Abstract
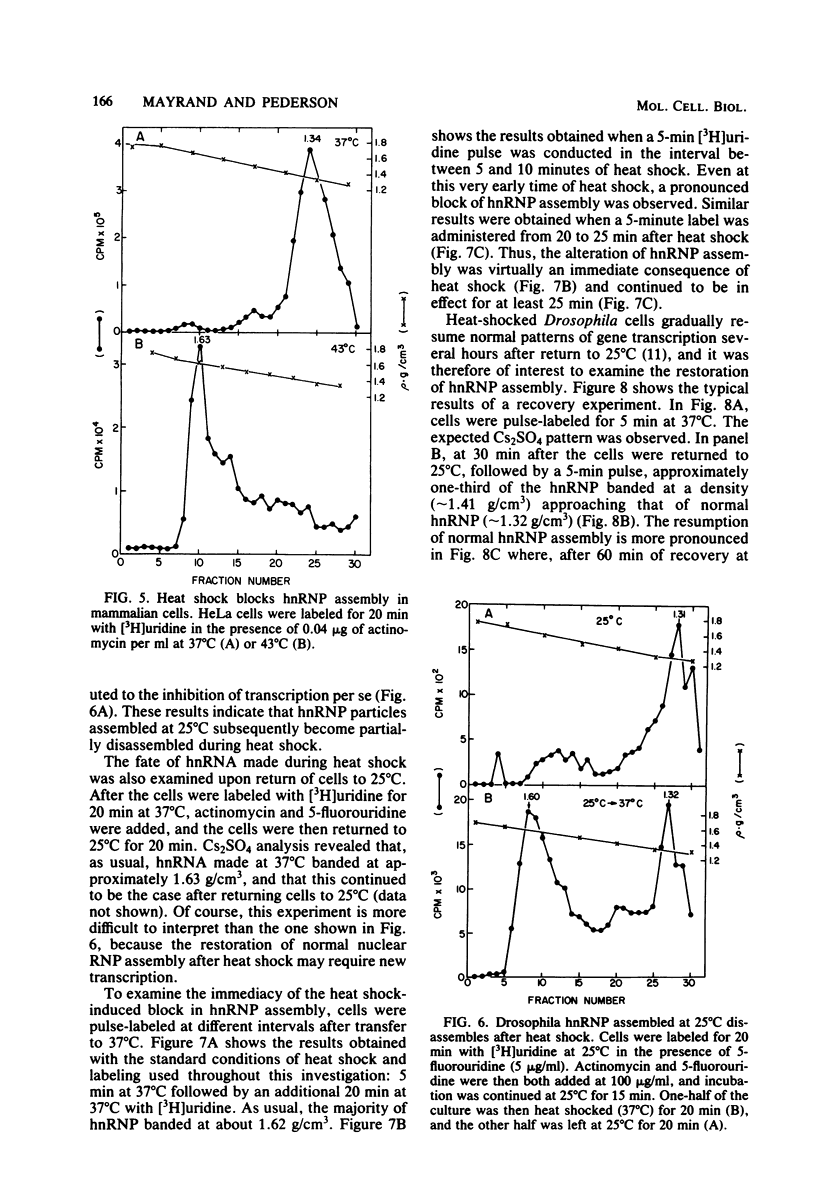
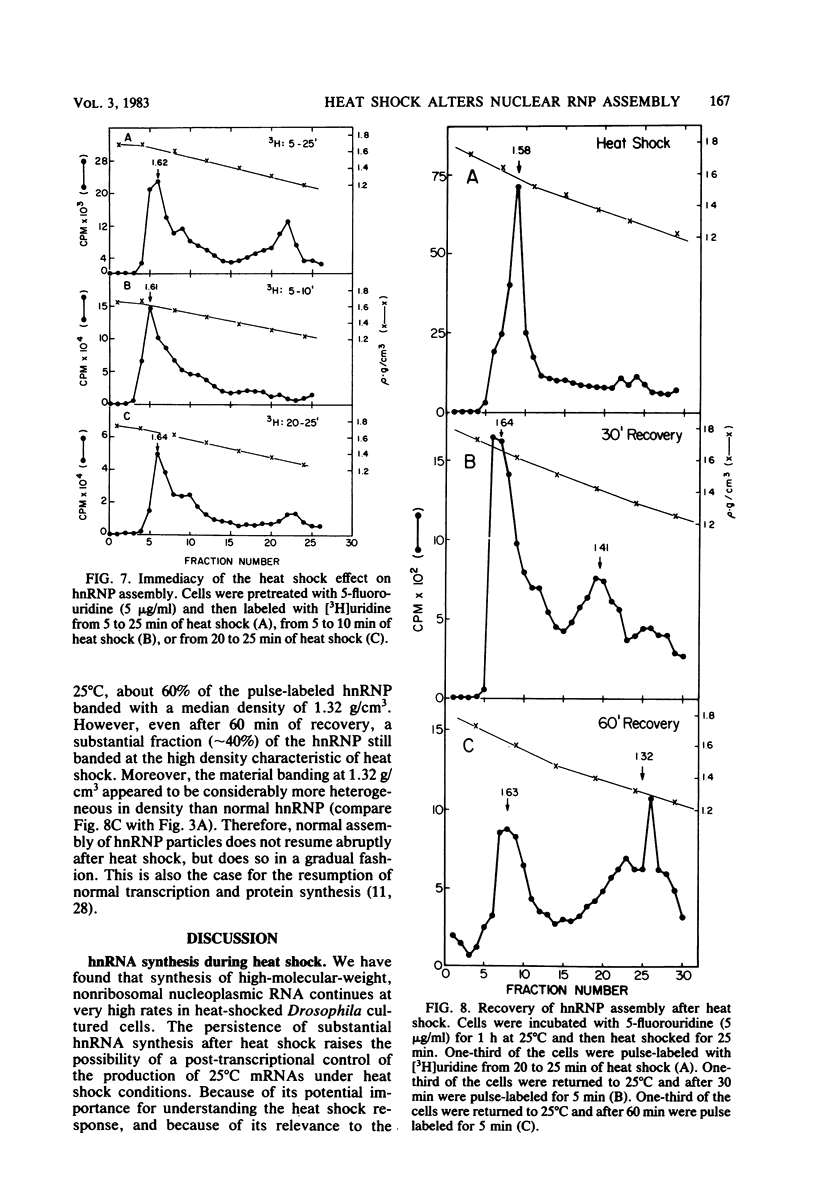
Heterogeneous nuclear RNA is normally complexed with a specific set of proteins, forming ribonucleoprotein particles termed hnRNP. These particles are likely to be involved in mRNA processing. We have found that the structure of hnRNP is profoundly altered during the heat shock response in Drosophila cultured cells. Although hnRNA continues to be synthesized at a near-normal rate during heat shock, its assembly into hnRNP is incomplete, as evidenced by a greatly decreased protein content of the particles in Cs2SO4 density gradients. RNA-protein cross-linking conducted in vivo (Mayrand and Pederson, Proc. Natl. Acad. Sci. U.S.A. 78:2208-2212, 1981) also reveals that hnRNA made during heat shock is complexed with greatly reduced amounts of protein. The block of hnRNP assembly occurs immediately upon heat shock, even before the onset of heat shock protein synthesis. Additional experiments reveal that hnRNP assembled normally at 25 degrees C subsequently disassembles during heat shock. The capacity for normal hnRNP assembly is gradually restored after heat-shocked cells are returned to 25 degrees C. Heat-shocked mammalian cells also show a similar block in hnRNP assembly. We suggest that incomplete assembly of hnRNP during heat shock leads to abortive processing of most mRNA precursors and favors the processing or export (or both) of others whose pathway of nuclear maturation is less dependent on, or even independent of, normal hnRNP particle structure. This hypothesis is compatible with a large number of previous observations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Berendes H. D. Factors involved in the expression of gene activity in polytene chromosomes. Chromosoma. 1968;24(4):418–437. doi: 10.1007/BF00285017. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. The effect of heat shock on RNA synthesis in Drosophila tissues. Cell. 1976 May;8(1):43–50. doi: 10.1016/0092-8674(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Meyer L. M., Pederson T. Small nuclear RNA U2 is base-paired to heterogeneous nuclear RNA. Science. 1982 Jul 30;217(4558):456–458. doi: 10.1126/science.6178162. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Base-pairing interactions between small nuclear RNAs and nuclear RNA precursors as revealed by psoralen cross-linking in vivo. Cell. 1981 Nov;26(3 Pt 1):363–370. doi: 10.1016/0092-8674(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Meselson M. Transcription at two heat shock loci in Drosophila. Cell. 1977 Oct;12(2):441–451. doi: 10.1016/0092-8674(77)90120-9. [DOI] [PubMed] [Google Scholar]
