Abstract
The airway epithelium plays an essential role in innate immunity to lung pathogens. Ribonucleoprotein particles primarily composed of major vault protein (MVP) are highly expressed in cells that encounter xenobiotics. However, a clear biologic function for MVP is not established. We report here that MVP is rapidly recruited to lipid rafts when human lung epithelial cells are infected with Pseudomonas aeruginosa, and maximal recruitment is dependent on bacterial binding to the cystic fibrosis transmembrane conductance regulator. MVP was also essential for optimal epithelial cell internalization and clearance of P. aeruginosa. These results suggest that MVP makes a substantial contribution to epithelial cell–mediated resistance to infection.
The innate immune response to foreign pathogens involves a rapidly initiated, complex array of cellular changes and activation of signaling pathways after contact of pathogenic microbes with epithelial surfaces. Cellular uptake of pathogens, induction of an inflammatory cascade, and eventual apoptosis of cells to restore tissue homeostasis are essential for the clearance of many pathogens (1, 2). One setting that allows for an in-depth study of innate immunity in the airway is the condition cystic fibrosis (CF), in which hypersusceptibility to infection with Pseudomonas aeruginosa results partly from a failure to properly activate the normal host immune response (3–5).
Different mutations in the CF transmembrane conductance regulator (CFTR) gene have been found, many resulting in a deficiency in the innate immune response to P. aeruginosa (6–8). Most CF patients (>80%) develop chronic P. aeruginosa lung infections, with respiratory function decline over 10 to 25 years, and premature mortality in the third to fourth decade of life (3, 6). Among its other functions, CFTR acts as a receptor for the outer core oligosaccharide of the P. aeruginosa lipopolysaccharide (LPS) (5). In humans with WT-CFTR, rapid binding to P. aeruginosa initiates an innate immune response that includes bacterial ingestion by epithelial cells, nuclear factor κB (NF-κB) activation, cytokine secretion, and eventual epithelial cell apoptosis (8–11). These responses are associated with maximal resistance to P. aeruginosa infection. In CF, the absence of functional CFTR prevents these rapid innate immune responses to infection, resulting in failure to clear P. aeruginosa. Infection is further exacerbated by additional features of CF, including poor mucociliary clearance due to dehydrated airway surface liquid and thickened mucus (6), as well as the propensity of the organism to grow as mucoid microcolonies within anaerobic mucus plugs (12).
P. aeruginosa infection initiates effective innate immune responses after contact with lung epithelial cells by inducing formation of lipid rafts and/or caveolae membrane micro-domains (13, 14) that contain WT-CFTR (14). To determine the molecular events that ensue from this initial epithelial cell contact, we performed an analysis of proteins recruited to lipid rafts generated during 15 min of infection with P. aeruginosa. Rafts were isolated from WT-CFTR– expressing human lung epithelial cell lysates, and proteins in these rafts were compared with proteins in rafts from uninfected cells (15). About 150 proteins identified by matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometry were found exclusively in lipid rafts of P. aeruginosa–infected cells (table S1), potentially having an impact on the various cellular responses that follow CFTR binding of P. aeruginosa. We identified one of these proteins with an abundant number of detected peptides as the major vault protein (MVP), also referred to as the lung resistance–related protein (16).
MVP is highly expressed in lung and intestinal epithelia, dendritic cells, and macrophages, and multiple copies of MVP assemble into 13-megadalton barrel-like ribonucleoprotein complexes known as vaults that also contain two minor proteins [vault poly(ADP-ribose) polymerase (vPARP) and telomerase-associated protein 1 (TEP1)] and untranslated vault RNA sequences (vRNAs) (17). Although vaults have been proposed to have a role in drug resistance, nucleocytoplasmic transport, and regulation of signaling (18–24), a definitive function for MVP or vaults has yet to be assigned as MVP knockout mice (MVP−/−) do not have phenotypes consistent with these in vitro observations (17, 25).
