Abstract
Immunoglobulin heavy chain (Igh) genes are assembled by sequential rearrangements of diversity (DH) and variable (VH) gene segments. Three critical constraints govern VH recombination. These include timing (VH recombination follows DH recombination), precision (VHs recombine only to DJH junctions) and allele specificity (VH recombination is restricted to DJH recombined alleles). We provide a model for these universal features of VH recombination. Analyses of DJH recombined alleles revealed that DJH junctions were selectively epigenetically marked, became nuclease sensitive and bound RAG proteins, thereby permitting DH-associated recombination signal sequences to initiate the second step of Igh gene assembly. We propose that VH recombination is precise because these changes did not extend to germline DH gene segments located 5′ of the DJH junction.
INTRODUCTION
Genes that encode antigen receptors of lymphocytes are assembled via DNA recombination events that juxtapose gene segments spread over several megabases of the genome. V(D)J recombination, as this process is known, is precisely coordinated to the lineage- and developmental stage of lymphocytes1,2. Thus, immunoglobulin (Ig) genes rearrange in the B lymphocyte lineage whereas T cell receptor genes rearrange in the T lymphocyte lineage. Within the B lineage, Ig heavy chain (Igh) genes rearrange first, followed by Ig light chain (Igk, Igl) genes; similarly, within the T lineage Tcrb chain genes rearrange first followed by Tcra genes. The loci that rearrange first in each lineage (Igh and Tcrb) consist of variable (V), diversity (D) and joining (J) gene segments and require two recombination events to generate fully recombined alleles. In each case, D to J recombination precedes V recombination to the pre-formed DJ junction to produce VDJ recombined alleles. Thus, understanding antigen receptor gene assembly involves uncovering mechanisms that (a) select a locus for rearrangement and (b) impose the order of V(D)J recombination at the Igh and Tcrb loci.
V(D)J recombination requires recruitment of the recombination activating gene products, RAG1 and RAG2, to loci destined for rearrangement. Thereafter, RAG1-RAG2 introduce double-strand breaks at special recombination signal sequences (RSSs) that flank gene segments to initiate recombination. The accessibility of a locus to RAG recombinase determines the choice of the antigen receptor gene that will recombine. This is termed the accessibility hypothesis3. Accessibility, in turn, is regulated by cis-acting accessibility control elements (ACEs) which coincide with promoters and enhancers within antigen receptor loci4. At one level, therefore, the order of B cell antigen receptor gene rearrangements can be viewed as Igh accessibility preceding Igk accessibility, and within the Igh locus, DH gene segments becoming accessible before the VH gene segments.
From the earliest formulation of the accessibility hypothesis chromatin structure has been considered to be a key determinant of locus accessibility5,6; however, molecular features that distinguish between accessible and inaccessible loci are just beginning to be understood7–9. All antigen receptor loci contain acetylated histones prior to initiation of recombination in the appropriate lymphocyte lineage and at the appropriate developmental stage1,4,10. Where examined, rearrangeable loci are also marked with activation-associated histone methylation, such as di- or tri- methylation of lysine 4 of histone H3 (H3K4me2, me3). Conversely, the repressive histone modification H3 lysine 9 di-methylation (H3K9me2) is reduced prior to recombination11,12. Moreover, recruitment of the H3K9 methyl transferase G9a to recombination substrate attenuates recombination thereby providing direct evidence of the inhibitory effects of this modification13. The function of specific positive modifications in V(D)J recombination remains unclear, however, because it is difficult to modulate these marks independently of one another and assess the effects on recombination. The recognition that PHD domain of RAG2 binds H3K4me3 leads to a model where epigenetic histone modifications mark a locus for RAG1-RAG2 recruitment14–16.
The Igh locus comprises approximately 150 VH gene segments, 8–12 DH gene segments and 4 JH gene segments17. The initial activation of DH (rather than VH) recombination and the preferential usage of certain DH gene segments are explained by several observations. First, analyses of RAG-deficient pro-B cells show that only the 5′-and 3′-most DH gene segments (DFL16.1 and DQ52 respectively) and the region encompassing the JH gene segments extending until the Cμ exons have hallmarks of active chromatin11,18. These include the presence of activating histone modifications, nuclease sensitivity and pockets of DNA demethylation (R. Selimyan, I.I, R.Su., F.W.A., R.Se, et al., submitted for publication). The absence of such marks at the VH locus leads to a model that VH gene segments are relatively inaccessible to recombinase at this stage19. Second, the JH region exhibits the greatest density of RAG proteins within the Igh locus20; in contrast, RAG proteins are undetectable at VH genes in pro-B cells. Thus, recombinase is perfectly positioned to initiate DH rather than VH recombination. Third, the 3′ end of the Igh locus has been proposed to fold into a 3-loop structure that places the 5′- and 3′-most DH gene segments closest to the RAG-rich recombination center21. This spatial configuration maximizes the chance of JH-associated RAG proteins to find complementary DH-RSSs in the first recombination step. Fourth, a recombination barrier element has been recently identified 5′ of DFL16.1 that prevents VH recombination to germline DH gene segments22. Binding sites for the insulator protein CTCF within this element are essential for barrier activity23.
With plausible models for the regulation of DH recombination in place, it is imperative to study the second step of Igh gene assembly. VH recombination is regulated at multiple levels, such as preferential recombination of proximal VH gene families, IL-7 responsiveness of the VHJ558 genes located at the 5′ end of the locus, and feedback inhibition of VH recombination24,25. Before these features of VH gene segment selectivity come into play, however, three general aspects of VH recombination must be addressed. First, why does VH recombination always follow DH recombination? Second, why does VH recombination occur selectively on DJH recombined alleles? Third, what is the mechanism that directs VH gene segments to recombine to the DJH junction? The exquisite precision of this latter point is noteworthy because the closest unrearranged DH gene segment 5′ of a DJH junction is located only 4 kb away; yet, VH gene segments from more than a megabase away find the DJH junction and not the adjacent germline DH gene segment.
We reasoned that answers to these questions likely lay in the chromatin structure and RAG recruitment profile of DJH recombined alleles, and therefore analyzed changes that occur after the first step of Igh recombination. We demonstrate that DJH junctions were selectively activated as measured by highly localized changes in histone modifications and nuclease sensitivity. The absence of activating histone modifications on Eμ-deficient DJH recombined alleles points to a key role for Eμ in mediating these changes and explains the loss of VH to DJH recombination on Eμ-deleted alleles. We also show that RAG1-RAG2 binding is re-distributed towards the DJH junction on WT alleles and does not extend to the closest unrearranged DH gene segment. We propose DH recombination brings the associated DH-RSS to the recombination center, thereby permitting its use to initiate the second step of Igh gene assembly. Because RAG proteins are not present at upstream germline DH gene segments to initiate recombination, VH genes recombine specifically to the DJH junctions.
