Abstract
Allogeneic transplant access can be severely limited for patients of racial and ethnic minorities without suitable sibling donors. Whether cord blood (CB) transplantation can extend transplant access due to the reduced stringency of required HLA-match is not proven. We prospectively evaluated availability of unrelated donors (URD) and CB according to patient ancestry in 553 patients without suitable sibling donors. URDs had priority if adequate donors were available. Otherwise ≥ 4/6 HLA-matched CB grafts were chosen utilizing double units to augment graft dose. Patients had highly diverse ancestries including 35% non-Europeans. In 525 patients undergoing combined searches, 10/10 HLA-matched URDs were identified in 53% of those with European ancestry, but only 21% of patients with non-European origins (p < 0.001). However, the majority of both groups had 5–6/6 CB units. The 269 URD transplant recipients were predominantly European, with non-European patients accounting for only 23%. By contrast, 56% of CB transplant recipients had non-European ancestries (p < 0.001). Of 26 patients without any suitable stem cell source, 73% had non-European ancestries (p < 0.001). Their median weight was significantly higher than CB transplant recipients (p < 0.001), partially accounting for their lack of a CB graft. Availability of CB significantly extends allo-transplant access, especially in non-European patients, and has the greatest potential to provide a suitable stem cell source regardless of race or ethnicity. Minority patients in need of allografts, but without suitable matched sibling donors, should be referred for combined URD and CB searches to optimize transplant access.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation may be curative for patients with lethal hematologic malignancies. Thus, it represents one of the major achievements of modern hematology1. It is therefore imperative that all eligible patients have equal access to this therapy. However, less than 30% of patients requiring an allograft have a suitable human leukocyte antigen (HLA)-matched sibling donor1. While unrelated donor (URD) registries can supply grafts for those without a suitable sibling donor, the strict HLA-match needed to minimize the chance of lethal or debilitating transplant complications limits the donor pool2,3. Patients of racial and ethnic minorities are disadvantaged due to their relatively low numbers in volunteer registries and an increased likelihood that potential donors will be unavailable upon request4–6. Highly polymorphic HLA loci pose additional challenges in some groups, such as patients of African ancestry4.
In 1989 Gluckman and colleagues reported the use of HLA-identical sibling donor cord blood (CB) as a stem cell source for transplantation7. Subsequent series have confirmed the efficacy of unrelated donor CB transplantation8–13, including leukemia-free survival at least comparable to that provided by 8/8 HLA-matched URD bone marrow transplantation in children14. Promising survival has also been reported in adults15–17, with two recent reports suggesting double unit CB transplantation may be associated with comparable survival to that of HLA-matched sibling donor and URD transplantation in patients with hematologic malignancies18,19. A remarkable feature of this neonatal stem cell source is a greatly reduced incidence of graft-versus-host disease (GVHD) for the degree of donor-recipient HLA-mismatch8–13. Therefore, public CB banking was originally proposed as a means to provide a stem cell source that could extend transplant access20. That CB can extend transplant access to minorities has been suggested but this hypothesis has never been formally tested, and cannot be assumed given the relatively small size of the global CB inventory21. Hence, we conducted a prospective study evaluating donor availability according to patient ancestry in the highly diverse population referred to our Center in New York City.
PATIENTS AND METHODS
Study Population
Patients were candidates for allogeneic hematopoietic stem cell transplantation for the treatment of high-risk hematologic malignancies or severe aplastic anemia. They had no suitable HLA-matched related donors, were eligible for either URD or CB transplantation, provided informed consent for a formal donor search, and were transplanted or had their search closed during the study period. Institutional Review Board approval was obtained to analyze patient data including those patients whose preliminary search failed to identify any potentially suitable URD or CB units to request for typing. Adopted patients with unknown ancestry, patients whose searches were ongoing without a donor chosen, and searches for second transplants were excluded. Data on the searches for 553 patients were collected prospectively between October 1, 2005 and December 31, 2009.