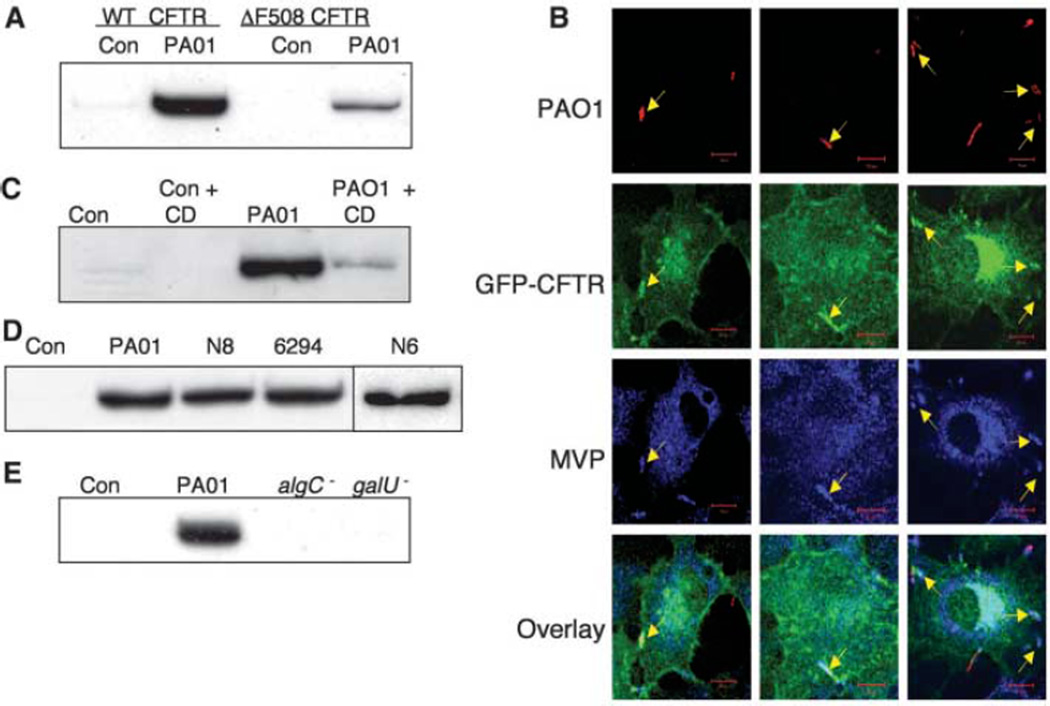
Western blot confirmed the rapid recruitment of MVP to lipid rafts during P. aeruginosa infection (Fig. 1A). Fifteen minutes after infection, the proportion of total cellular MVP in rafts isolated from airway epithelial cells expressing WT-CFTR increased dramatically, whereas the proportion of total cellular MVP recruited to rafts in P. aeruginosa–infected isogenic airway cells expressing only the ΔF508 CFTR protein increased to only 30% of the levels of WT-CFTR cells (Fig. 1A). Total MVP expression was identical in the two cell lines, and 10 to 15% of the total cellular MVP protein was found in rafts of infected WT-CFTR cells. Of the minor vault components, vPARP was detected by immunoblot and vRNA was detected by reverse transcription polymerase chain reaction (RT-PCR) in raft fractions from P. aeruginosa–infected cells (fig. S1). Although TEP1 was not detected in the raft fractions of infected cells, this protein was readily detected in cell lysates (fig. S1). Because TEP1 is the vault component that binds vRNA, the finding of vRNA in the raft fractions suggested that TEP1 was also present at undetectable levels and that the complete vault structure was recruited to lipid rafts after infection. Green fluorescent protein (GFP)–tagged WT-CFTR was stably transfected into the IB3-1 CF cell line (ΔF508/W1282X CFTR alleles), and immunofluorescence staining of P. aeruginosa–infected cells showed colocalization of bacteria, MVP, and CFTR (Fig. 1B), which indicated that all three components were recruited to the same cellular site during infection. However, coimmunoprecipitation experiments using lysates from infected cells failed to detect a stable interaction between CFTR and MVP. Thus, it appears that the presence of WT-CFTR augments MVP recruitment to membrane microdomains in response to P. aeruginosa infection without direct physical association.
Fig. 1. Recruitment of MVP to lipid rafts of airway epithelial cells by P. aeruginosa.