- Holmgren R., Corces V., Morimoto R., Blackman R., Meselson M. Sequence homologies in the 5' regions of four Drosophila heat-shock genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3775–3778. doi: 10.1073/pnas.78.6.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren R., Livak K., Morimoto R., Freund R., Meselson M. Studies of cloned sequences from four Drosophila heat shock loci. Cell. 1979 Dec;18(4):1359–1370. doi: 10.1016/0092-8674(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Houghton M., Jackson I. J., Porter A. G., Doel S. M., Catlin G. H., Barber C., Carey N. H. The absence of introns within a human fibroblast interferon gene. Nucleic Acids Res. 1981 Jan 24;9(2):247–266. doi: 10.1093/nar/9.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Isolation and characterization of ribonucleoprotein particles containing heterogeneous nuclear RNA. Methods Cell Biol. 1978;17:377–399. doi: 10.1016/s0091-679x(08)61155-3. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Lakhotia S. C., Mukherjee T. Specific activation of puff 93D of Drosophila melanogaster by benzamide and the effect of benzamide treatment on the heat shock induced puffing activity. Chromosoma. 1980;81(1):125–136. doi: 10.1007/BF00292427. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Adelman J., Franke A. E., Houck C. M., Gross M., Najarian R., Goeddel D. V. Human fibroblast interferon gene lacks introns. Nucleic Acids Res. 1981 Mar 11;9(5):1045–1052. doi: 10.1093/nar/9.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel J. A., Ransom L. J., Graham M. L., Pardue M. L. Transcription and metabolism of RNA from the Drosophila melanogaster heat shock puff site 93D. Chromosoma. 1980;80(3):237–252. doi: 10.1007/BF00292683. [DOI] [PubMed] [Google Scholar]
- Lengyel J., Penman S. hnRNA size and processing as related to different DNA content in two dipterans: Drosophila and Aedes. Cell. 1975 Jul;5(3):281–290. doi: 10.1016/0092-8674(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Levis R., Penman S. 5'-terminal structures of poly(A)+ cytoplasmic messenger RNA and of poly(A)+ and poly(A)- heterogeneous nuclear RNA of cells of the dipteran Drosophila melanogaster. J Mol Biol. 1978 Apr 25;120(4):487–515. doi: 10.1016/0022-2836(78)90350-9. [DOI] [PubMed] [Google Scholar]