RESULTS
Localized activation of DJH junctions
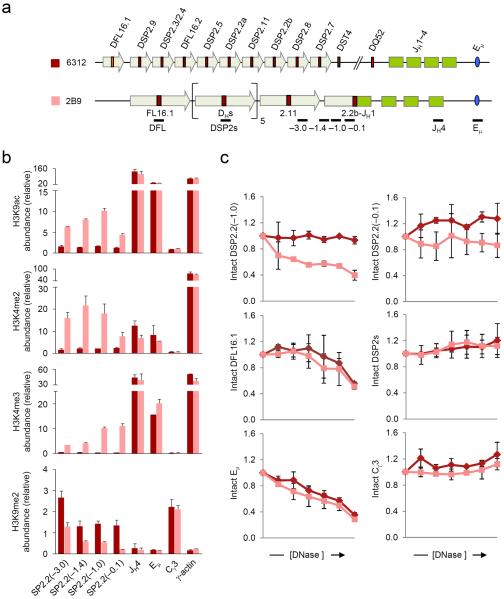
To analyze the state of DJH rearranged loci we generated a panel of cell lines that contain specific Igh rearrangements. For this we transiently transfected RAG2-deficient fetal liver-derived 6312 cells with a RAG2 expression vector and isolated single-cell clones with recombined Igh alleles. Because RAG2 was expressed transiently these clones were genetically stable thereafter. DH recombination was assayed by PCR (Supplementary Fig. 1) and we used representative clones in chromatin assays. We first examined the changes that accompany rearrangement of a DSP gene segment located in the middle of the DH cluster. One allele in 2B9 cells has a DSP2.2b-JH1 rearrangement and the second allele has undergone VH recombination, thereby deleting all unrearranged DH gene segments (Fig. 1a). We designed primers specific to the 5′ region of the rearranged DSP2.2b gene segment and compared the histone modification state of the DJH rearranged allele to germline alleles in the parental cell line.
Figure 1. Chromatin accessibility at DSP2.2b-JH1 rearranged allele.
(a) Schematic of the germline Igh locus in 6312 cells and the DJH rearranged Igh locus in 6312-derivative 2B9 cells, which harbor a DSP2.2b-JH1 junction in one allele and a VH rearrangement in the other allele (not to scale). The positions of amplicons analyzed by real-time PCR are also shown. Block arrows represent DH-associated repeat sequences10,11. (b) ChIP assays were performed using antibodies for modified histones as indicated with chromatin obtained from 6312 (maroon bars) and 2B9 (pink bars) cells. The numbers within the parentheses indicate positions in kb 5′ of the DSP2.2b segment. All samples were assayed in duplicate by real-time PCR and relative abundance (y axis) for each amplicon in the immunoprecipitate was calculated as previously described11. γ-actin promoter and Cγ3 served as controls for active and inactive chromatin, respectively. Data show the average of 2 independent ChIP experiments and error bars indicate standard deviation. (c) DNase I sensitivity analyses of the DSP2.2b-JH1 allele (pink lines) compared to the germline Igh allele (maroon lines). 2×106 nuclei from 6312 and 2B9 cells were treated with increasing concentrations of DNase I (x axis, 0 to 2 units of DNase I) followed by purification of the genomic DNA. All samples were assayed in duplicate by quantitative real-time PCR and the proportion of intact DNA (y axis) at each DNase I concentration was determined for the indicated amplicon as previously described18. Eμ corresponds to the known DNase I hypersensitive site in the JH-Cμ intron, while Cγ3 is DNase I insensitive. Data show the average of 2 independent DNase I sensitivity experiments and error bars indicate standard deviation.
Chromatin immunoprecipitation (ChIP) assays revealed that activation-related H3K9ac and K3K4me2 modifications were increased in the 3 kb region 5′ of the DJH junction (Fig. 1b, pink bars) compared to germline alleles (maroon bars). The γ-actin promoter and Cγ3 constant region served as positive and negative controls, respectively. Sequences close to the DJH junction were also depleted of suppressive H3K9me2 modifications and enriched in transcription-associated H3K4me3 (Fig. 1b). Abundance of H3K9me2 modification increased whereas H3K4me3 modification decreased, 3 kb 5′ of the DJH junction. These changes provided plausible explanations for our earlier analyses of DJH transcription. In those studies we showed that DFL16.1 and DSP rearrangements activated promoters that were dormant in the germline configuration, resulting in increased RNA polymerase II (Pol II) recruitment and elevated sense- and anti-sense-oriented transcription from the DJH rearranged alleles11. However, both Pol II density and anti-sense transcripts decreased substantially within 2 kb 5′ of the DJH junction as compared to the peak near the DJH junction. We conclude that DH recombination leads to chromatin activation and transcription that is highly restricted close to the DJH junction.
Since the most prominent ACE at the Igh locus is the intronic enhancer Eμ, we determined if the changes in the abundance of histone modifications at the DJH junction were dependent on Eμ by analyzing the status of DJH junctions in v-abl transformed pro-B cell lines from Eμ-deficient mice. We performed ChIP assays using antibodies to H3K9ac and H3K4me3 on three Eμ-deficient cell lines: FA3, which had two Igh alleles in the germline configuration; FA8, which had a DSP2.8-JH3 rearrangement in one allele and a DQ52-JH2 rearrangement in the other allele; and FA10, which had a DSP2.7-JH2 rearranged allele and a germline allele. We used the Eμ-sufficient 6312 cell line as control for these positive modifications at the Igh locus. We found that in the absence of Eμ, both the germline and the DJH rearranged loci were completely devoid of these active histone marks, indicating that activating histone modifications associated with DJH junctions required the intronic enhancer Eμ (Supplementary Fig. 2).
To get an independent measure of activation, we assayed DNase I sensitivity of the DSP2.2b-JH1 allele in 2B9 cells using a PCR assay. The Eμ-associated DNase I hypersensitive site and inactive Cγ3 sequences served as positive and negative controls, respectively. We found increased sensitivity of an amplicon 1 kb 5′ of the rearranged DSP2.2b gene segment compared to unrearranged DSP2.2b in 6312 cells (Fig. 1c). An amplicon closer to the DJH junction was also more DNase I sensitive in 2B9 cells than in the parental cells, although overall sensitivity at this site was not as great as at the −1 kb region, suggesting that the −1 kb region represented a weak DNase I hypersensitive site. However, increased DNase I sensitivity did not extend to upstream unrearranged DSP2 gene segments (Fig. 1c, labeled DSP2s), which were comparably insensitive in 2B9 and 6312 cells. These observations indicate that the chromatin state of a recombined DH gene segment is selectively altered relative to unrearranged DH gene segments on the same allele.