(A) Lysates of WT and homozygous ΔF508 CFTR human airway epithelial cells (CFT1-LCFSN and CFT1-LC3, respectively) left uninfected (Con) or infected with P. aeruginosa strain PA01-V (PA01) were separated on discontinuous sucrose gradients. Proteins in raft fractions were precipitated and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis with antibodies directed against human MVP. (B) IB3-1 CF cells (ΔF508/W1282X) transfected with WT-CFTR with an N-terminal GFP tag were infected with CFP-expressing P. aeruginosa, then fixed and stained for MVP. Confocal microscopy identified (arrows) bacteria (red), CFTR (green), and MVP (blue) at the site of bacterial contact with the cell membrane. Scale bar, 10 µm. Overlay shows the merged image of the three individual channels. (C) MVP in rafts of WT-CFTR cells left uninfected (Con) or infected with P. aeruginosa (PA01) in the presence or absence of 5 mM cyclodextrin (CD). (D) MVP in rafts of WT-CFTR cells left uninfected or infected with strain PA01-V or clinical isolates of P. aeruginosa from two CF patients (N6, N8) or from a corneal infection (6294). (E) MVP in rafts of WT-CFTR cells left uninfected or infected with strain PA01-V or LPS mutants of PA01-V (algC− or galU−). The LPS mutants lack the CFTR-binding domain on the bacterial cell surface and do not promote MVP entry into lipid rafts after P. aeruginosa infection.
Disruption of lipid rafts by cholesterol extraction using methyl-β-cyclodextrin (Fig. 1C) or solubilization of rafts using octyl-d-glucoside (fig. S2) markedly reduced the level of MVP in the raft fractions. Multiple strains of P. aeruginosa, including strain PA01-V; two early isolates from the lungs of a CF patient (N6, N8); and an isolate from a corneal infection (6294) induced recruitment of MVP to rafts (Fig. 1D). P. aeruginosa strains that express a truncated form of LPS, lacking a full outer core [mutations in the galU or algC genes (5, 10)] and thus incapable of binding to CFTR, did not induce raft localization of MVP in cells with WT-CFTR (Fig. 1E). This result indicates that binding of P. aeruginosa LPS outer-core oligosaccharide to CFTR is required for recruitment of MVP to lipid rafts.
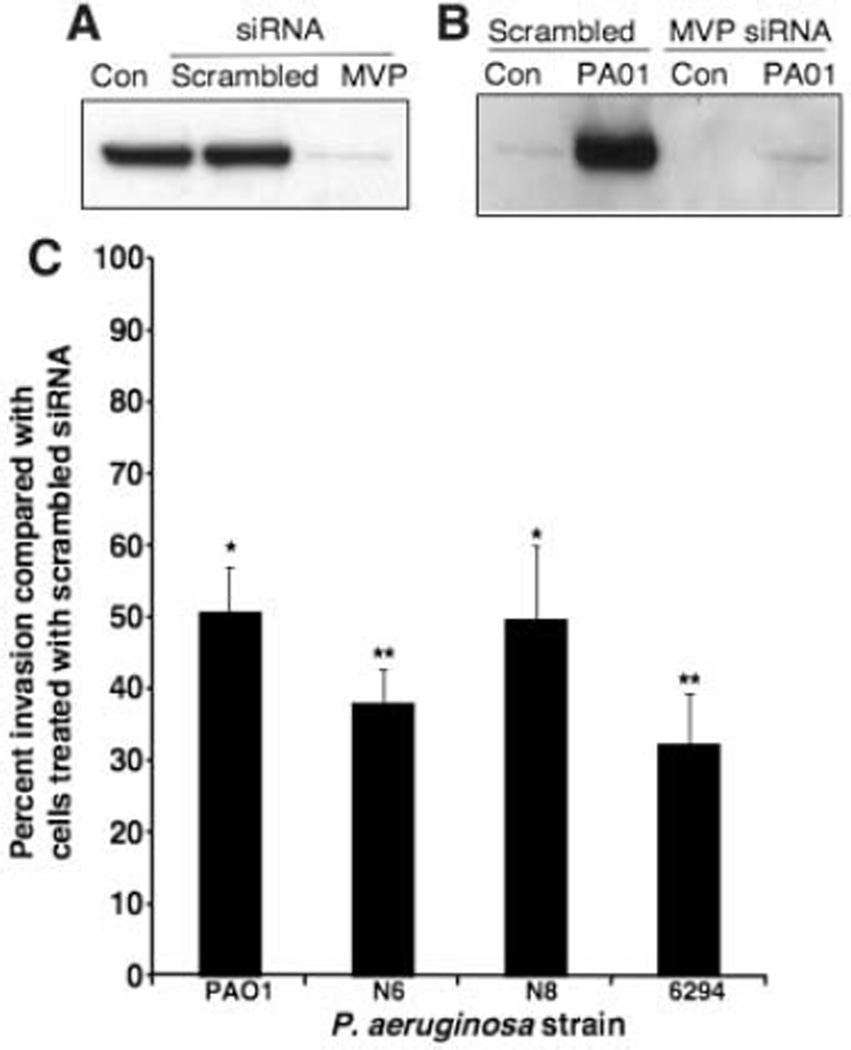
Previously characterized CFTR-dependent epithelial cell responses to P. aeruginosa include bacterial uptake, NF-κB activation, interleukin 8 (IL-8) secretion, and induction of apoptosis (4, 8, 10, 11, 26). To determine the potential role of MVP in these responses to P. aeruginosa, we reduced MVP cellular expression by more than 90% using MVP-specific small interfering RNA (siRNA) (Fig. 2A). Control cells were transfected with a scrambled siRNA sequence. MVP knockdown decreased recruitment of this protein to lipid rafts 15 min after P. aeruginosa infection (Fig. 2B). Internalization of four different P. aeruginosa strains was consequently reduced to 35 to 50% of the levels seen with control cells (Fig. 2C). MVP knockdown had no effect on NF-κB activation, IL-8 secretion, or apoptosis induced by P. aeruginosa (fig. S3, A to C). Thus, of these four components of the innate immune response, MVP appears to be involved only in the bacterial uptake pathway. It is also possible that, due to the high expression levels of MVP in airway cells, the residual 5 to 10% of MVP present after siRNA treatment was sufficient for these other signaling and response pathways or that other proteins could substitute for MVP in these responses.