- Levis R., Penman S. The metabolism of poly (A)+ and poly(A)-hnRNA in cultured Drosophila cells studied with a rapid uridine pulse-chase. Cell. 1977 May;11(1):105–113. doi: 10.1016/0092-8674(77)90321-x. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Freund R., Schweber M., Wensink P. C., Meselson M. Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5613–5617. doi: 10.1073/pnas.75.11.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGIO R., SIEKEVITZ P., PALADE G. E. STUDIES ON ISOLATED NUCLEI. II. ISOLATION AND CHEMICAL CHARACTERIZATION OF NUCLEOLAR AND NUCLEOPLASMIC SUBFRACTIONS. J Cell Biol. 1963 Aug;18:293–312. doi: 10.1083/jcb.18.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2208–2212. doi: 10.1073/pnas.78.4.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- PETRALLI J. K., MERIGAN T. C., WILBUR J. R. CIRCULATING INTERFERON AFTER MEASLES VACCINATION. N Engl J Med. 1965 Jul 22;273:198–201. doi: 10.1056/NEJM196507222730405. [DOI] [PubMed] [Google Scholar]
- Pederson T., Bhorjee J. S. Evidence for a role of RNA in eukaryotic chromosome structure. Metabolically stable, small nuclear RNA species are covalently linked to chromosomal DNA in HeLa cells. J Mol Biol. 1979 Mar 15;128(4):451–480. doi: 10.1016/0022-2836(79)90288-2. [DOI] [PubMed] [Google Scholar]
- Pederson T., Davis N. G. Messenger RNA processing and nuclear structure: isolation of nuclear ribonucleoprotein particles containing beta-globin messenger RNA precursors. J Cell Biol. 1980 Oct;87(1):47–54. doi: 10.1083/jcb.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Munroe S. H. Ribonucleoprotein organization of eukaryotic RNA. XV. Different nucleoprotein structures of globin messenger RNA sequences in nuclear and polyribosomal ribonucleoprotein particles. J Mol Biol. 1981 Aug 25;150(4):509–524. doi: 10.1016/0022-2836(81)90377-6. [DOI] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- RUIZ-GOMEZ J., ISAACS A. Interferon production by different viruses. Virology. 1963 Jan;19:8–12. doi: 10.1016/0042-6822(63)90018-7. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Derynck R., Fiers W. Evidence for a unique human fibroblast interferon (IFN-beta 1) chromosomal gene, devoid of intervening sequences. Nucleic Acids Res. 1981 Feb 11;9(3):461–471. doi: 10.1093/nar/9.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Pederson T. Small nuclear ribonucleoproteins of Drosophila: identification of U1 RNA-associated proteins and their behavior during heat shock. Mol Cell Biol. 1982 Aug;2(8):914–920. doi: 10.1128/mcb.2.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]