC Chancellor’s Discovery Fund for Multidisciplinary Research awarded to S.A.S.
Footnotes
A similar pattern of results were found when using raw amplitude and magnitude T-scores (i.e., when non-responses of 0 amplitude were included in condition averages).
In the present study, we tested for linear effects. However, the overall repeated measures ANOVA indicated a weak trend for a Condition × Cue × RSA interaction, F(2, 216) = 1.88, p = .16, ηp2 = .02.
When all individuals taking psychiatric medications were excluded from the analyses (N = 10), the Condition × Cue × RSA interaction became a trend, F(1, 97) = 3.75, p = .06, ηp2 = .04, although the partial correlation between RSA and UISI potentiation remained significant (r = −.27, p = .01).
There was no effect of gender in the present analyses. When gender was included as a covariate, the Condition × Cue × RSA interaction remained significant, F(1, 107) = 4.28, p = .04, ηp2 = .04, as did the correlation between RSA and startle amplitude during UISI (r = −.27, p = .006).
References
- Allen JJB, Chambers AS, Towers DN. The many metrices of cardiac chronotrophy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;1:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Cacioppo JT, Eckberg DL, Grossman P, Kaufmann PG, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behavioural Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Blom EH, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99:604–611. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Boomsma DL, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta Geneticae Medicae Gemellologiae. 1990;39:181–191. doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- Bornas X, Liabres J, Noguera M, Lopez AM, Barcelo F, Tortella-Feliu M, Fullana MA. Self-implication and heart rate variability during simulated exposure to flight-related stimuli. Biological Psychology. 2005;70:182–187. doi: 10.1016/j.biopsycho.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ, Dawson ME, Schell AM, Bohmet AH. Startle modification: Implication for neuroscience, cognitive science, and clinical science. Cambridge University Press; New York: 1999. Affect and the startle reflex. [Google Scholar]
- Butler EA, Wihelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosomatic Medicine. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Casadei B, Moon J, Johnston J, Caiazza A, Sleight P. Is respiratory sinus arrhythmia a good index of cardiac vagal tone in exercise? Journal of Applied Physiology. 1996;81:556–564. doi: 10.1152/jappl.1996.81.2.556. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. When do individual differences matter? A paradoxical theory of personality coherence. Psychological Inquiry. 1993;4:247–271. [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Cooper WH, Withey MJ. The strong situation hypothesis. Personality and Social Psychology Review. 2009;13:62–72. doi: 10.1177/1088868308329378. [DOI] [PubMed] [Google Scholar]
- Craske MG, Glover D, DeCola J. Predicted versus unpredicted panic attacks: Acute versus general distress. Journal of Abnormal Psychology. 1995;104:214–223. doi: 10.1037//0021-843x.104.1.214. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:12–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biological Psychology. 2007;74:286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J, Geyer MA, Braff DL. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biological Psychology. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychological Bulletin. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Anxiety and autonomic flexibility: A cardiovascular approach. Biological Psychology. 1998;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- Goedhart AD, Van Der Sluis S, Houtveen JH, Wilemsen G, Geus EJC. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology. 2007;44:203–215. doi: 10.1111/j.1469-8986.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–204. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Heller R, Hirschhorn E, Kling MA, Pine DS, Schulkin J, Vythilingam M. Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biological Psychiatry. 2011;69:549–555. doi: 10.1016/j.biopsych.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1990;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim H, Davis LL, Pizzagalli DA. The worried mind: Autonomic and prefrontal activation during worrying. Emotion. 2005;5:464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, De Geus EJC. Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiration depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39:427–436. doi: 10.1017.S0048577202394022. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Johnsen BH, Hansen AL, Sollers JJ, Murison R, Thayer JF. Heart rate variability is inversely related to cortisol reactivity during cognitive stress. Psychosomatic Medicine. 2002;64:289. [Google Scholar]
- Johnsen BH, Thayer JF, Laberg JC, Wormnes B, Raadal M, Skaret E, Berg E. Attentional and physiological characteristics of patients with dental anxiety. Journal of Anxiety Disorders. 2003;17:75–87. doi: 10.1016/s0887-6185(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovačić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen J, Ravaja N, Näätänen P, Keltikangas-Järvinen L. The relationship of respiratory sinus arrhythmia to the co-activation of autonomic and facial responses during the Rorschach test. Psychophysiology. 2000;37:242–250. [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biological Psychology. 2011;89:349–364. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Macfie HJ, Bratchell N, Greenhoff K, Vallis LY. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. Journal of Sensory Studies. 1989;4:129–148. [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: A review. Cognition and Emotion. 2009;23:2–209. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: Implications for panic disorder. International Journal of Psychophysiology. 2009;71:109–117. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Mischel W. The interaction of person and situation. In: Magnusson D, Endler NS, editors. Personality at the crossroads: Current issues in interactional psychology. Lawrence Erlbaum Associates; Hillside, NJ: 1977. [Google Scholar]
- Mineka S, Cook M, Miller S. Fear conditioned with escapable and inescapable shock: Effects of a feedback stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:307–323. [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable vs. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Best SR, Orr SP, Marmar CR. Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. Journal of Traumatic Stress. 2003;16:471–479. doi: 10.1023/A:1025758411370. [DOI] [PubMed] [Google Scholar]
- Porges SW, Bohrer RE. Analyses of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Press; New York, NY: 1990. [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Roots of mental illness in children. Annals of the New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. In: Fox NA, editor. Monographs of the Society for Research in Child Development. Blackwell Publishing; Boston: 1994. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Development and Psychopathology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Raskin DC. Respiratory and heart rate components of attention. Journal of Experimental Psychology. 1969;81:497–503. doi: 10.1037/h0027921. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discrimability, and perceived magnitude of painful electrical shock. Perception & Psychophysics. 1987;42:257–268. doi: 10.3758/bf03203077. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Sollers JJ, Vila J, Thayer JF. The rhythm of the heart in the blink of an eye: Emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology. 2003;40:306–313. doi: 10.1111/1469-8986.00032. [DOI] [PubMed] [Google Scholar]
- Sargunaraj D, Lehrer PM, Hochron SM, Rausch L, Edelberg R, Porges SW. Cardiac rhythm effects of .125-Hz paced breathing through a resistive load: Implications for paced breathing therapy and the polyvagal theory. Applied Psychophysiology and Biofeedback. 1996;21:131–147. doi: 10.1007/BF02284692. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Souza GGL, Mendonca-De-Souza ACF, Barros EM, Coutinho EFS, Oliveria L, Mendlowicz MV, Volchan E. Resilience and vagal tone predict cardiac recovery from acute psychological stress. Stress. 2007;10:374–386. doi: 10.1080/10253890701419886. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Watson DL, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Berger R, Balon R, Srinivasan K. Relationship between age and heart rate variability in supine and standing postures: A study of spectral analysis of heart rate. Pediatric Cardiology. 1994;15:14–20. doi: 10.1007/BF00797000. [DOI] [PubMed] [Google Scholar]