URD and CB Searches
The majority of patients underwent combined search for both URD and CB units. URD searches and grafts were facilitated by the National Marrow Donor Program (NMDP). CB searches were conducted through the Bone Marrow Donors Worldwide, the NMDP, and the New York Blood Center (NYBC), and both domestic and international units were considered for potential use. MSKCC policy is to type potential URD recipients and their donors at 10 HLA-alleles given the progressive decrease in survival with an allele or antigen mismatch at each of the HLA-A,-B,-C,-DRB1 loci when transplanting URD grafts, and that HLA-DQ mismatches are detrimental when present with other mismatches2,3. Further, our institution is investigating T-cell depletion as a means to abrogate severe GVHD, especially if mismatch. Therefore, adequate donor-recipient HLA-match was defined as ≥ 8/10 HLA-A,-B,-C,-DRB1,-DQ for a T-cell depleted graft. Patients requiring unmodified grafts in the setting of very high risk disease or reduced intensity conditioning required ≥ 9/10 HLA-match for an unmodified URD graft in order to reduce the risk of life-threatening GVHD.
By contrast, adequate CB units were ≥ 4/6 HLA-A,-B antigen, -DRB1 allele matched to the recipient with a cryopreserved total nucleated cell (TNC) dose ≥ 1.5 × 107/kg/unit. Double unit grafts were used to augment engraftment, reduce transplant-related mortality, potentially reduce relapse22,23, and improve survival in all patients15–17. Unit-unit HLA-match was not considered. CB transplant recipients preferably had at least one additional back-up unit identified before transplant. URDs had priority as the stem cell source. CB was chosen if no suitably HLA-matched URDs were available within the required time period according to physician assessment of transplant urgency. Patients were transplanted with high-dose or reduced intensity conditioning according to age, diagnosis, prior therapy, and co-morbidities.
Assessment of Patient Ancestry
Patient ancestry information was collected by physicians and transplant coordinators using direct patient interview to determine the country of birth of the patient, each parent, and each grand-parent for as many generations as known or needed to establish the ancestry. In addition, patients self-identified themselves as Black and/or Hispanic. We used similar categories as previous reports4,24, but additionally divided patients of European ancestry, however, into north-western, eastern, southern, and mixed Europeans. Moreover, non-European ancestries were divided into Asian, African (Hispanic and non-Hispanic), white Hispanic, Middle Eastern, and mixed non-European groups. Patients of African ancestry included African-Americans, and immigrants from Africa or the Caribbean. Non-European mixes included any patient with at least partial non-European origins even if partly European (but excluded those who self-identified as Black), and frequently included complex racial mixes. Information concerning the ancestry or race of URDs and maternal donors of CB was incomplete, precluding analyses of recipient-donor ancestral matching.
Statistical Analysis
Tests of association between independent binary variables were performed using Fisher’s exact test; the paired binary data were examined using McNemar’s test. For continuous variables, the test of equality between independent groups was based on the Wilcoxon rank sum test. For multiple observations per subject, the Wilcoxon test incorporating clustering was employed. The time to secure an URD versus a CB graft was not analyzed as URD were given priority, and therefore URD and CB were not given equal weight at the search initiation. Also, it is already established that cryopreserved CB is available more quickly than URD25.
RESULTS
Patient Ancestry and Demographics
Our patients had highly diverse ancestries. Only 112 (20%) of the 553 patients were of north-western European ancestry, whereas 81 (15%) had eastern, 60 (11%) southern, and 105 (19%) mixed European backgrounds. North-western Europeans were predominantly of English, Irish, Scottish, German, and/or Scandinavian origins. The majority of southern Europeans originated from southern Italy. European mixes were most frequently a mix of north-western and southern European (e.g. Sicilian Italian-Irish), or north-western and eastern European (e.g. English-Polish) ancestries. Of the 195 (35%) patients of non-European origins, 46 (8%) had Asian, 64 (12%) African (55 non-Hispanic and 9 Hispanic), 51 (9%) white Hispanic, 11 (2%) Middle Eastern, and 23 (4%) mixed non-European ancestries. There were no Pacific Islanders. Ten of the mixed non-Europeans had part Native American ancestry, however. The majority of patients (n = 445, 80%) were born in the United States.
