Abstract
Objective
Lower concentrations of adiponectin have been linked to subsequent risk of coronary heart disease in healthy individuals. Whether similar relationships exist for the development of systemic atherosclerosis, such as peripheral artery disease (PAD), is uncertain. We investigated the association between total adiponectin and risk of lower extremity PAD.
Methods and Results
We performed a prospective, nested case-control study among 18,225 male participants of the Health Professionals Follow-up Study who were free of diagnosed cardiovascular disease at the time of blood draw (1993-1995). During 14 years of follow-up, 143 men developed PAD. Using risk set sampling, controls were selected in a 3:1 ratio and matched on age, smoking status, fasting status, and date of blood draw (n=429). Median (interquartile range) adiponectin concentrations at baseline were lower among cases compared to controls (4.1 [3.2-5.5] vs. 5.4 [3.8-7.5] μg/mL; P<0.001). A log-linear inverse association was evident over the full spectrum of adiponectin concentrations with PAD risk after controlling for baseline cardiovascular risk factors using restricted spline conditional logistic regression. Adiponectin was associated with a 42% lower risk of PAD per SD increase in natural log-transformed adiponectin (Relative risk [RR], 0.58; 95% confidence interval [CI], 0.45-0.74) after adjustment for cardiovascular risk factors. The RR was attenuated (RR, 0.68; 95% CI, 0.51-0.92) after further accounting for high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, and cystatin C. Additional adjustment for hemoglobin A1c, triglycerides, and gamma-glutamyltransferase had little impact on this association (RR, 0.68; 95% CI, 0.50-0.92).
Conclusion
Total adiponectin is inversely associated with risk of symptomatic lower extremity PAD in men.
Keywords: Adiponectin, Atherosclerosis, Biomarker, Epidemiology, Peripheral Artery Disease
Introduction
Adiponectin is an adipocyte-derived protein that has gained considerable research interest because of its pleiotropic effects on insulin sensitivity, atherosclerosis and inflammation.1 In addition to a consistently lower risk of type 2 diabetes,2 higher adiponectin concentrations have also been associated with lower risk of cardiovascular disease (CVD) in several studies.3-5 More recent epidemiological reports, however, observed weaker inverse associations6, 7 after adjustment for high-density lipoprotein (HDL) cholesterol,8-10 questioning the putatively protective and independent role of adiponectin in atherosclerotic diseases.
Peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis that affects an estimated 10 million U.S. adults and is associated with reduced functional capacity11 and increased risk for cardiovascular morbidity and mortality.12, 13 Although cholesterol and inflammatory risk factors are also strong predictors in this form of CVD,14, 15 PAD is characterized by progressive luminal obstruction in peripheral arteries and may be less related to thrombosis or plaque rupture than are myocardial infarction (MI) or ischemic stroke.16, 17 This raises the possibility that factors with anti-atherosclerotic and anti-inflammatory properties, like adiponectin, may be of particular importance in the development of this type of CVD. To date, this association has only been investigated in a single prospective study limited to women, with findings of 65% lower risk of PAD across extreme tertiles of adiponectin.18
To evaluate the association of total adiponectin with risk of PAD in the lower extremities, we studied men enrolled in a longstanding and well-characterized cohort of men with information on numerous risk factors of atherosclerosis and inflammation and over a decade of follow-up for incident PAD.
Results
Baseline characteristics of cases and controls are presented in Table 1. Although we matched cases to controls on current smoking status, cases had greater total pack-years of smoking and also were more likely to have a history of diabetes, hypertension and hypercholesterolemia than controls. Cases had significantly lower concentrations of adiponectin and HDL cholesterol and higher levels of LDL cholesterol, CRP, triglycerides, HbA1c, creatinine and cystatin C. BMI levels were virtually identical at baseline among case and controls.
Table 1.