Fig. 2. MVP function in the interaction of airway epithelial cells with P. aeruginosa.
(A) Whole-cell lysates from WT-CFTR cells (CFT1-LCFSN) that had been treated with either a scrambled siRNA sequence or MVP-specific siRNA were subjected to electrophoresis and immunoblot analysis with MVP-specific antibodies. Specific siRNA treatment reduced the level of MVP by >90%. (B) WT-CFTR cells were treated with scrambled or MVP-specific siRNA then left uninfected (Con) or infected for 15 min with P. aeruginosa PA01-V (PA01). Cell lysates were separated on discontinuous sucrose gradients. Raft fractions were precipitated and subjected to immunoblot analysis with MVP-specific antibodies. (C) Scrambled or MVP-specific siRNA-treated WT-CFTR cells were incubated with the indicated P. aeruginosa strains, and the amount of bacterial internalization was determined. The internalization of P. aeruginosa by cells treated with MVP-specific siRNA is plotted as a percentage of that obtained for scrambled siRNA-treated cells. Error bars represent SEMs. *P < 0.05, **P < 0.01 by a two-tailed t test comparison with internalization by scrambled siRNA-treated cells.
Proteomic analysis of raft proteins recovered from control or MVP siRNA-treated cells showed that MVP knockdown reduced the levels of other proteins recruited to the membrane microdomains after P. aeruginosa infection (table S2). The majority of the proteins reduced in infected, MVP siRNA-treated cells are involved in raft formation or cell signaling processes, consistent with the conclusion that MVP is critical for formation of stable membrane microdomains and/or raft recruitment of cell signaling molecules after P. aeruginosa infection. Compared with rafts from P. aeruginosa–infected control cells, rafts from P. aeruginosa–infected MVP knockdown cells had increased levels of several myosin isoforms and tropomyosin 3 (table S3).
To establish an in vivo role for MVP in host resistance to P. aeruginosa, we infected WT and MVP−/− mice intranasally with ~1.5 × 107 P. aeruginosa strain PA01-Vand removed the lungs after 6 hours to measure total and internalized bacteria. Similar experiments using transgenic CF mice have shown that lack of CFTR results in decreased cellular internalization of bacteria and an increase in total bacteria in the lungs (9). Six hours after infection, virtually no innate immune cells, such as polymorphonuclear leukocytes or macrophages, were found to be associated with bacteria in WT or MVP−/− mice, but they were found associated with bacteria 24 hours post infection (fig. S4). Bacteria could be detected 6 hours post infection associated with epithelial cells expressing epidermal growth factor receptor (fig. S4), which indicated that bacterial internalization was primarily due to uptake by the epithelial cells.