Patients had a median age of 47 years (range 0.9–73). Diagnoses included acute leukemia (n = 294; 53%), myelodysplasia/myeloproliferative disorders (n = 83; 15%), non-Hodgkin lymphoma or chronic lymphocytic/prolymphocytic leukemia (n = 119; 22%), Hodgkin lymphoma (n = 36; 7%), multiple myeloma (n = 12; 2%), aplastic anemia (n = 7; 1%), or other malignancies (n = 2; < 1%).
Results of Combined URD and CB Searches
Nearly all patients (n = 525, 95%) had combined URD and CB formal searches which allowed direct comparison of the best matched URD, and best matched and adequately dosed CB units, identified for the same patients (Table 1). The remaining 28 patients had suitably matched URDs and a CB search was not performed. We found that fully matched (10/10) URD were identified for the majority of those with north-western (62%) or eastern European (59%) backgrounds, and half of those with mixed European ancestries (50%). However, a 10/10 HLA-matched URD was identified in only 33% of southern European patients (p = 0.001). Furthermore, while 60% of patients with Middle Eastern ancestry had 10/10 HLA-matched URD identified, this was only the case for 19% of Asian, 8% of African, and 21% of white Hispanic patients. By contrast, CB extended stem cell availability to patients of both European and non-European origins.
Table 1.
Formal Search Results Showing the Best HLA-Matched URD or Best CB Units in Patients who Underwent Combined Searches (n = 525): URD Predominantly Serves Patients of North-western, Eastern, or Mixed European Ancestry, Whereas CB Extended Access to a Stem Cell Source to Both Europeans and Non-Europeans.
| Patients of European Ancestries | ||||
|---|---|---|---|---|
| North-western European (n = 104) | Eastern European (n = 76) | Southern European (N = 60) | European Mix (n = 101) | |
| Best URD | ||||
| 10/10 | 64 (62%) | 45 (59%) | 20 (33%) | 51 (50%) |
| 9/10 | 28 (27%) | 20 (26%) | 20 (33%) | 31 (31%) |
| ≤ 8/10 | 12 (12%) | 11 (14%) | 20 (33%) | 19 (19%) |
| Best CB* | ||||
| 5–6/6 | 88 (85%) | 62 (82%) | 36 (60%) | 84 (83%) |
| 4/6 | 15 (14%) | 12 (16%) | 15 (25%) | 14 (14%) |
| No CB | 1 (1%) | 2 (3%) | 9 (15%) | 3 (3%) |
| Patients of Non-European Ancestries | |||||
|---|---|---|---|---|---|
| Asian (n = 42) | African (n = 61) | White Hispanic (n = 48) | Middle Eastern (n = 10) | Non-European Mix (n = 23) | |
| Best URD | |||||
| 10/10 | 8 (19%) | 5 (8%) | 10 (21%) | 6 (60%) | 9 (39%) |
| 9/10 | 6 (14%) | 20 (33%) | 14 (29%) | 1 (10%) | 8 (35%) |
| ≤ 8/10 | 28 (67%) | 36 (59%) | 24 (50%) | 3 (30%) | 6 (26%) |
| Best CB* | |||||
| 5–6/6 | 36 (86%) | 35 (57%) | 33 (69%) | 8 (80%) | 19 (83%) |
| 4/6 | 6 (14%) | 14 (23%) | 10 (21%) | 2 (20%) | 2 (9%) |
| No CB | 0 | 12 (20%) | 5 (10%) | 0 | 2 (9%) |
Best CB units were defined according to HLA-match but also had to have an adequate TNC dose of at least 1.5 × 107/kg/unit.