Baseline Characteristics of Men with Incident Lower Extremity Peripheral Artery Disease (Cases) and Matched Controls
| Characteristic | Cases (n=143) | Controls (n=429) | P |
|---|---|---|---|
| Age, y | 65.4 (8.1) | 65.3 (8.1) | Matched |
| Smoking status, n (%) | |||
| Current | 32 (22) | 90 (21) | |
| Past | 78 (55) | 242 (56) | Matched |
| Never | 23 (16) | 82 (19) | |
| Total adiponectin, μg/mL | 4.1 (3.2-5.5) | 5.4 (3.8-7.5) | <0.001 |
| HDL cholesterol, mg/dL | 41.7 (11.5) | 48.5 (14.2) | <0.001 |
| LDL cholesterol, mg/dL | 139.2 (34.8) | 131.4 (33.3) | 0.04 |
| Triglycerides, mg/dL | 143 (105-195) | 115 (80-165) | 0.001 |
| Hemoglobin A1c*, % | 5.56 (5.34-5.96) | 5.41 (5.24-5.59) | <0.001 |
| C-reactive protein, mg/dL | 2.24 (1.18-3.52) | 1.18 (0.51-2.26) | 0.02 |
| Cystatin C, mg/dL | 1.04 (0.91-1.22) | 0.96 (0.86-1.07) | <0.001 |
| Creatinine, mg/dL | 0.89 (0.80-1.02) | 0.87 (0.78-0.96) | 0.04 |
| eGFR, mL·min−1·1.73 m−2 ** | 86.8 (74.3-96.4) | 89.9 (82.3-96.3) | 0.02 |
| Gamma-glutamyltransferase, U/L | 27 (20-36) | 23.0 (17-31) | 0.15 |
| Pack-years of smoking, y | 26 (5-45) | 18 (1-35) | <0.001 |
| History of diabetes, n (%) | 28 (20) | 16 (4) | <0.001 |
| History of hypertension, n (%) | 70 (49) | 130 (30) | <0.001 |
| History of hypercholesterolemia, n (%) | 82 (57) | 187 (44) | 0.005 |
| Parental history of myocardial infarction before age 60 y, n (%) |
22 (15) | 44 (10) | 0.09 |
| Body mass index, kg/m2 | 25.8 (3.3) | 25.6 (4.4) | 0.48 |
| Physical activity, MET-h/wk | 22.7 (8.0-43.8) | 27.4 (10.3-52.8) | 0.003 |
| Alcohol consumption, g/day | 7.6 (0.9-17.8) | 9.8 (1.8-20.3) | 0.55 |
Data are expressed as mean (standard deviation), median (interquartile range) or number (percentage). P values are derived from generalized linear mixed models for continuous variables and Cochran-Mantel-Haenszel tests for categorical variables controlling for matched sets. Age, smoking status, fasting status and month of blood sampling were matching variables. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; MET-h, metabolic equivalent task-hours.
Data was missing for one case and four controls.
eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation.
We next examined the association between total adiponectin and specific cardiovascular risk factors among controls. Spearman age-adjusted partial correlation coefficients demonstrated positive associations between adiponectin and HDL (0.50, P<0.001), and LDL cholesterol (0.11, P=0.03), and negative associations between adiponectin and triglycerides (−0.43, P<0.001), BMI (−0.37, P<0.001), CRP (−0.23, P<0.001), HbA1c (−0.22, P<0.001) and γGT (−0.18, P<0.001); there were no significant associations with creatinine, cystatin C, eGFR, physical activity or alcohol consumption (all P>0.29). Adiponectin concentrations were also positively correlated with age (0.15, P=0.001). Results were similar if cases and controls were combined with additional adjustment for case-control status.
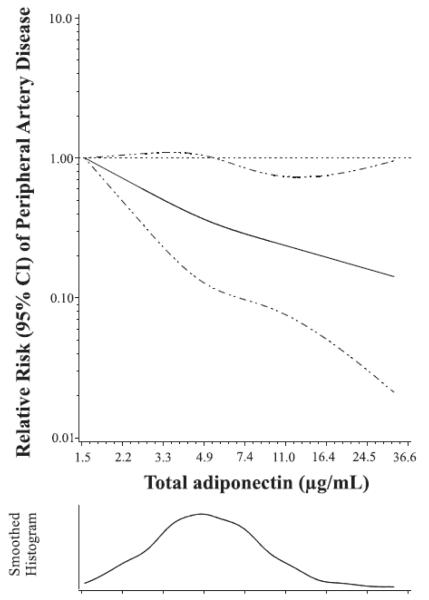
Table 2 displays the RRs of PAD for total adiponectin as both a categorical and continuous variable from conditional logistic regression. Subjects in the highest quartile of adiponectin had an 80% lower risk of PAD compared with the lowest quartile after adjustment for medical history and behavioral factors. Additional adjustment for HDL cholesterol, LDL cholesterol, CRP and cystatin C attenuated the association, but it remained significantly inverse. In all multivariable models, we observed a consistent linear trend for lower risk with increasing quartiles of adiponectin (All Ptrend <0.01). The adjusted restricted spline curve confirmed the log-linear inverse association over the full spectrum of adiponectin concentrations with risk of PAD (Plinear=0.009; multivariable model 2; Figure).