To further corroborate the idea that DJH junctions were locally activated, we examined the histone modification status of unrearranged DH gene segments in cells that contained DJH recombined alleles. Unrearranged DSP2 gene segments in 2B9 cells were inactive by several criteria; they lacked H3K9ac and H3K4me3 and retained H3K9me2 (Fig. 2b, labeled DSP2s). The same was true in a different cell line, 2F1, that had undergone DQ52 to JH1 rearrangement in one allele and DSP2.2a to JH2 rearrangement in the second allele (Fig. 2a,c). We also interrogated sequences around DFL16.1 in these cells to determine whether DH rearrangements affected the peak of activation at the 5′ end of the DH-Cμ domain. We found that neither DSP2.2b nor DQ52 rearrangement affected the activation state of DFL16.1 positively or negatively (Fig. 2, amplicons labeled DFL). Consistent with this observation, DNase I sensitivity of the unrearranged DFL16.1 was not altered on DSP2.2b-rearranged alleles (Fig. 1c). We conclude that DH recombination leads to highly localized histone modification and accessibility changes at DJH junctions that do not extend to germline DH gene segments that lie upstream.
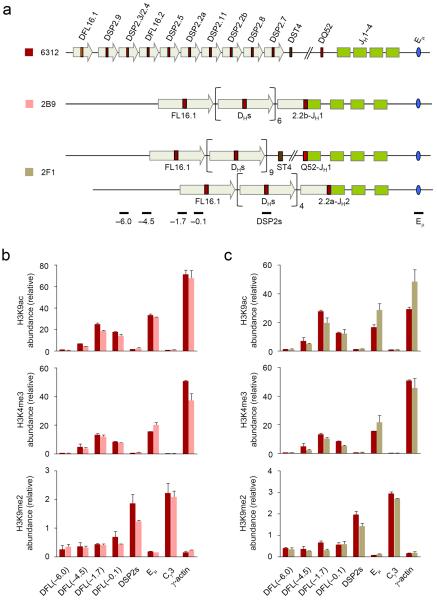
Figure 2. Histone modifications at unrearranged upstream DH gene segments in DJH rearranged Igh alleles.
(a) Schematic of the germline Igh locus in 6312 cells and the rearranged loci in the 6312-derivatives 2B9 and 2F1 cells are shown (not to scale). 2B9 cells have a DSP2.2b-JH1 rearrangement on one allele and a VH rearrangement on the second allele; 2F1 cells have DQ52-JH1 and DSP2.2a-JH2 rearrangements. These configurations leave 7 germline DH gene segments in 2B9 cells (1xDFL16.1, 1xDFL16.2 and 5xDSP2); 2F1 cells have 10 germline DH gene segments (1xDFL16.1, 1xDFL16.2 and 8xDSP2) in the DQ52-JH1 rearranged allele and 5 germline DH gene segments (1xDFL16.1, 1xDFL16.2 and 3xDSP2) in the DSP2.2a-JH2 rearranged allele. The positions of amplicons analyzed by real-time PCR are also shown. (b, c) ChIP assay was performed using antibodies for modified histones as indicated with chromatin obtained from 6312 (maroon bars), 2B9 (b, pink bars) and 2F1 (c, light green bars) cells. Real-time PCR assays were performed in duplicate for the indicated amplicons to determine the chromatin status of upstream unrearranged DH gene segments that remain on DJH rearranged alleles. Numbers within parentheses indicate the position of amplicons in kb 5′ of DFL16.1. Data show the average of 2 independent ChIP experiments and error bars indicate standard deviation.
Chromatin changes at uniquely located DH gene segments
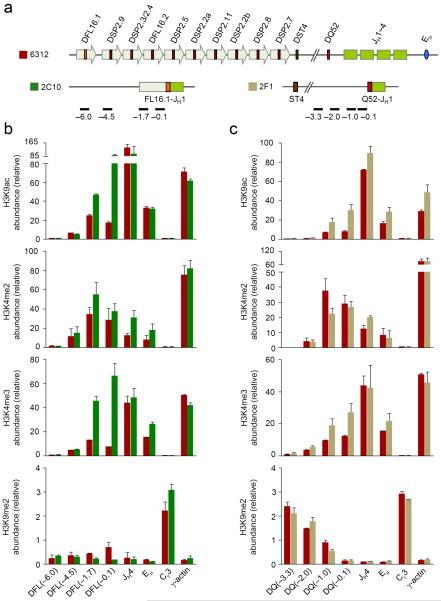
Unlike the intervening DSP2 gene segments, the 5′-most and 3′-most DH gene segments are associated with active chromatin marks in the germline configuration. To determine whether rearrangements of these gene segments also lead to additional local chromatin activation, we investigated the state of a DFL16.1-JH1 recombined allele in 2C10 cells and a DQ52-JH1 recombined allele in 2F1 cells (Fig. 3a). The sequences upstream of DFL16.1 were lost in the second allele of 2C10 cells, which had undergone VH to DJH recombination, and the sequences immediately upstream of DQ52 were lost in the second allele of 2F1 cells, which had rearranged an upstream DH gene segment (DSP2.2a-JH2). This configuration of rearrangements in the two cell lines allowed us to unequivocally probe the chromatin state surrounding rearranged DFL16.1 and DQ52 gene segments.
Figure 3. Histone modifications at DFL16.1- and DQ52-rearranged Igh alleles.
(a) Schematic of the germline Igh locus in 6312 cells and the DJH-rearranged loci in 6312-derived 2C10 and 2F1 cells are shown (not to scale) along with the positions of amplicons analyzed by real-time PCR; 2C10 cells have a DFL16.1-JH1 rearrangement in one allele and a VH rearrangement in the second allele which does not score for the DFL16.1 specific primers used in part b). 2F1 cells have a DQ52-JH1 rearrangement in one allele and a DSP2.2a-JH2 rearrangement in the second allele which does not score with the DQ52-specific primers used in part c). (b, c) ChIP assays were performed using antibodies for modified histones as indicated, with chromatin obtained from 6312 cells (maroon bars) and derivative cell lines 2C10 (b, green bars) and 2F1 (c, light green bars). Numbers within parentheses indicate positions in kb 5′ of the rearranged DH gene segment. Real-time PCR assays were performed in duplicate for the indicated amplicons and data show the average of 2 independent ChIP experiments with error bars indicating standard deviation.