Overall, the difference in URD and CB availability according to European versus non-European ancestry in patients undergoing combined searches is summarized in Table 2. European ancestry patients were more likely to have a fully matched URD identified than those of non-European origins (53% versus 21%, p < 0.001). In addition, Europeans were more likely to have 5–6/6 HLA-matched CB units (identified in 79%) than to have 10/10 HLA-matched URD (identified in 53%, p < 0.001). In non-European patients, CB units matched at 5–6/6 HLA loci were also more frequently identified than fully matched URDs (71% versus 21%, p < 0.001).
Table 2.
Summary Comparison of URD and CB Availability for Patients Undergoing Combined Searches According to European versus Non-European Ancestry: European Patients were Much More Likely to Secure a 10/10 HLA-matched URD Than Non-Europeans Whereas 5–6/6 CB Were Available for Both Groups.
| Europeans (n = 341) |
Non-Europeans (n = 184) |
p | |
|---|---|---|---|
| Best URD | |||
| 10/10 (n = 218) | 180 (53%) | 38 (21%) | < 0.001 |
| 9/10 (n = 148) | 99 (29%) | 49 (27%) | |
| ≤ 8/10 (n = 159) | 62 (18%) | 97 (53%) | |
| Best CB | |||
| 5–6/6 (n = 401) | 270 (79%) | 131 (71%) | |
| 6/6 5/6 |
74 196 |
13 118 |
|
| 4/6 (n = 90) | 56 (16%) | 34 (18%) | |
| No CB (n = 34) | 15 (4%) | 19 (10%) |
Abbreviations: URD, adult unrelated volunteer donor; CB, cord blood.
Transplant Analysis
Of the 553 study population, 359 (65%) underwent transplantation. Three-quarters (n = 269, 75%) used URDs (median age 48 years, range 1–73), whereas 25% (n = 90) required CB (median age 37 years, range 1–68). Twenty-six patients (5% of the total study population) were not transplanted specifically due to a lack of a suitable URD or CB graft. The remaining 168 patients (30% of the total) were not transplanted with URD or CB for other reasons, predominantly due to refractory disease or treatment complications (n = 110). The proportions of patients of European (n = 103, 29%) and non-European (n = 65, 33%) backgrounds that could not be transplanted for reasons other than not having a graft were similar (p = 0.240).
URD Transplant Recipients
Notably, URD transplant recipients were predominantly European (n = 208, 77%), with the largest sub-group having north-western European ancestry (Table 3, Figures 1 and 2). Non-European patients combined (n = 61) comprised only 23% of URD transplants. The majority (n = 163, 61%) of the 269 URD transplant recipients received 10/10 HLA-matched grafts, whereas 81 (30%) received 9/10, and 25 (9%) received 8/10 HLA-matched grafts. The percentage of north-western Europeans that received 10/10 URD grafts (n = 49, 68%) was similar to that of other Europeans (n = 87, 64%, p = 0.646), but significantly higher than non-Europeans (n = 27, 44%, p = 0.008). Among non-European patients, while 9 (69%) Asian patients received 10/10 URD, this was only the case in 3 (14%) African, and 6 (40%) white Hispanic patients. URD grafts were obtained from North America (n = 175, 65%), Europe (n = 74, 28%), and elsewhere (n = 20, 7%), with no relationship between patient ancestry and need for a non-American donor.
Table 3.
Ancestral Composition of URD and CB Transplant Recipients or Those Without a Suitable Graft: URD Transplantation Predominantly Serves Europeans, Whereas CB Extends Transplant Access to Patients of Non-European Ancestry.
| Patient Ancestry (Total = 385) |
URD Transplants (n = 269) |
CB Transplants (n = 90) |
No Graft (n = 26) |
|---|---|---|---|
| Europeans (n = 255) | 208 (77%) | 40 (44%) | 7 (27%) |
| North-western (n = 79) | 72 | 6 | 1 |
| Eastern (n = 57) | 48 | 9 | 0 |
| Southern (n = 40) | 26 | 10 | 4 |
| Mix (n = 79) | 62 | 15 | 2 |
| Non-Europeans (n = 130) | 61 (23%) | 50 (56%) | 19 (73%) |
| Asian (n = 30) | 13 | 17 | 0 |
| African (n = 50) | 22 | 15 | 13 |
| White Hispanic (n = 29) | 15 | 10 | 4 |
| Middle Eastern (n = 8) | 5 | 3 | 0 |
| Non-European Mix (n = 13) | 6 | 5 | 2 |
Abbreviations: URD, adult unrelated volunteer donor; CB, cord blood.