Table 2.
Relative Risks (95% Confidence Intervals) of Lower Extremity Peripheral Artery Disease According to Total Adiponectin
| Continuous Adiponectin (per Each SD Increase in ln-Transformed Variable) |
Quartile of Adiponectin, Median (Range), μg/mL |
|||||
|---|---|---|---|---|---|---|
| 2.84 (1.54-3.62) | 4.28 (3.63-5.05) | 5.98 (5.06-7.16) | 9.02 (7.17-31.9) | P trend | ||
| Cases, n | 143 | 53 | 44 | 27 | 19 | |
| Controls, n | 429 | 90 | 99 | 116 | 124 | |
| Matching variables | 0.58 (0.47-0.72) | 1.00 (Reference) | 0.73 (0.45-1.19) | 0.39 (0.23-0.66) | 0.24 (0.13-0.44) | <0.0001 |
| Multivariable model 1 | 0.58 (0.45-0.74) | 1.00 (Reference) | 0.62 (0.35-1.10) | 0.43 (0.24-0.79) | 0.20 (0.10-0.42) | <0.0001 |
| Multivariable model 2 | 0.68 (0.51-0.92) | 1.00 (Reference) | 0.47 (0.24-0.91) | 0.52 (0.27-1.00) | 0.28 (0.12-0.67) | 0.005 |
| Multivariable model 3 | 0.68 (0.50-0.92) | 1.00 (Reference) | 0.48 (0.25-0.94) | 0.55 (0.28-1.08) | 0.28 (0.12-0.66) | 0.006 |
Relative risks are derived from conditional logistic regression models. Matching variables were age, smoking status, fasting status and date of blood draw. Ptrend is calculated by treating the median of natural log-transformed adiponectin concentrations of each quartile as a continuous variable. Multivariable model 1 is adjusted for pack-years of smoking, history of type 2 diabetes, history of hypertension, history of hypercholesterolemia, parental history of myocardial infarction before age 60 y, body mass index and physical activity. Multivariable model 2 is model 1 + high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein and cystatin C. Multivariable model 3 is model 2 + hemoglobin A1c, triglycerides and gamma-glutamyltransferase.
Figure.
Dose-response relationship between total adiponectin and relative risk of peripheral artery disease (PAD).
Figure depicts the adjusted relative risk of PAD as a function of natural log-transformed plasma adiponectin concentrations which were then back-transformed to the original values for ease of interpretation. Data are fitted by a spline conditional logistic regression model with three knots. Age, smoking status, fasting status and date of blood sampling were matching variables. The model is adjusted for high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, cystatin C, pack-years of smoking, history of type 2 diabetes, history of hypertension, and history of hypercholesterolemia, parental history of myocardial infarction before age 60 y, body mass index, and physical activity. The 95% confidence interval is indicated by the dashed lines.
We also examined the RR associated per each SD increment in continuous natural log-transformed total adiponectin in successive models including potentially intermediary variables. The multivariable-adjusted RR was 0.58 (95% CI, 0.45-0.74; multivariable model 1), and only modestly attenuated to 0.67 (95% CI, 0.51-0.89) after additionally accounting for both HDL and LDL cholesterol and to 0.68 (95% CI, 0.51-0.92) after also including CRP and cystatin C (multivariable model 2). Despite their correlations with adiponectin and associations with case-control status, adjustment for triglycerides, HbA1c and γGT did not materially influence the association between adiponectin and PAD when added to the model (RR 0.68; 95% CI 0.50-0.92; multivariable model 3).