H3K9ac abundance was substantially higher close to the recombined DFL16.1-JH1 junction compared to the same location in germline configuration (Fig. 3b). Increased H3K9ac also was evident at DFL16.1 (−1.7), the approximate position of the H3K9ac peak in the unrearranged state, but dropped off rapidly thereafter. The same trend was evident with H3K4me3 modification (Fig. 3b). We observed no major changes in H3K4me2 or H3K9me2 modifications around the rearranged DFL16.1 compared to germline DFL16.1 (Fig. 3b). The net result of these changes was that, in addition to increased abundance of activation modifications, the peak of modifications moved to the DJH junction rather than being located 1.7 kb 5′ of DFL16.1. DQ52 rearrangement also increased local H3K9ac and H3K4me3 modifications (Fig. 3c), however, the fold change was much less compared to DFL16.1 or DSP2 rearrangements. This result is probably because germline DQ52 already contains abundant activating histone modifications due to its proximity to the JH region and the nearby PQ52 promoter. The restriction of activation marks close to DQ52 was most dramatically exemplified by the sharp increase in suppressive H3K9me2 modifications within 2 kb 5′ of the DJH junction (Fig. 3c). Even though we noted changes in the abundance of histone modifications at DJH junctions involving DFL16.1 and DQ52, we did not observe any changes in DNase I sensitivity at or near these junctions (Supplementary Figs. 3,4) compared to corresponding locations near the germline DH gene segments. These observations demonstrate that chromatin alterations in response to DJH recombination are highly localized regardless of DH gene usage.
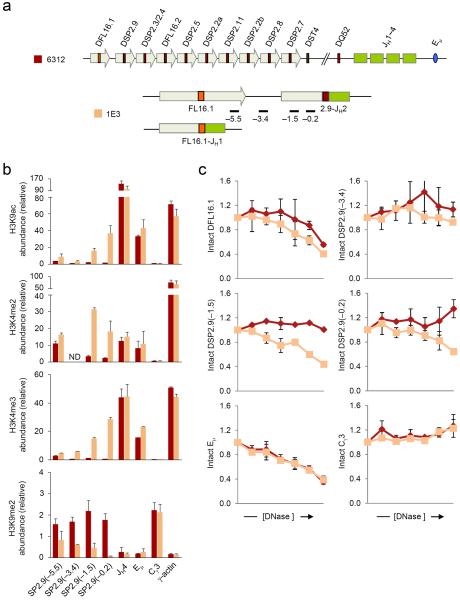
We hypothesize that localized changes that distinguish DJH junctions from upstream unrearranged DH gene segments provide a plausible mechanism for targeting VH recombination to the DJH junction. In this regard DSP2.9 occupies a special position within the DH cluster. As the first DH gene segment 3′ of DFL16.1, it is closest to the pocket of active chromatin at the 5′ end of the germline DH cluster other than DFL16.1 itself. It is therefore possible that chromatin changes associated with DSP2.9 recombination could lead to a large domain of activated chromatin that encompassed both DSP2.9 (rearranged) and DFL16.1 (unrearranged) gene segments. Alternatively, a DSP2.9 rearranged allele may contain two distinct mini-domains of active chromatin. To distinguish between these alternatives we examined the structure of a DSP2.9-JH2 rearranged allele in 1E3 cells, which have a DFL16.1-JH1 rearrangement in the other allele (Fig. 4a). Remarkably, we again noted highly localized increase in H3K9ac and transcription-associated H3K4me3 at the DJH junction, which dropped off rapidly 3.4 kb 5′ of DSP2.9 (Fig. 4b); this amplicon is located about 3.4 kb 3′ of DFL16.1. Conversely, inhibitory H3K9me2 marks were absent at the recombined junction compared to germline DSP2.9 (Fig. 4b). Though the pattern of H3K4me2 modification was less precisely restricted to the DSP2.9-JH2 junction, overall DSP2.9 rearrangement also resulted in local enhancement of activating histone modifications at the DJH junction.
Figure 4. Chromatin accessibility at DSP2.9-JH2 rearranged allele.
(a) Schematic of the germline Igh locus in 6312 cells and the DJH-rearranged loci in 1E3 cells are shown (not to scale) along with the positions of amplicons analyzed by real-time PCR. 1E3 cells have a DSP2.9-JH2 junction in one allele and a DFL16.1-JH1 junction in the second allele which would not score for the DSP2.9-specific primers used in this assay. (b) ChIP assays were performed using antibodies for modified histones as indicated, with chromatin from 6312 (maroon bars) and 1E3 (orange bars) cells. Numbers within parentheses indicate positions in kb 5′ of the DSP2.9 segment. Real-time PCR assays were performed in duplicate for the indicated amplicons. Data show the average of 2 independent ChIP experiments and error bars indicate standard deviation. ND stands for `not determined'. (c) DNase I sensitivity analyses of the DSP2.9-JH2 allele (orange lines) compared to the germline Igh allele (maroon lines). 2×106 nuclei from 6312 and 1E3 cells were treated with increasing concentrations of DNase I (x axis, 0 to 2 units of DNase I) followed by purification of the genomic DNA. All samples were assayed in duplicate by quantitative real-time PCR assay and the proportion of intact DNA (y axis) at each DNase I concentration was determined for the indicated amplicon as previously described18. Eμ corresponds to the known DNase I hypersensitive site in the JH-Cμ intron, while Cγ3 is DNase I insensitive. Data show the average of 2 independent DNase I sensitivity experiments; error bars indicate standard deviation.
These epigenetic changes were accompanied by increased DNase I sensitivity of the sequences 5′ of the DJH junction (Fig. 4c). An amplicon 1.5 kb 5′ of the rearranged DSP2.9 was substantially more sensitive to DNase I digestion than the corresponding region around germline DSP2.9. This increased sensitivity of the rearranged allele was not apparent 3.4 kb 5′ of DSP2.9. Additionally, DNase I sensitivity of germline DFL16.1 was comparable between DSP2.9 rearranged and unrearranged alleles. We conclude that DSP2.9 behaves exactly like other DH gene segments analyzed in this study and DSP2.9 rearrangement does not significantly alter the chromatin state around DFL16.1.
Chromatin modifications at DJH junctions in primary Pro-B cells
To determine whether DJH rearrangement-associated chromatin changes were present in primary pro-B cells, we performed micro-ChIP on bone marrow pro-B cells isolated by flow cytometry. To account for the heterogeneity of bone marrow pro-B cells, we used a pan-DSP primer that hybridized to all 6 DSP gene segments and one that was unique to DFL16.1, together with a JH1 primer (Fig. 5a) to assay the histone modification state of DJH1 junctions by real-time PCR. We found that H3K9ac as well as H3K4me3 marks were substantially higher at DFL16.1-JH1 and DSP2-JH1 junctions compared to the corresponding germline DH segments (Fig. 5b). γ-actin promoter and β-globin amplicons served as positive and negative controls. To examine the chromatin state of DJH junctions that utilized other JH gene segments, we used a reverse primer located 3′ of JH4; for these assays the PCR reaction was followed by Southern blotting (Fig. 5c) and quantification of the signals from the Southern blots (Fig. 5d). We compared PCR products from 0.1–0.2 ng of ChIP material to a serial dilution of input material (2.0, 1.0 and 0.5 ng for DJH junctions; 0.8, 0.2 and 0.05 ng for germline fragments). In two independent H3K4me3 ChIPs and one H3K9ac ChIP we found that DJH junctions, but not germline DH gene segments, were enriched in ChIP DNA compared to input DNA. Taken together, both assays demonstrate that DJH junctions are selectively targeted for epigenetic modifications implicated in RAG1-RAG2 recruitment.