Figure 1. Comparison of Ancestry Composition of URD and CB Transplant Recipients and Those Without a Graft.
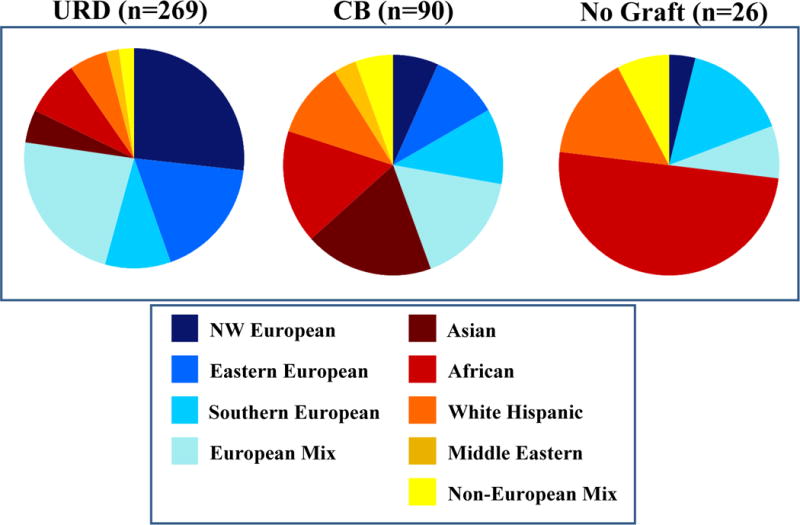
Greater than 50% of CB transplant recipients were non-European. Patients with African ancestry were the least likely to secure a suitable graft.
Figure 2. Comparison of Transplant Type or Lack of Graft for Individual Ancestry Groups.
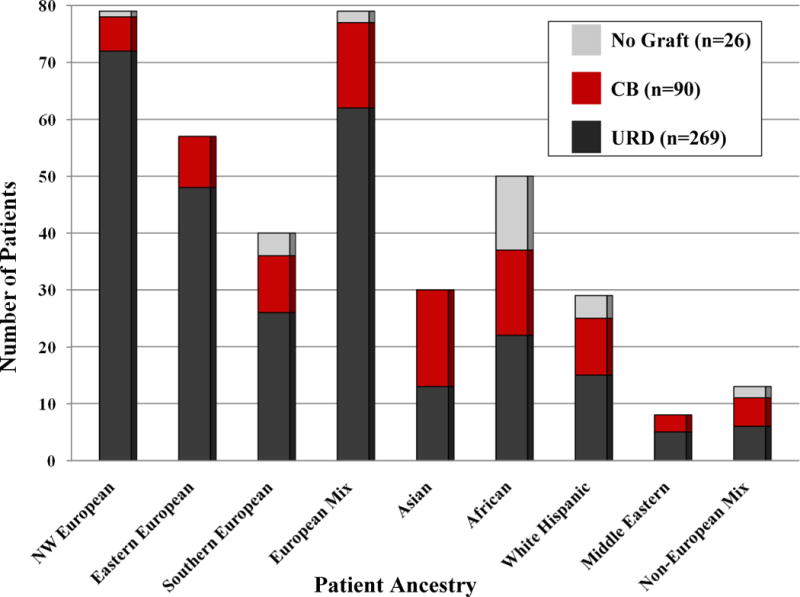
URD transplantation predominantly serves Europeans. CB significantly extends transplant access to patients of eastern, southern, and mixed European ancestry, as well as Asian, African, white Hispanic, and mixed non-European patients.
