Abstract
Multidrug resistance (MDR) is a major cause of failure in cancer chemotherapy. Tocopheryl polyethylene glycol 1000 succinate (TPGS) has been extensively explored for the treatment of MDR in cancer because of its ability to inhibit P-glycoprotein. Here, we have established multifunctional nanoparticles (MFNPs) using a single-molecule modification of TPGS, which can deliver a hydrophobic drug, paclitaxel (PTX), and a hydrophilic drug, fluorouracil (5-FU), and overcome MDR in cancer. Our data indicated that, when delivered into a PTX-resistant cell line using MFNPs, the combination of PTX and 5-FU was more cytotoxic than each agent individually.
Keywords: multidrug resistance, TPGS, paclitaxel, multifunctional nanoparticles
The development of multidrug resistance (MDR) to a variety of chemotherapeutic agents is one of the major challenges to effective cancer treatment.1 Of the various mechanisms mediating MDR, resistance conferred by the P-glycoprotein (P-gp) transporter is the best characterized and is of considerable clinical importance. P-gp functions by increasing the eflux of drugs out of the cell against a concentration gradient, thereby decreasing intracellular levels of the drugs below their level of effectiveness, a phenomenon that leads to drug resistance.2 An efficient strategy in overcoming MDR using highly selective P-gp inhibitors has become attractive. For example, tocopheryl polyethylene glycol 1000 succinate (TPGS) has been extensively investigated for use as an inhibitor of P-gp.3–5
Similarly, the development of nanocarriers of combined drugs that exhibit a high loading efficiency presents a significant challenge, particularly when the drugs being codelivered are hydrophobic and hydrophilic. The nanocarriers are designed to deliver combined drugs to the target cells; each individual drug resides within the signaling pathways in the cancer cells, causing synergistic effects. However, if the drugs are high-affinity substrates for P-gp [e.g., paclitaxel (PTX)], an additional agent, specifically a P-gp inhibitor, should be codelivered in the nanocarriers. Here, we demonstrate that multifunctional nano-particles (MFNPs) could be created through a single-molecule modification of TPGS [i.e., conjugation of fluorouracil (5-FU) to TPGS]. As the 5-FU–TPGS conjugate and PTX are assembled into MFNPs, the segment of TPGS serves several functions: (1) carrying the hydrophilic drug (5-FU), (2) delivering a hydrophobic drug (PTX), (3) inhibiting P-gp to reverse MDR, and (4) stabilizing the MFNP.
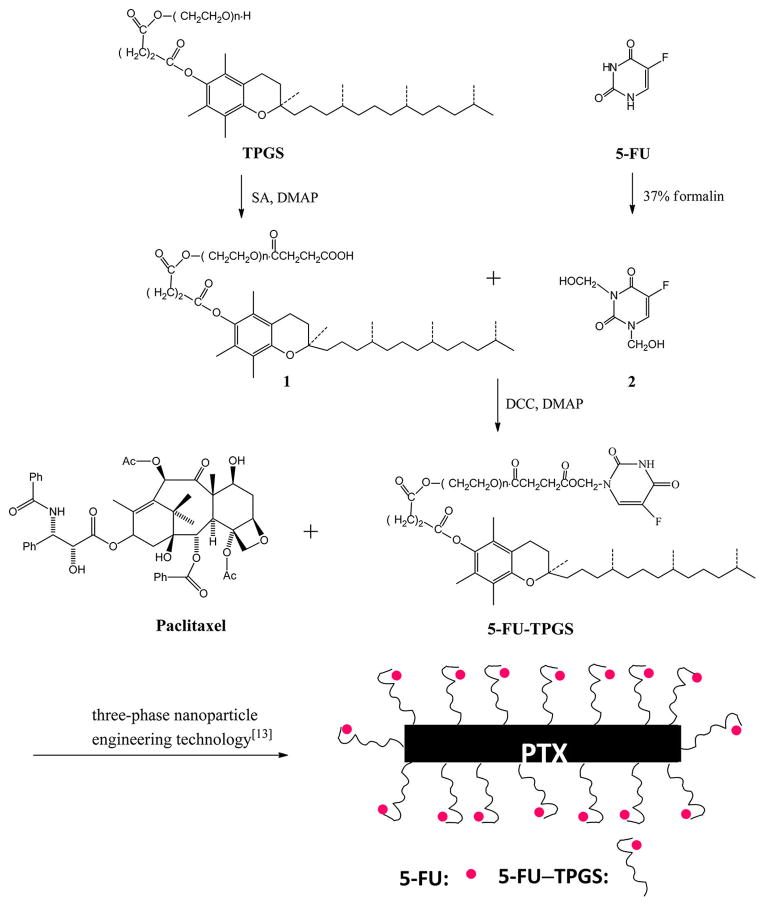
Various derivatives of 5-fluorouracil (5-FU) have been used as antitumor agents in recent years. For example, N-acylox-ymethyl-5-fluorouracil exhibits strong antitumor activity.6 Therefore, we have conjugated TPGS with N-hydroxymethyl-5-fluorouracil via an ester bond to produce a 5-fluorouracil–TPGS conjugate (5-FU–TPGS), which contains an acyloxy-methyl group at position N-1. To create this conjugate, succinoylated TPGS (1) was synthesized according to the methods described by S.-S. Feng.7 During this process, 5-FU was treated with formalin to generate 1,3-dihydroxymethyl-5-fluorouracil (2). Next, succinoylated TPGS (1, 1.60 g, 1 mmol), 1,3-dihydroxymethyl-5-fluorouracil (2, 0.23 g, 1.2 mmol), and DCC (0.25 g, 1.2 mmol) in the presence of a catalytic amount of DMAP in anhydrous CH3CN were stirred at room temperature under a nitrogen atmosphere for 24 h. The resulting mixture was filtered to remove N,N-dicyclohexylurea (DCU) and evaporated under reduced pressure until it was dry. The residue was purified using silica-gel column chromatograpy, eluting with a chloroform/methanol solution with increasing methanol content. After the solvent was removed in vacuo, a 5-FU–TPGS conjugate was obtained, appearing as a waxy white solid. The chemical structure of the synthesized 5-FU–TPGS conjugate was confirmed by 1H NMR (Figure S1 of the Supporting Information) and mass (Figure S2 of the Supporting Information) spectra.