We performed several sensitivity analyses. We first stratified by HDL cholesterol level since it was strongly correlated with adiponectin and attenuated the effect estimate for adiponectin to the greatest degree. The adjusted RRs for PAD per SD increment in adiponectin were 0.80 (95% CI: 0.32-2.03; n=76 cases) among men with HDL cholesterol levels ≤40 mg/dL and 0.65 (95% CI: 0.39-1.06; n=67 cases), respectively among men with levels >40 mg/dL. Similar results were obtained when cystatin C was substituted for eGFR, as gross measure of kidney function, or when subjects with imputed HbA1c were excluded. We also repeated the analysis excluding 18 cases based only on a confirmed physician’s diagnosis (RR, 0.70; 95% CI, 0.51-0.95) or with prevalent diabetes (baseline history of type 2 diabetes or HbA1c ≥6.5%) (RR, 0.64; 95% CI, 0.45-0.93).
Finally, we found no significant interactions between total adiponectin and time to diagnosis after initial blood collection, predicted risk of PAD (based on multivariable model 2 without adiponectin), age, current smoking, and fasting status. We also did not observe an interaction with potential biochemical markers of PAD risk (All Pinteraction>0.10).
Meta-Analysis of Prospective Studies on Total Adiponectin and Risk of PAD
The previous prospective study reported an RR of 0.35 (95% CI, 0.17-0.74) when the lowest tertile of total adiponectin concentrations was compared to the highest. The RR comparing the first and third tertiles in this study was 0.28 (95% CI, 0.13-0.59). When tertile-specific estimates from the two studies were meta-analyzed, the RR across tertiles was 0.31 (95% CI, 0.13-0.49) with no evidence for heterogeneity among the findings of these studies (χ21=0.14; Pheterogeneity=0.71; I2=0.0%).
Discussion
In this nested case-control study, we found a strong inverse association between total adiponectin and risk of lower extremity PAD in otherwise healthy men. This association was apparent over the entire range of adiponectin concentrations and persisted even after controlling for traditional biochemical risk factors such as HDL cholesterol, LDL cholesterol, CRP and other established PAD risk factors including cumulative lifelong smoking, hypertension, hypercholesterolemia and diabetes. No interactions were observed between adiponectin and lipids, and markers of glycemic control, inflammation, or kidney function.
To our knowledge, only one prospective study has investigated the effect of adiponectin on risk of incident PAD.18 Similar to our findings in men, data from the Women’s Health Study showed a strong inverse association between total and high-molecular weight adiponectin with risk of symptomatic PAD (defined as intermittent claudication or PAD revascularization) among 110 women with incident PAD and 230 controls. When tertile-specific estimates from that study and ours were meta-analyzed, we observed a 69% lower risk, again suggesting a particularly strong inverse association of adiponectin with PAD, even relative to other forms of CVD.
Several potential mechanisms could explain the lower risk of PAD associated with higher adiponectin concentrations. Adiponectin may suppress smooth muscle cell proliferation and foam cell formation of macrophages and inhibit monocytic cell adhesion to endothelial cells. It may suppress inflammatory pathways in endothelial cells through down regulation of the nuclear factor kappa B (NF-κB) pathway, a key regulator in tumor necrosis factor alpha (TNF-α) and other cytokines.1, 27 Adiponectin also reduces the development of atherosclerosis in mice prone to its development, 28 whereas in humans, low adiponectin may predict coronary artery disease severity and progression.29, 30 Also, adiponectin may lower the risk of atherosclerosis through its effect on insulin sensitivity,31 and subsequently lower risk of type 2 diabetes.2
Although compelling experimental evidence suggests a protective role of adiponectin in atherosclerosis, prospective data on adiponectin and risk of forms of CVD other than PAD seem less consistent.3-10 There are a number of possible explanations for this discrepancy. First, heterogeneity in case definitions may play a role: combining atherosclerotic events inversely related to adiponectin with other cardiovascular outcomes less immediately related to atherosclerosis, such as heart failure and fatal CVD (including sudden cardiac death). Second, the discrepancy may relate to the paradoxically increased risk of all-cause and CVD mortality associated with adiponectin mostly observed among older adults32-34 and patients with prevalent heart failure,35 CVD36, 37, PAD38 or on hemodialysis39 but also among apparently healthy men free of coronary artery disease.37 Adiponectin has pleiotropic roles beyond its known insulin-sensitizing actions, including