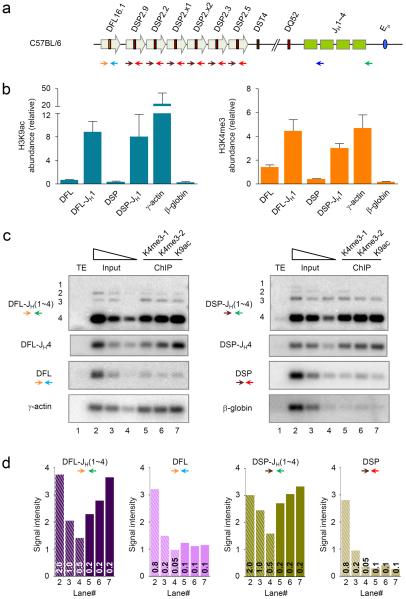
Figure 5. Chromatin accessibility at DJH junctions in primary pro-B cells.
(a) Schematic of the C57BL/6 Igh locus and the location of primers (colored arrows) used for PCR are shown. (b, c) Micro-ChIP assays were performed with 2−2.5×105 pro-B cells isolated by flow cytometry from the bone marrow of C57BL/6 mice using antibodies specific for modified histones as indicated. We assayed the ChIP samples by real-time PCR (b) or by Southern blot of indicated PCR products (c). (b) DJH1 junctions were scored with forward primers that annealed 5′ of either the DFL16.1 segment (orange) or all the DSP2 segments (brown) and a reverse primer that annealed 3′ of JH1 (blue; amplicons labeled DFL-JH1 and DSP-JH1). Germline DFL16.1 and DSP2 segments were assayed using the same forward primers and a reverse primer that annealed 3′ of either DFL16.1 (light blue) or all the DSP2 (red) gene segments respectively (amplicons labeled DFL and DSP). γ-actin promoter and β-globin locus served as positive and negative controls respectively for the ChIP assay. y axis shows the relative abundance of amplicons in the ChIP material compared to the same quantity of DNA from input material. Data show the average of 2 independent ChIP experiments using each antibody from a single isolation of bone marrow pro-B cells; error bars indicate standard deviation. (c) PCR amplification was carried out on micro-ChIP samples using the same DH region forward primers and a reverse primer that annealed 3′ of JH4 (green). Following amplification the products were fractionated by agarose gel electrophoresis, transferred to nylon membranes which were probed with radioactively labeled oligonucleotides listed in Supplementary Table 1. Serially diluted input DNA (lanes 2–4; 2, 1 and 0.5 ng for top two panels in each column; 800, 200 and 50 pg for bottom two panels in each column) was compared to either 200 pg (DJH junctions) or 100 pg (germline DH, γ-actin and β-globin) of ChIP DNA from two independent H3K4me3 (lanes 5 and 6) and one anti-H3K9ac (lane 7) ChIPs. γ-actin promoter and β-globin locus served as positive and negative controls, respectively, for the ChIP assay (bottom panels). A lower exposure of the Southern blot is shown for the DJH4 PCR products (second panel from top in each column) since signal from these bands was saturated with the exposure time of the top panel. (d) Quantitation of cumulative signal intensity of the PCR products from each lane of the Southern blots for DJH junctions and germline DH gene segments shown in (c). The amount of template DNA (in ng) used in the PCR reactions is shown at the base of the bars; lanes 2–4 correspond to a serial dilution of input DNA and lanes 5–7 correspond to ChIP DNA from two H3K4me3 and one H3K9ac ChIPs. Data represent 3 independent PCR assays and Southern blots using different quantities of template DNA.
Restricted recruitment of recombinase to DJH junctions
To determine whether DJH junction-localized changes in histone modifications correlated with recombinase recruitment, we used chromatin immunoprecipitation to locate RAG1 on DJH recombined alleles. We started with a pro-B cell line that lacks endogenous RAG1 and is transgenic for the catalytically inactive RAG1(D708A) mutant (D345 cells), in which the Igh locus is in the germline configuration20. We transiently expressed RAG1 in D345 cells and identified single-cell clones that had undergone DH recombination. We used 3 such lines in our assays (Fig. 6a); clone 3E had a DSP2.2-JH2 rearranged allele, while clones 1C6 and 2C11 had DFL16.1-JH1 and DFL16.1-JH4 rearrangements; the other allele in all three clones was in germline configuration.
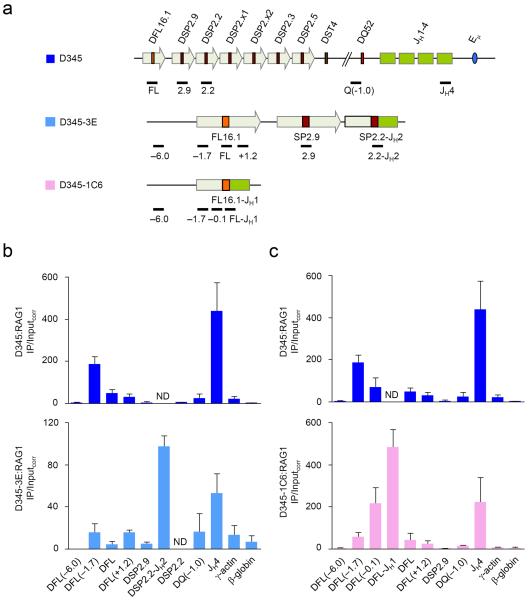
Figure 6. RAG1 association with DJH rearranged Igh alleles.
(a) Schematic of the germline Igh locus in Bcl2-Tg RAG1/D708A-Tg (D345) pro-B cells20 and the DJH junctions in the D345-derivatives 3E and 1C6 cells are shown (not to scale) along with the positions of amplicons analyzed by real-time PCR. 3E cells have a DSP2.2-JH2 junction and 1C6 cells have a DFL16.1-JH1 junction. The second allele in these cells is in germline configuration. (b, c) ChIP assays were performed with a RAG1-specific antibody in D345 (dark blue bars), 3E (b; light blue bars) and 1C6 (c; pink bars) cells. The numbers within the parentheses indicate positions in kb 5′ (−) or 3′ (+) of the indicated gene segment. Amplicons for JH4 and β-globin served as positive and negative controls for RAG1 binding. All amplicons were assayed in duplicate by quantitative real-time PCR and IP/Inputcorr was calculated as before20. Data show the average of 2 or 3 independent ChIP experiments; error bars represent standard deviation between experiments. ND stands for `not determined'.