Compared with the level of bacteria internalized by epithelial cells in WT mice, lung epithelial cells in MVP−/− mice internalized 55% less of the total bacteria in their lungs (Fig. 3A). This deficiency in uptake resulted in a 3.5-fold increase in bacterial burden in the lungs of MVP−/− mice compared with WT mice (Fig. 3B), which indicated a role for MVP in P. aeruginosa ingestion and clearance from the lung. MVP loss resulted in increased mortality from P. aeruginosa infection by a factor of almost 3 [Fig. 3C; hazard ratio = 2.96, 95% confidence interval (CI) 1.27 to 13.1; P = 0.018). Thus, MVP contributes to resistance against P. aeruginosa lung infection; this conclusion is consistent with the findings that CFTR-dependent recruitment of MVP to rafts after P. aeruginosa infection facilitates optimal innate immune responses to this pathogen.
Fig. 3. In vivo role of MVP in bacterial internalization, clearance and resistance to lethality.
(A) Six hours after intranasal inoculation with ~1.5 × 107 P. aeruginosa strain PA01-V, WT and MVP−/− mice were killed (n = 9 WT, 12 MVP−/−), and the lungs were removed and dissociated. The internalized percentage of total bacteria found in the lung is plotted. Bars represent mean values. *P < 0.0001 by unpaired two-tailed t test. (B) The lung suspensions from the same mice used in (A) were analyzed for total bacterial load. The number of bacteria per gram of lung is plotted. Error bars represent standard error of the mean. *P < 0.01 by unpaired two-tailed t test. (C) Survival curves over 96 hours after intranasal inoculation with ~4 × 106 colony-forming units of P. aeruginosa strain PA01-V (n = 17 WT, 15 MVP−/−). Hazard ratio = 2.96, 95% CI 1.27 to 13.1; P = 0.018.
Bacterial internalization by respiratory epithelial cells is an important initial step in clearance of P. aeruginosa (5, 8, 9) and numerous other microbes with a propensity to cause pneumonia (27–29), which indicates that uptake of bacteria by these cells enhances resistance to infection. Although the lack of MVP could cause increased susceptibility to infection through other means, the fact that the MVP−/− mice have normal dendritic cell function (30) suggests that the general phagocytic cell response would be unaltered in these mice, and that differences in outcomes would likely stem from an inappropriate epithelial cell response. We have identified a role for MVP in the response to lung infection and have validated this phenotype in MVP−/− mice. MVP, the predominant component of the vault complex, has been suggested to be a scaffold protein in signaling pathways and to be involved in intracellular transport (18, 19, 23, 24), and its recruitment to lipid rafts is likely required to facilitate incorporation into rafts of additional proteins essential for responses to infection. Alternatively, MVP may stabilize or internalize the components of bacterial-induced lipid rafts by linking raft proteins to cytoskeletal elements such as tubulin or actin (31) or via raft-independent effects on innate immunity mediated by another function of MVP, such as the regulation of extracellular signal–regulated kinase (ERK) signaling (24).
There is great interest in finding human genes with nucleotide polymorphisms that modify innate immune responses, including those involved in CF lung disease (7). Identification and association of MVP with the cellular responses to pathogens such as P. aeruginosa suggests that investigation of sequence variations in the mvp gene or possibly other genes encoding vault components could help to define additional roles for MVP-containing structures in innate immunity.