CB Transplant Recipients
In marked contrast to URD transplant recipients, only 6 (7%) CB transplant recipients had north-western European ancestry. Notably, over half (n = 50, 56%) of the CB recipients had non-European origins, and included 19% Asian, 17% African, 11% white Hispanic, 3% Middle Eastern, and 6% non-European mix patients (Table 3, Figures 1 and 2). Thus, as compared to URD transplants, CB transplant recipients were more likely to have non-European origins (56% versus 23%, p < 0.001). The majority of CB transplant recipients received CB because they did not have any suitable URDs. Of patients eligible for transplant (i.e those in Table 3 that excludes those not able to be transplanted due to refractory disease or treatment complications), the availability of CB extended transplant access to 8% of north-western European, 16% of eastern European, 25% of southern European, and 19% of mixed European patients. For patients of non-European ancestry, the availability of CB had a greater impact by extending access to 57% of Asian, 30% of African, 34% of white Hispanic, 38% of Middle Eastern, and 38% of mixed non-European patients. Units were obtained from domestic (n = 129, 72%) or international (n = 51, 28%) banks, with no relationship between European versus non-European ancestry and need for international units.
The median infused TNC doses of the double unit grafts were 2.56 × 107/kg (range 1.42–12.79) for the larger and 1.94 × 107/kg (range 0.91–7.09) or the smaller unit o compare the TNC doses available to patients according to ancestry but independent of the patient’s weight, the TNC/kg were back-calculated to give the total TNC/unit. We found European patients received units with a higher median total TNC post-thaw of 1.60 × 109/unit (range 0.64–2.97). This compared with 1.40 × 109/unit (range 0.49–4.07) in non-European patients (p = 0.018).
The donor-recipient HLA-match of the 90 CB grafts (180 units) was 5–6/6 in 58% (n = 105), and 4/6 in 42% (n = 75). European patients were not more likely to receive 5–6/6 HLA-matched units. Fifty (63%) of 80 units European patients received, and 55 (55%) of 90 units given to non-European patients, were 5–6/6 HLA-matched (p = 0.362).
Patients without URD or CB Grafts
Of the 26 patients (median age 51 years, range 14–68) not transplanted due to lack of any stem cell source, most (n = 19, 73%) had non-European origins (p < 0.001), with 50% of these patients having African ancestry (Table 3, Figures 1 and 2). Of the total study population, 1/112 (< 1%) north-western, 0/81 (0%) eastern, 4/60 (7%) southern, and 2/105 (2%) mixed European patients had no graft. This compared with 0/46 (0%) Asian, 13/64 (20%) African, 4/51 (8%) white Hispanic, and 2/23 (9%) mixed non-European background patients. Thus, patients of African ancestry had the greatest difficulty securing an adequate URD or CB graft. Notably, the median 82kg (range 50–151) weight of “no graft” patients was higher than the 66kg (range 7–119) of CB transplant recipients (p < 0.001).
DISCUSSION
New York City (NYC) has one of the most diverse populations in the world. For example, in the Borough of Queens alone an estimated 170 foreign languages are spoken26. The NYC population, therefore, generates patients with highly diverse ancestry and thus varying and unique racial4,27 and ancestral28 HLA-haplotypes. This has allowed our Center to directly compare the ability of the global inventory of URDs and CB units to provide suitable grafts to a variety of European, Asian, African, and white Hispanic patients. To study donor availability we used similar ancestry categories as previous reports4,24, but distinguished European sub-groups, African Hispanics from white Hispanics, and non-European mixes. This considerably broadens the reporting of patient origins beyond “white” versus “non-white”. While the attribution of ancestry remains complex, our study has the advantage that our physicians and dedicated transplant coordinators took a personal family history of the birthplace of the patients and their ancestors rather than relying on staff assessment of presumed race or ethnicity.