Using a RAG1-specific antibody in ChIP, we found that RAG1 was highly enriched in the JH region and completely depleted over most DH gene segments in D345 cells as previously shown (Fig. 6b,c)20. Interestingly, we noticed a small peak of RAG1 coincident with low amounts of activating histone modifications just 5′ of DFL16.1. RAG1 density peaked approximately 1.7 kb upstream of DFL16.1, as previously noted for H3K9ac and H3K4me3 peaks11. This peak may represent a “spill-over” of RAG proteins from the JH-associated recombination center because of the spatial proximity of DFL16.1 to the JH domain21
We found more RAG1 at the recombined DSP2.2-JH2 junction in 3E cells (Fig. 6b). However, RAG1 binding was close to background (represented by the β-globin amplicon) at upstream unrearranged DSP2.9 and DFL16.1 gene segments. The pattern of RAG2 recruitment in these cells was similar to that seen with RAG1 (Supplementary Fig. 5). Thus, both RAG1 and RAG2 were highly enriched at a recombined DSP2.2 gene segment. Similarly, RAG1 density was substantially higher at the recombined DFL16.1-JH1 junction in 1C6 cells, compared to unrearranged DFL16.1 gene segment in the same cells (Fig. 6c, compare amplicon labeled DFL-JH1 to those labeled DFL and DFL(+1.2)). The same pattern of RAG1 binding was noted in an independently derived DFL16.1 rearranged cell line (clone 2C11, Supplementary Fig. 6). We confirmed that the histone modification pattern of the D345 derivatives closely resembled the pattern of 6312-derived clones shown in Figs. 1–3 (Supplementary Fig. 7). We conclude that DH recombination leads to accumulation of RAG proteins at DJH junctions.
The high RAG1 density at DFL16.1-JH1 in 1C6 cells indicated that the RAG1 peak centered 5′ of DFL16.1 on unrearranged alleles shifted to the DFL16.1-JH junction after recombination (compare RAG1 at DFL16.1-JH1 to RAG1 at DFL(−1.7) which represents both recombined and germline alleles). This result is directly analogous to the shift in histone modification peaks seen on DFL16.1-JH1 recombined alleles (Fig. 3b). Moreover, we noted that RAG1 density was higher at DJH junctions compared to the germline JH4 region in both rearranged cell lines. Because RAG protein density in the germline Igh locus is concentrated over the JH gene segments, these observations suggest that RAG1 and RAG2 re-distribute towards the DJH junctions on rearranged alleles. We propose that re-focusing RAG1-RAG2 not only maximizes utilization of the DJH RSS for VH recombination, but also serves to limit the low but detectable occurrence of direct VH to JH rearrangements26
DISCUSSION
Three regulatory features are shared by all VH gene recombination. Firstly, VH recombination always follows DH recombination (timing). This timing could be mediated by a late-acting ACE associated with VH gene segments, such that DH recombination would have always occurred before this ACE was activated. However, such an ACE has not been identified. Secondly, VH recombination is selectively activated on alleles that have undergone DH recombination (allele specificity). This under-appreciated facet of VH recombination can be inferred from the state of Igh alleles in core RAG knock-in mice27,28. A substantial number of mature B cells that develop in these strains contain one Igh allele in germline configuration, compared to normal B cells where both Igh alleles are invariably rearranged. These observations indicate that recombination is preferentially completed on DJH recombined alleles. Thirdly, VH gene segments recombine precisely to the RSS associated with DJH junctions, while excluding virtually identical RSSs associated with germline DH gene segments on the same allele (precision). These universal features must be accounted for in any model for activation of VH recombination. Here we propose such a model based on our analyses of DJH recombined alleles.
We propose that VH recombination follows DH recombination because it is only after the formation of DJH junctions that RAG proteins have ready access to the 5′-RSS of DH genes. Prior to initiation of recombination RAG density is primarily located over the JH gene segments. In accordance with prevailing models of RSS synapsis and hairpin formation, an initiating RAG complex at a JH-RSS would seek and pair with a DH-RSS (because of 12/23 complementarity), thereby initiating DH recombination. After DH recombination RAG proteins are preferentially recruited to DJH junctions, permitting them to initiate the reaction at the 5′ DH-RSS; now the complementary RSS would be that of a VH gene segment, thereby leading to VH recombination. This model extends the recombination center model for germline antigen receptor loci to DJH recombined alleles. Essentially, DH recombination brings DH-RSSs into the recombination center to permit the second step of Igh gene assembly. The notion that DH RSSs become available to initiate recombination only after DJH recombination also provides a ready explanation for allele-specificity of VH recombination.
Our model circumvents the need to invoke independent activation and recruitment of RAG proteins to VH gene segments. Indeed, it is easy to imagine that RAG recruitment all along the 2.5 Mb VH locus would substantially increase RAG-induced DNA breaks and translocations. We hypothesize that restricting RAG presence to a discrete part of the Igh locus, while sequentially bringing in the right gene segments provides the correct recombination order while minimizing genomic instability. We do not suggest that RAG recruitment to the DJH RSS is sufficient to initiate VH recombination. Rather, RAG proteins bound to the DJH-associated RSS must find and gain access to a VH RSS in order for hairpin formation to occur. It is likely that locus conformation, mediated by looping and/or compaction, plays a role in spatially positioning VH gene segments in the vicinity of DJH-associated RAG proteins. Lack of such positioning is the likely explanation for reduced distal VH recombination in Pax5- or YY1-deficient pro-B cells29,30. Additionally, correctly positioned VH gene segments must also be in the appropriate chromatin state for RAG proteins to recognize the VH RSS and induce nicking. The permissive chromatin state required for VH access may be conferred in part by interleukin 7-dependent histone modifications19,31–34 and Pax5-dependent loss of H3K9me2 (ref. 12).
One caveat to the model is that DQ52-associated RSSs, that are RAG-rich prior to rearrangement, should be able to recombine with germline VH gene segments to produce VH-DQ52 junctions. Indeed, such rearrangements are in fact observed, but only when the spatial configuration of the Igh locus is altered. The first instance of VH to germline DH recombination was observed in mice where a VH gene segment was “knocked in” very close to DFL16.1 (ref. 35). This VH gene segment rearranged preferentially to DQ52 located 50 kb away, rather than to DFL16.1 located only 1.0 kb away. This product was likely generated by RAG binding at an unrearranged DQ52, followed by capture of the RSS associated with the knocked-in VH. We propose that synapsis between germline DQ52 and the knocked-in VH is possible in this situation because both gene segments lie within the same chromatin domain that is demarcated by CTCF and YY1 binding sites 5′ of DFL16.1 (refs. 21, 22). In the normal configuration of the Igh locus, RAG-bound complexes at DQ52 RSSs would be more effectively captured by JH RSSs because VH gene segments are located outside the DH domain21.