clearance of apoptotic cells.40 As a result, it may directly improve insulin sensitivity and endothelial function even as levels rise in response to ongoing processes that lead to cellular apoptosis and necrosis and presumably mortality.Regardless, in our population of otherwise healthy subjects, we found no indication that the association between adiponectin and PAD risk differed over our age range or over predicted probabilities of PAD, suggesting that adiponectin acts similarly on risk of PAD among individuals at lower and higher risk. Third, we cannot rule out a gender difference, since the independent association between adiponectin and other forms of CVD so far has predominantly been demonstrated in men4, 5 and not in women9, 31 even in mixed-gender studies7, 41 although results from the Women’s Health Study make this possibility less likely.18
The potentially confounding or modifying role of kidney disease in the association between adiponectin and risk of CVD has not been extensively investigated. Most previous studies of adiponectin and PAD18 or CVD4-10 have not included measures of kidney function. Impaired kidney function may increase circulating adiponectin levels,42 although we did not observe a correlation between adiponectin and estimates of kidney function. The association of adiponectin with risk of PAD did not differ by levels of creatinine, cystatin C, or eGFR in this study although most participants had normal kidney function and only very few (<5%) would have met criteria for moderate renal insufficiency (eGFR <60 mL · min−1 · 1.73 m−2 according to the CKD-EPI equation).
The association of adiponectin with PAD risk persisted after adjustment for HDL cholesterol, despite the strong positive correlation between the two variables at baseline. Given the attenuation of the association after HDL cholesterol adjustment noted by others3-10 and us, the effect of adiponectin on the vascular system may be mediated in part through HDL cholesterol metabolism. Indeed, adiponectin may accelerate reverse cholesterol transport by increasing HDL assembly in the liver through increased expression and secretion of apolipoprotein A-I and ATP-binding cassette transporter 1 (ABCA1) in the liver.43 Further, in macrophages, adiponectin leads to up-regulation of the expression of ABCA1 and increased HDL-mediated cholesterol efflux.44 Adiponectin may also have a direct role on HDL catabolism through apolipoprotein A-I metabolism.45
Strengths of the current study are the variety of biochemical and traditional risk factors that we included, the use of risk set sampling to select controls, the prospective design, the long-term follow-up, the homogeneity of our study population, and the use of adjudicated events with a pronounced atherosclerotic origin. Some potential limitations merit consideration. First, we used symptomatic PAD as an endpoint. Subclinical or asymptomatic PAD, which potentially could have been detected by ABI screening, may have been missed, similar to asymptomatic coronary or cerebral atherosclerosis in studies on MI or stroke. However, end points included in this analysis were confirmed by medical records, reducing the likelihood of false-positive cases albeit at the risk of false-negative non-cases. Moreover, this definition encompasses a degree of severity that is of unequivocal importance to both patients and physicians. Second, we studied men of predominantly Caucasian descent, although we have no reason to assume that adiponectin might be of less importance in other ethnicities.46, 47 Thirdly, adiponectin concentrations, and included covariables alike, were only determined at baseline, although intra-individual concentrations of adiponectin are reasonably stable over time,48 and changes over time would result in exposure misclassification and attenuate the results toward the null. Finally, our findings are observational and despite the wide variety of potentially confounding factors we adjusted for, unmeasured or residual confounding may be present. However, it should also be noted that some of the biochemical variables we adjusted for may be in the causal pathway, which would underestimate the true relationship between adiponectin and risk of PAD.
In conclusion, we observed a strong linear inverse association between total adiponectin and risk of symptomatic lower extremity PAD in men free of manifest atherosclerotic disease. The association appeared to be independent of important biochemical or traditional clinical risk factors of CVD. These findings suggest a prominent role of adiponectin in the initiation and progression of atherosclerotic diseases such as PAD. Future mechanistic studies and prospective studies with well-characterized individuals are warranted to better understand the role of adiponectin in atherosclerosis.