We demonstrate that despite the 6.7 million URDs registered with the NMDP, and an additional 5 million donors in affiliated registries worldwide29, URD transplantation predominantly serves patients of north-western, eastern, and mixed European ancestry. However, southern European, Asian, African, white Hispanic and mixed non-European patients were much less likely to secure a suitable URD. These groups together constituted 46% of our patients. Furthermore, patients of non-European backgrounds, especially African ancestries, were more likely to receive a mismatched URD transplant, with a higher chance of compromising transplant outcome2,3. We, therefore, show that URD registries predominantly serve patients of European origins, with the exception of southern Europeans, and confirm the suggestion of the 2002 General Accounting Office Report to the US Congress that “equal access to a match may not be attainable” according to race. From a practical standpoint our findings demonstrate that transplant centers should be mindful of the patient’s ancestry during URD searches given the decreased likelihood of search success for patients with southern European and non-European origins.
CB can be available substantially faster than URDs25, an advantage for patients requiring urgent transplantation. We now show for the first time that, due to the relative tolerance of HLA-disparity, CB has a further advantage: despite a relatively small global inventory of approximately 400,000 public units21, CB significantly extends transplant access to all patients, especially those of southern European and non-European ancestry. This is in marked contrast to URD transplantation, and is despite the median weight of 66kg (range 7–119) in our CB transplant recipients. Due to the addition of CB as an alternative stem cell source, only 5% of our diverse patient population undergoing URD searches could not be transplanted due to lack of a graft. Furthermore, given that approximately one-quarter of allograft referrals have a sibling donor, the addition of CB to volunteer donors means that the lack of a suitable stem cell source is now a rare barrier to transplant. An additional finding at the opposite end of the donor availability spectrum is striking: Europeans who had 10/10 HLA-matched URD were even more likely to have 5–6/6 HLA-matched CB units, with 22% of European patients having 6/6 HLA-matched CB units, for example. This is relevant given the reporting of higher leukemia-free survival after 6/6 HLA-matched CB transplantation compared with 8/8 HLA-allele matched URD bone marrow transplantation in children14. Thus, a randomized study between 5–6/6 matched CB and 10/10 URD may be feasible in the future.
However, despite an encouraging extension of access, CB had limitations. Independent of recipient weight, patients of non-European origins were more likely to receive units with a lower TNC dose. Further, while no Asian patient lacked a suitable CB graft, one-fifth of African ancestry patients, and a significant number of patients with southern European, white Hispanic, and mixed non-European ancestries, did not have suitable CB units. These groups likely have a lesser representation in the global CB inventory. Such patients, especially those with an adult weight, are less likely to identify an adequately HLA-matched and dosed CB graft, even when utilizing double units. Therefore, based on these findings, prior reports suggesting that only a small inventory o CB units should be adequate30,31 are not correct. An increased inventory of adequately dosed and high quality units from racially diverse populations is required to rectify this inequity. Units collected from African births have lower TNC counts32, contributing to a lower inventory of adequately dosed units for African ancestry patients. This is a particular challenge that must be addressed.
We can now demonstrate that CB is extending transplant access to racial and ethnic minorities. Given the recent promising survival after pediatric and adult CB transplantation for hematologic malignancies, this is a major advance in the field. It is known that increasing URD registry size will not result in an appreciable increase in donor availability for populations such as African Americans due to their varied genetic composition4,6. Extending search time also does not increase the likelihood of securing a suitable donor33. Our study suggests that with adequate funding CB has the greatest chance of providing a stem cell source regardless of race or ethnicity. This is compelling support for increased funding of public CB banks and should prompt studies to estimate the size of the CB inventory needed to provide stem cells to all. Furthermore, patients from racial and ethnic minorities in need of allografts, but without suitable matched sibling donors, should be promptly referred for combined URD and CB searches to optimize identification of a suitable donor and timely transplantation.
Acknowledgments
Andromachi Scaradavou, M.D., is the Medical Director of the National Cord Blood Program of the New York Blood Center as well as an Attending Physician at the Memorial Sloan-Kettering Cancer Center. The authors have no other relevant conflicts of interest to disclose.