A second instance of VH to germline DQ52 rearrangement was observed22 on Igh alleles mutated at the two CTCF-binding elements upstream of DFL16.1 (ref. 23). These modifications remove the newly identified looping or barrier sites 5′ of DFL16.1 that sequester all DH gene segments in one chromatin domain21,22. In the absence of the normal looping or barrier sites perhaps the DH domain extends into the proximal VH region, thereby incorporating one or more VH gene segments into the DH domain. Functionally, this would be analogous to the proposed structure generated on the allele with the knocked-in VH gene segment described above. Therefore, VH RSS(s) would be available for synapsis with the DQ52 RSS leading to proximal VH to germline DQ52 rearrangements.
Finally, our observations provide a plausible mechanism for the precision of VH recombination to DJH junctions but not to germline DH-RSSs that lie 4 kb upstream. Specifically, we found that activating histone modifications and nuclease sensitivity of DJH junctions did not extend even 4 kb to the closest unrearranged DH gene segment. Because these changes occur in recombinase-deficient cells, our working hypothesis is that these changes direct RAG recruitment to DJH junctions while avoiding germline DH gene segments that lie 5′. Consistent with this idea, direct analysis of RAG binding also showed highest amounts of RAG1-RAG2 at DJH junctions and very little at germline DH gene segments. Interestingly, RAG density appeared to shift from its pre-rearrangement position over JH gene segments to focused accumulation at DJH junctions, thereby further accentuating the use of the DJH-RSS in the next recombination step. We propose that the exquisite specificity of VH recombination to DJH junctions is imposed by the localized changes in chromatin structure and consequent restriction of RAG proteins to DJH junctions.
Several mechanisms can be considered by which chromatin changes are restricted to DJH junctions. First, since large portions of the DH region are actively maintained in silent (H3K9me2-marked) chromatin11, it is possible that some of these heterochromatin-associated enzymes are brought along with the recombining DH gene segment. After rearrangement though, the DH promoter is activated by proximity to Eμ and results in activation/transcription-associated histone modifications close to the DJH junction. However, these modifications cannot spread 5′ due to silencing activities located there. Secondly, DH promoters activate bi-directional transcription after rearrangement11. It is possible that elevated levels of antisense transcripts that are generated from the DH promoter may play a role in maintaining the heterochromatic state of upstream germline DH gene segments. Third, DH promoters may function as boundary elements. In this scenario, though the DH gene segment recombines into the highly active JH region, the positive effect of this part of the locus is prevented from spreading into the upstream germline DH gene segments by the newly active rearranged DH promoter. Further studies are needed to determine the factors that restrict chromatin structural changes to DJH junctions.
METHODS
Cell Culture
Rag2−/− 6312 cells and its derivative cell lines 2B9, 2C10, 2F1 and 1E3 as well as Bcl2-Tg RAG1 D708A-Tg/D345 cells and its derivatives 3E, 1C6 and 2C11 were grown in RPMI media supplemented with fetal bovine serum, antibiotics and 2-mercaptoethanol. The DJH-rearranged derivative cells were generated by transient transfection of a RAG2 expression vector into 6312 cells or a RAG1 expression vector into D345 cells, followed by single cell cloning and characterization of DJH junctions by PCR. Briefly, a bicistronic retroviral vector that coexpressed RAG2 and GFP was transfected into 6312 cells or retroviral vectors expressing RAG1 and RFP were co-transfected into D345 cells using Amaxa nucleofector (Cell Line Nucleofector Kit V, Program W-01). 24 h after transfection, GFP-positive 6312 cells and RFP-positive D345 cells were sorted and grown in RPMI culture medium. After 4 to 7 days of culture, GFP-negative 6312 and RFP-negative D345 cells were single-cell sorted into 96-well dishes for expansion. Clones were screened for the presence of DJH recombined alleles by PCR and chosen to represent a range of DH segment usage. Generation of the v-Abl transformed 6312 and D345 cells has been described previously20,36.
Chromatin Immunoprecipitaion (ChIP)
ChIPs for modified histones and RAG1 were performed as previously described11,20. Antibodies for ChIP were purchased from the following sources: H3K9ac (06–942) and H3K9me2 (07–441) from Millipore; H3K4me2 (39141) and H3K4me3 (39159) from Active Motif. Hybridoma cell lines producing monoclonal antibodies for RAG1 (#23) or RAG2 (#11) were generated by Epitomics, Inc (Supplementary Fig. 8). Rabbits were immunized with either strep-RAG1 core (murine RAG1 amino acids 377–1008 fused to strep-tagII37) or murine RAG2 amino acids 1–490 fused to a hexahistidine tag. Hybridoma supernatants were screened against MBP-RAG1 core (murine RAG1 amino acids 384–1008 fused to maltose binding protein at the N-terminus and a hexahistidine tag at the C-terminus38) or murine RAG2 amino acids 1–490 fused to maltose binding protein. All RAG proteins were purified from bacteria. Input DNA and the immunoprecipitated DNA were quantified fluorometrically using PicoGreen (Molecular Probes/Life Technologies). 200–400 pg of DNA was used in each real-time PCR reaction performed in duplicates and each ChIP was performed in duplicate or triplicate. The relative abundance of specific sequences in the immunoprecipitate relative to input was analyzed as described11 by real-time PCR using the primers listed in Supplementary Table 1. The abundance (IP/Inputcorr) of RAG1 at specific genomic loci was calculated as described before20.
DNase I sensitivity
DNase I sensitivity assays were performed as previously described18. Briefly, nuclei from 2×106 cells were treated with increasing amounts of DNase I (0 to 2 units) followed by purification of the genomic DNA. Real-time PCR assay for each treated sample was performed in duplicate using primers listed in Supplementary Table 1. Sensitivity was determined using two independent DNase I-treated samples for each cell line.