Methods
Study Population
We followed participants enrolled in the Health Professionals Follow-up Study, a prospective cohort of 51,529 US male health care professionals aged 40 to 75 years at baseline in 1986. In 1993, a blood sample was requested from all living participants, and 18,225 subjects provided samples. Responders were somewhat younger but were otherwise similar to non-responders. Drawing from the cohort that provided blood samples, and after the exclusion of participants with a history of CVD (defined as any report of cerebrovascular disease, coronary heart disease, and PAD) before 1994, we identified 143 participants with incident PAD between the date of blood collection and January 31, 2008. Controls were randomly selected from participants with a blood sample who did not have a history of CVD at baseline and who were alive and free of PAD at time of diagnosis of the matched case according to risk set sampling.19 For controls, we used a 3:1 ratio and matched on age, smoking status, fasting status and month and year of blood collection resulting in 429 controls available for the analysis. All subjects gave written informed consent, and the institutional review boards of the Harvard School of Public Health and Beth Israel Deaconess Medical Center approved the study protocol.
Ascertainment of Lower Extremity PAD
Participants reported the occurrence of diagnosed medical conditions, including claudication and revascularization for arterial disease of the leg on biennial mailed questionnaires. Lower extremity PAD cases were defined as arterial disease below the aortic bifurcation (i.e., excluding abdominal aortic aneurysm and renal artery stenosis) and were verified with medical records as previously described.20 In short, cases were confirmed if records showed one of the following (in order of severity/certainty): (1) report of amputation, bypass, or other revascularization procedure for occlusive artery disease (n=87; 64%), (2) angiogram or Doppler ultrasound showing at least 50% stenosis of at least one artery with congruent symptoms in the ipsilateral limb (n=15; 10%), (3) ankle-brachial index (ABI) <0.90 (n=23; 16%), or (4) physician’s diagnosis (n=18; 13%). We performed sensitivity analyses excluding cases with physician diagnosis alone.
Measurement of Biochemical Variables
Blood samples were collected as previously described.4 In short, liquid EDTA tubes, placed on ice packs, stored in styrofoam containers, were returned to our laboratory via overnight courier, and centrifuged and aliquoted for storage in liquid nitrogen freezers (−130°C or colder). Fifty-nine percent of the participants in the present analysis provided fasting blood samples (defined as ≥8 hours since last meal). All analyses were performed in a laboratory certified by the Centers for Disease Control and Prevention/ National Heart, Lung, and Blood Institute Lipid Standardization Program with commercially available analytic systems. Total adiponectin was measured using an ELISA method from ALPCO Diagnostics Inc. (Salem, NH) with a coefficient of variation of 10%. The determination of HDL cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides were simultaneously performed using reagents and calibrators from Roche Diagnostics (Indianapolis, IN). Measurements of gamma-glutamyltransferase (γGT) and creatinine were based on enzymatic assays using reagents and calibrators from Roche; measurement of hemoglobin A1c (HbA1c) was based on turbidimetric immunoinhibition using packed red cells and measurement of high-sensitivity C-reactive protein (CRP) and cystatin C were based on an immunoturbidimetric assay, using reagents and calibrators from DiaSorin (Stillwater, MN) and Genzyme (Stillwater, MN), respectively. Coefficients of variation for these biochemical markers were 8% or less.
Assessment of Medical History, Anthropometric Data, and Lifestyle Factors
Men reported their medical history, anthropometric data, and lifestyle habits by questionnaire in 1994. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Physical activity was expressed as metabolic equivalent task–hours based on self-reported types and durations of activities over the previous year. Previous studies demonstrated that the validity and reproducibility of these self-reported measures is high.21-24 Estimated glomerular filtration rate (eGFR) was calculated from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.25
Statistical Analyses
Continuous data are summarized as either mean ± standard deviation (SD) for normally distributed variables or median and interquartile range for non-normally distributed variables. Categorical data are expressed as percentages. We used generalized linear mixed models and Cochran-Mantel-Haenszel tests to compare continuous variables and categorical variables by case/control status, respectively, accounting for clustering by matching status. Associations between adiponectin and PAD risk factors were examined using age-adjusted Spearman partial correlation coefficients.