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the Memorial Sloan-Kettering Cancer Center Society (J.N.B.), the Translational and Integrative Medicine Research Grant (J.N.B.), and P01 CA23766 from the National Institutes of Health (J.N.B., N.A.K., E.B.P., J.W.Y., M.RM.vdB.). The authors further wish to thank the helpful review of Dr Peter Bach, Dr Cladd Stevens, Dr Victoria Barker, Dr Anthony Barker, and Anne-Marie Gonzales, Marissa Lubin, and Michelle Abboud for their assistance with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–30. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–83. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60:778–83. [PubMed] [Google Scholar]
- 5.Confer DL. The National Marrow Donor Program. Meeting the needs of the medically underserved. Cancer. 2001;91:274–8. doi: 10.1002/1097-0142(20010101)91:1+<274::aid-cncr18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Kollman C, Abella E, Baitty RL, et al. Assessment of optimal size and composition of the U.S. National Registry of hematopoietic stem cell donors. Transplantation. 2004;78:89–95. doi: 10.1097/01.tp.0000132327.40702.97. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 10.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen M, Rubinstein P, Zhang MJ, et al. Comparison of outcomes after transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukemia. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 15.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after non-myeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker JN, Abboud M, Rice RD, et al. A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponce D, Zheng J, Gonzales AM, et al. Disease-free survival after cord blood transplantation is not different to that after related or unrelated donor transplantation in patients with hematologic malignancies. Blood. 2009;114 Abstract 906. [Google Scholar]
- 19.Brunstein CG, Gutman JA, DeFor TE, et al. Reduced relapse and similar progression-free survival after double umbilical cord blood transplantation (DUCBT): comparison of outcomes between sibling, unrelated adult and unrelated DUCB hematopoietic stem cell donors. Blood. 2009;114:276. [Google Scholar]
- 20.Rubinstein P, Rosenfield RE, Adamson JW, et al. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679–1690. [PubMed] [Google Scholar]
- 21.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94:451–4. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–63. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 24.Hurley CK, Fernandez-Vina M, Hildebrand WH, et al. A high degree of HLA disparity arises from limited allelic diversity: analysis of 1775 unrelated bone marrow transplant donor-recipient pairs. Hum Immunol. 2007;68:30–40. doi: 10.1016/j.humimm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Barker JN, Krepski TP, DeFor TE, et al. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 26.Santos F. NY Times. New York: 2008. Mayor Orders NY to Expand Language Help. [Google Scholar]
- 27.Mori M, Beatty PG, Graves M, et al. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997;64:1017–27. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 28.Mack SJ, Tu B, Lazaro A, et al. HLA-A, -B, -C, and -DRB1 allele and haplotype frequencies distinguish Eastern European Americans from the general European American population. Tissue Antigens. 2009;73:17–32. doi: 10.1111/j.1399-0039.2008.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Confer D, Robinett P. The US National Marrow Donor Program role in unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42(Suppl 1):S3–S5. doi: 10.1038/bmt.2008.102. [DOI] [PubMed] [Google Scholar]
- 30.Beatty PG, Boucher KM, Mori M, et al. Probability of finding HLA-mismatched related or unrelated marrow or cord blood donors. Hum Immunol. 2000;61:834–40. doi: 10.1016/s0198-8859(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 31.Querol S, Mufti GJ, Marsh SG, et al. Cord blood stem cells for hematopoietic stem cell transplantation in the UK: how big should the bank be? Haematologica. 2009;94:536–41. doi: 10.3324/haematol.2008.002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtzberg J, Cairo MS, Fraser JK, et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–55. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 33.Dehn J, Arora M, Spellman S, et al. Unrelated donor hematopoietic cell transplantation: factors associated with a better HLA match. Biol Blood Marrow Transplant. 2008;14:1334–40. doi: 10.1016/j.bbmt.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