Micro-ChIP from primary pro-B cells
Bone marrow from 16 C57BL/6 mice was labeled with biotinylated antibodies for Mac-1 (553309), Gr-1 (553125), Ter119 (553672), CD3ε (553060), IgM (553406), Ly-6C (557359) and DX-5 (553856) purchased from BD Biosciences. The labeled cells were then bound to streptavidin microbeads (130-048-102) and depleted by passing through LD columns (130-042-901), both from Miltenyi Biotec. The flow through fraction was then stained with B220-FITC, CD19-PE-Cy7, CD43-PE (553088, 552854 and 553271 respectively from BD Biosciences) and AA4.1-APC (17-5892, eBioscience) and cells expressing all four markers were sorted on a BD FACSAria cell sorter. The total yield of 1.3×106 pro-B cells was divided into 5 tubes, one of which was used as input material while the other four tubes were used to perform micro-ChIP in duplicate using antibodies against histone H3K9ac and H3K4me3. The micro-ChIP protocol described by Dahl and Collas was adopted with minor modifications39. 100 to 200 pg of ChIP samples was analyzed by real-time PCR (Fig. 5a) or by Southern blot of PCR products (Fig. 5b) to determine the enrichment of specific targets. For Southern blots, we performed one round of PCR of 35 cycles (for DJH rearrangement analysis) or of 32 cycles (for germline fragments). The sequence of primers and probes used in the assay are listed in Supplementary Table 1. Animal experiments were reviewed and approved by the NIA/IRP Animal Care and Use Committee (Animal Studies Protocol # 338-LMBI-2013)
Statistical analysis
The average values and standard deviations for all ChIP experiments as well as DNase I sensitivity assays were calculated in Microsoft Office Excel 2007. Graphs were generated in either GraphPad Prism 5 or Microsoft Office Excel 2007.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institute on Aging, Baltimore, MD, and by NIH grants to F.W.A. (AI20047) and D.G.S. (AI32524). F.W.A. and D.G.S. are Investigators of the Howard Hughes Medical Institute.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
REFERENCES
- 1.Bergman Y, Cedar H. Epigenetic control of recombination in the immune system. Semin Immunol. 2010;22:323–329. doi: 10.1016/j.smim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 3.Perlot T, Alt FW. Cis-regulatory elements and epigenetic changes control genomic rearrangements of the IgH locus. Adv Immunol. 2008;99:1–32. doi: 10.1016/S0065-2776(08)00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas LR, Cobb RM, Oltz EM. Dynamic regulation of antigen receptor gene assembly. Adv Exp Med Biol. 2009;650:103–115. doi: 10.1007/978-1-4419-0296-2_9. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell TK, et al. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986;324:585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. [PubMed] [Google Scholar]
- 7.Osipovich O, Oltz EM. Regulation of antigen receptor gene assembly by genetic-epigenetic crosstalk. Semin Immunol. 2010;22:313–322. doi: 10.1016/j.smim.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spicuglia S, Pekowska A, Zacarias-Cabeza J, Ferrier P. Epigenetic control of Tcrb gene rearrangement. Semin Immunol. 2010;22:330–336. doi: 10.1016/j.smim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Subrahmanyam R, Sen R. Epigenetic Features that Regulate IgH Locus Recombination and Expression. Curr Top Microbiol Immunol. 2011;356:39–63. doi: 10.1007/82_2011_153. [DOI] [PubMed] [Google Scholar]
- 10.Subrahmanyam R, Sen R. RAGs' eye view of the immunoglobulin heavy chain gene locus. Semin Immunol. 2010;22:337–345. doi: 10.1016/j.smim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty T, et al. Repeat organization and epigenetic regulation of the DHCmu domain of the immunoglobulin heavy-chain gene locus. Mol Cell. 2007;27:842–850. doi: 10.1016/j.molcel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Johnson K, et al. B cell-specific loss of histone 3 lysine 9 methylation in the V(H) locus depends on Pax5. Nat Immunol. 2004;5:853–861. doi: 10.1038/ni1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osipovich O, et al. Targeted inhibition of V(D)J recombination by a histone methyltransferase. Nat Immunol. 2004;5:309–316. doi: 10.1038/ni1042. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds Hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27:561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews AG, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramon-Maiques S, et al. The plant homeodomain finger of RAG2 recognizes histone H3 methylated at both lysine-4 and arginine-2. Proc Natl Acad Sci U S A. 2007;104:18993–18998. doi: 10.1073/pnas.0709170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty T, et al. A 220-nucleotide deletion of the intronic enhancer reveals an epigenetic hierarchy in immunoglobulin heavy chain locus activation. J Exp Med. 2009;206:1019–1027. doi: 10.1084/jem.20081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury D, Sen R. Stepwise activation of the immunoglobulin mu heavy chain gene locus. EMBO J. 2001;20:6394–6403. doi: 10.1093/emboj/20.22.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–431. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C, et al. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell. 2011;147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem. 2010;285:9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degner-Leisso SC, Feeney AJ. Epigenetic and 3-dimensional regulation of V(D)J rearrangement of immunoglobulin genes. Semin Immunol. 2010;22:346–352. doi: 10.1016/j.smim.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewitt SL, Chaumeil J, Skok JA. Chromosome dynamics and the regulation of V(D)J recombination. Immunol Rev. 2010;237:43–54. doi: 10.1111/j.1600-065X.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 26.Koralov SB, Novobrantseva TI, Hochedlinger K, Jaenisch R, Rajewsky K. Direct in vivo VH to JH rearrangement violating the 12/23 rule. J Exp Med. 2005;201:341–348. doi: 10.1084/jem.20041577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akamatsu Y, et al. Deletion of the RAG2 C terminus leads to impaired lymphoid development in mice. Proc Natl Acad Sci U S A. 2003;100:1209–1214. doi: 10.1073/pnas.0237043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley DD, et al. Impaired V(D)J recombination and lymphocyte development in core RAG1-expressing mice. J Exp Med. 2003;198:1439–1450. doi: 10.1084/jem.20030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesslein DG, et al. Pax5 is required for recombination of transcribed, acetylated, 5' IgH V gene segments. Genes Dev. 2003;17:37–42. doi: 10.1101/gad.1031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, et al. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolino E, et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 33.Stanton ML, Brodeur PH. Stat5 mediates the IL-7-induced accessibility of a representative D-Distal VH gene. J Immunol. 2005;174:3164–3168. doi: 10.4049/jimmunol.174.6.3164. [DOI] [PubMed] [Google Scholar]
- 34.Xu CR, Schaffer L, Head SR, Feeney AJ. Reciprocal patterns of methylation of H3K36 and H3K27 on proximal vs. distal IgVH genes are modulated by IL-7 and Pax5. Proc Natl Acad Sci U S A. 2008;105:8685–8690. doi: 10.1073/pnas.0711758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates JG, Cado D, Nolla H, Schlissel MS. Chromosomal position of a VH gene segment determines its activation and inactivation as a substrate for V(D)J recombination. J Exp Med. 2007;204:3247–3256. doi: 10.1084/jem.20071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 37.Ciubotaru M, et al. RAG1-DNA binding in V(D)J recombination. Specificity and DNA-induced conformational changes revealed by fluorescence and CD spectroscopy. J Biol Chem. 2003;278:5584–5596. doi: 10.1074/jbc.M209758200. [DOI] [PubMed] [Google Scholar]
- 38.Fugmann SD, Villey IJ, Ptaszek LM, Schatz DG. Identification of two catalytic residues in RAG1 that define a single active site within the RAG1/RAG2 protein complex. Mol Cell. 2000;5:97–107. doi: 10.1016/s1097-2765(00)80406-2. [DOI] [PubMed] [Google Scholar]
- 39.Dahl JA, Collas P. A rapid micro chromatin immunoprecipitation assay (microChIP) Nat Protoc. 2008;3:1032–1045. doi: 10.1038/nprot.2008.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.