To examine the association between adiponectin and subsequent PAD risk, we treated adiponectin as both a categorical (in quartiles) and continuous (per SD increase) variable. We used conditional logistic regression to estimate the odds ratio and 95% confidence interval (CI) for future PAD associated with increasing concentrations of adiponectin. Because the controls were selected using risk set sampling, the odds ratios derived from conditional logistic regression provide unbiased estimates of the incidence rate ratio as a measure of relative risk (RR). Besides the matching variables, multivariable-adjusted RRs were adjusted for pack-years of smoking, history of type 2 diabetes, hypertension, and hypercholesterolemia, parental history of MI, BMI and physical activity (multivariable model 1), HDL cholesterol, LDL cholesterol and natural log-transformed CRP and cystatin C (multivariable model 2) and other confounders or potential mediators: triglyceride, HbA1c and γGT (all natural log-transformed) (multivariable model 3). Adjustment for smoking intensity did not provide further information after accounting for pack-years, nor did adjustment for incident type 2 diabetes that occurred after baseline. To test for a linear trend in the categorical analysis, we treated the median natural log-transformed value of each adiponectin quartile as a linear variable. We examined the potential dose-response relationship between adiponectin and risk for PAD by fitting restricted cubic splines to a conditional logistic regression model.26 Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Red blood cells with which to measure HbA1c were unavailable in 1 case and 4 controls. These values were imputed using BMI and age.
We tested interactions with cross-product terms between (continuous) natural log-transformed adiponectin concentrations and selected variables in conditional and unconditional logistic regression models with additional adjustment for the matching factors. To determine whether adiponectin interacted with overall risk of PAD, we ascertained predicted probabilities of PAD for each individual in a logistic regression model using multivariable model 2 without adiponectin and examined interaction of adiponectin with this probability.
Finally, we meta-analyzed our data with the only previous prospective study on adiponectin and risk of PAD published to date to our knowledge.18 Heterogeneity was assessed by standard χ2 tests and the I2 statistic. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and STATA version 11 (StataCorp, College Station, Texas). A two-sided P value of <0.05 was considered statistically significant.
Significance.
Lower extremity peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis which has received considerably less clinical and research attention than coronary or cerebrovascular disease. Although cholesterol and inflammatory risk factors are also strong predictors in this form of cardiovascular disease (CVD), PAD may be less related to thrombosis or plaque rupture. This raises the possibility that factors with anti-atherosclerotic and anti-inflammatory properties, like adiponectin, may be of particular importance in the etiology of this predominantly atherosclerotic type of CVD. To study this, we prospectively followed middle-aged male health professionals without existing CVD. After traditional cardiovascular risk factors were taken into account, each standard deviation increase in total adiponectin was associated with a 32% lower risk of symptomatic PAD. Given the inconsistencies in independent associations between adiponectin and other forms of CVD, this finding, if confirmed, suggests a more prominent role of adiponectin in the development of (peripheral) atherosclerosis.
Acknowledgments
Sources of Funding This work was supported by research grants R01 HL091874, R01 DE017176, HL35464 and CA55075 from National Institute of Health.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1–12. doi: 10.1016/j.cccn.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 4.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 5.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: A population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: Results from the 18-year follow-up of a large cohort from southern germany. J Am Coll Cardiol. 2006;48:1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Hu FB, Girman CJ, Rifai N, Manson JE, Rexrode KM, Rimm EB. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219:322–329. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cote M, Cartier A, Reuwer AQ, Arsenault BJ, Lemieux I, Despres JP, Wareham NJ, Kastelein JJ, Boekholdt SM, Khaw KT. Adiponectin and risk of coronary heart disease in apparently healthy men and women (from the epic-norfolk prospective population study) Am J Cardiol. 2011;108:367–373. doi: 10.1016/j.amjcard.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: Morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s heart disease: A textbook of cardiovascular medicine. Saunders Elsevier; Philadelphia, PA: 2011. pp. 1338–1358. [Google Scholar]