Supplementary Material
Acknowledgments
This work was funded by NIH grants R01 HL 58398-08 (G.B.P.) and R37 HL 32854-22 (D.E.G.) and by the EC FP6 RIBOREG Project (LSHG-CT-2003503022) from the European Commission (EACW). We thank A. Koh and G. Priebe for assistance with mouse studies.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/317/5834/130/DC1
Materials and Methods
Figs. S1 to S4
Tables S1 to S3
References
References and Notes
- 1.Grassme H, et al. Science. 2000;290:527. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 2.Mulvey MA, et al. Science. 1998;282:1494. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 3.Kerem B, Kerem E. Eur. J. Hum. Genet. 1996;4:65. doi: 10.1159/000472174. [DOI] [PubMed] [Google Scholar]
- 4.Pier GB, Grout M, Zaidi TS, Goldberg JB. Am. J. Respir. Crit. Care Med. 1996;154:S175. doi: 10.1164/ajrccm/154.4_Pt_2.S175. [DOI] [PubMed] [Google Scholar]
- 5.Pier GB, et al. Science. 1996;271:64. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher RC. Eur. Respir. J. 2004;23:146. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 7.Drumm ML, et al. N. Engl. J. Med. 2005;353:1443. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 8.Pier GB, Grout M, Zaidi TS. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12088. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder TH, et al. J. Immunol. 2001;166:7410. doi: 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder TH, et al. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6907. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon CL, Kowalski MP, Stopak KS, Pier GB. Am. J. Respir. Cell Mol. Biol. 2003;29:188. doi: 10.1165/rcmb.4898. [DOI] [PubMed] [Google Scholar]
- 12.Worlitzsch D, et al. J. Clin. Invest. 2002;109:317. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassme H, et al. Nat. Med. 2003;9:322. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski MP, Pier GB. J. Immunol. 2004;172:418. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 15.Materials and methods are available as supporting material on Science Online.
- 16.Scheffer GL, et al. Nat. Med. 1995;1:578. doi: 10.1038/nm0695-578. [DOI] [PubMed] [Google Scholar]
- 17.Mossink MH, van Zon A, Scheper RJ, Sonneveld P, Wiemer EA. Oncogene. 2003;22:7458. doi: 10.1038/sj.onc.1206947. [DOI] [PubMed] [Google Scholar]
- 18.Chung JH, Ginn-Pease ME, Eng C. Cancer Res. 2005;65:4108. doi: 10.1158/0008-5472.CAN-05-0124. [DOI] [PubMed] [Google Scholar]
- 19.Yu Z, et al. J. Biol. Chem. 2002;277:40247. doi: 10.1074/jbc.M207608200. [DOI] [PubMed] [Google Scholar]
- 20.Kickhoefer VA, et al. J. Biol. Chem. 1998;273:8971. doi: 10.1074/jbc.273.15.8971. [DOI] [PubMed] [Google Scholar]
- 21.Steiner E, et al. J. Cell Sci. 2006;119:459. doi: 10.1242/jcs.02773. [DOI] [PubMed] [Google Scholar]
- 22.Yi C, et al. Cancer Res. 2005;65:5835. doi: 10.1158/0008-5472.CAN-05-0423. [DOI] [PubMed] [Google Scholar]
- 23.Xiang Z, et al. Biochem. Biophys. Res. Commun. 2006;347:288. doi: 10.1016/j.bbrc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 24.Kolli S, Zito CI, Mossink MH, Wiemer EA, Bennett AM. J. Biol. Chem. 2004;279:29374. doi: 10.1074/jbc.M313955200. [DOI] [PubMed] [Google Scholar]
- 25.Mossink MH, et al. Cancer Res. 2002;62:7298. [PubMed] [Google Scholar]
- 26.Reiniger N, Ichikawa JK, Pier GB. Infect. Immun. 2005;73:6822. doi: 10.1128/IAI.73.10.6822-6830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes G, Alvarez D, Saus C, Alberti S. Infect. Immun. 2002;70:1075. doi: 10.1128/IAI.70.3.1075-1080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrager HM, Alberti S, Cywes C, Dougherty GJ, Wessels MR. J. Clin. Invest. 1998;101:1708. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St Geme 3rd JW, Falkow S. J. Infect. Dis. 1992;165(suppl. 1):S117. doi: 10.1093/infdis/165-supplement_1-s117. [DOI] [PubMed] [Google Scholar]
- 30.Mossink MH, et al. Immunology. 2003;110:58. doi: 10.1046/j.1365-2567.2003.01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann C, Golkaramnay E, Inman E, Rome L, Volknandt W. J. Cell Biol. 1999;144:1163. doi: 10.1083/jcb.144.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.