- 17.Dhaliwal G, Mukherjee D. Peripheral arterial disease: Epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16:36–44. doi: 10.1055/s-0031-1278244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho DY, Cook NR, Britton KA, Kim E, Creager MA, Ridker PM, Pradhan AD. High-molecular-weight and total adiponectin levels and incident symptomatic peripheral artery disease in women / clinical perspective. Circulation. 2011;124:2303–2311. doi: 10.1161/CIRCULATIONAHA.111.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 20.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Ascherio A, Rimm E, Giovannucci E, Colditz G, Rosner B, Willett W, Sacks F, Stampfer M. A prospective study of nutritional factors and hypertension among us men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 24.Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60:74–79. doi: 10.2337/db10-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial nf-kappab signaling through a camp-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 29.von Eynatten M, Schneider JG, Humpert PM, Kreuzer J, Kuecherer H, Katus HA, Nawroth PP, Dugi KA. Serum adiponectin levels are an independent predictor of the extent of coronary artery disease in men. J Am Coll Cardiol. 2006;47:2124–2126. doi: 10.1016/j.jacc.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 32.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 33.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab. 2008;93:3357–3364. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kizer JR, Benkeser D, Arnold AM, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Defilippi CR, Newman AB, Djousse L. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: The cardiovascular health study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 36.Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CDA, Snijder MB, Bouter LM, Matsuzawa Y, Shimomura I, Heine RJ. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 37.Hascoet S, Elbaz M, Bongard V, Bouisset F, Verdier C, Vindis C, Genoux A, Taraszkiewicz D, Perret B, Galinier M, Carrié D, Ferrières J, Ruidavets JB. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler Thromb Vasc Biol. 2013;33:e19–e29. doi: 10.1161/ATVBAHA.112.300079. [DOI] [PubMed] [Google Scholar]
- 38.Dieplinger B, Haltmayer M, Poelz W, Mueller T. Value of adiponectin as predictor of 5-year all-cause mortality in patients with symptomatic peripheral arterial disease: Results from the linz peripheral arterial disease (lipad) study. Clin Chim Acta. 2009;408:87–91. doi: 10.1016/j.cca.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 40.Fraser DA, Tenner AJ. Directing an appropriate immune response: The role of defense collagens and other soluble pattern recognition molecules. Curr Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 41.Urbonaviciene G, Frystyk J, Flyvbjerg A, Henneberg EW, Lindholt JS. Association of serum adiponectin with risk for cardiovascular events in patients with peripheral arterial disease. Atherosclerosis. 2010;210:619–624. doi: 10.1016/j.atherosclerosis.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Iwashima Y, Horio T, Kumada M, Suzuki Y, Kihara S, Rakugi H, Kawano Y, Funahashi T, Ogihara T. Adiponectin and renal function, and implication as a risk of cardiovascular disease. Am J Cardiol. 2006;98:1603–1608. doi: 10.1016/j.amjcard.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 43.Matsuura F, Oku H, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Maeda N, Tsujii K, Ishigami M, Nishida M, Hirano K, Kihara S, Hori M, Shimomura I, Yamashita S. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun. 2007;358:1091–1095. doi: 10.1016/j.bbrc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 44.Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, Nakatani K, Nakaoka H, Kawase R, Yuasa-Kawase M, Masuda D, Ohama T, Maeda N, Nakagawa-Toyama Y, Ishigami M, Nishida M, Kihara S, Shimomura I, Yamashita S. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Verges B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, Gambert P. Adiponectin is an important determinant of apoa-i catabolism. Arterioscler Thromb Vasc Biol. 2006;26:1364–1369. doi: 10.1161/01.ATV.0000219611.50066.bd. [DOI] [PubMed] [Google Scholar]
- 46.Chen CY, Asakura M, Asanuma H, Hasegawa T, Tanaka J, Toh N, Min KD, Kanzaki H, Takahama H, Amaki M, Itoh Y, Ichien G, Okumoto Y, Funahashi T, Kim J, Kitakaze M. Plasma adiponectin levels predict cardiovascular events in the observational arita cohort study in japan: The importance of the plasma adiponectin levels. Hypertens Res. 2012 doi: 10.1038/hr.2012.42. [DOI] [PubMed] [Google Scholar]
- 47.Zhang BC, Liu WJ, Che WL, Xu YW. Serum total adiponectin level and risk of cardiovascular disease in han chinese populations: A meta-analysis of 17 case-control studies. Clin Endocrinol (Oxf) 2012;77:370–378. doi: 10.1111/j.1365-2265.2011.04260.x. doi: 310.1111/j.1365-2265.2011.04260.x. [DOI] [PubMed] [Google Scholar]
- 48.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: Stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–652. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]