Like other nonionic surfactants, TPGS is composed of a hydrophobic tail (vitamin E) and a hydrophilic head (PEG1000). Because the 5-FU–TPGS conjugate was synthesized by coupling 5-FU to the hydrophilic headgroup, PEG1000, the hydrophobicity of vitamin E remains intact. We expected that PTX-loaded MFNPs (diagramed in Figure 1) could be assembled through a hydrophobic interaction between PTX and the hydrophobic tail of 5-FU–TPGS, which would be stabilized by the hydrophilic portion of the particle.
Figure 1.
Schematic diagram of the assembly of MFNP with PTX and 5-FU–TPGS.
Before assembling the MFNPs, we characterized 5-FU–TPGS to determine its ability to inhibit P-gp and antitumor activity. Rhodamine 123 (RH123), a fluorescent dye, is an established substrate of P-gp, which has been commonly used to examine the ability of inhibitors to block P-gp-mediated transport.8 Previous studies have shown that H460/TaxR cells derived from human, non-small cell lung cancer overexpress P-gp and are resistant to PTX.9 Therefore, this cell line was used to demonstrate the effects of 5-FU–TPGS on P-gp inhibition. Through the quantification of the uptake of RH123, we determined if the synthesized conjugate maintained the ability to inhibit P-gp. Low levels of uptake of RH123 by H460/TaxR cells and an increase of >10-fold in uptake when the cells were treated with 15 μM TPGS or 5-FU–TPGS were observed (Figure 2a). This result confirms that the 5-FU–TPGS conjugate exhibits a P-gp-inhibitory ability similar to that of TPGS in an MDR cell line.
Figure 2.
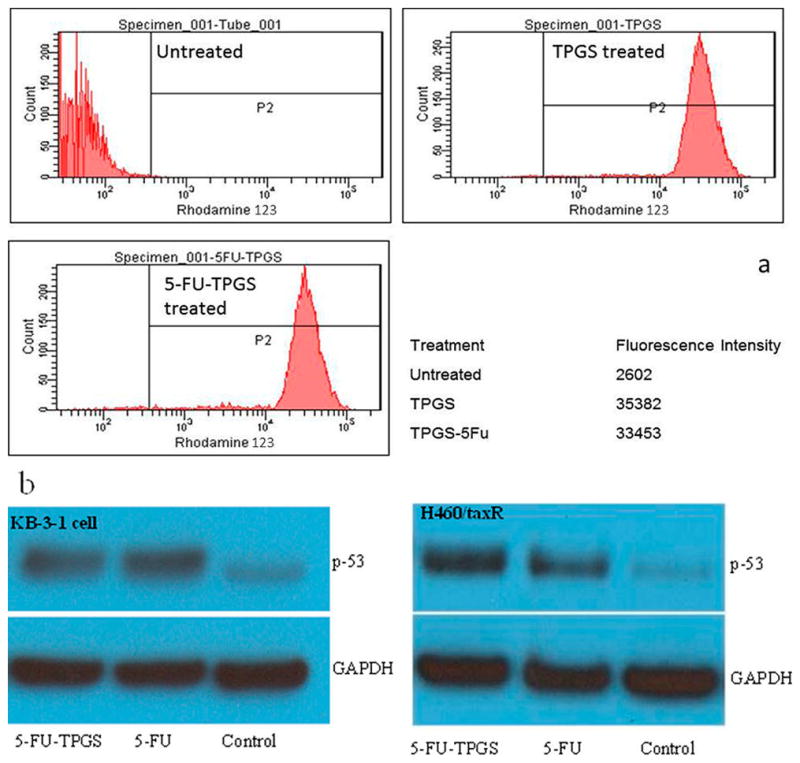
Functional assay of the 5-FU–TPGS conjugate. (a) Effect of the 5-FU–TPGS conjugate on P-gp inhibition determined by the flow-cytometric assessment of the uptake of rhodamine 123 (RH123) in H460/TaxR cells. (b) Western blot for the detection of p53 expression in response to 5-FU and 5-FU–TPGS in KB-3-1 and H460/TaxR cells.
Similarly, the upregulation of protein 53 (p53) by 5-FU is a critical component of the drug that should be maintained by the MFNPs. p53 is a well-established tumor suppressor that is upregulated by 5-FU.10,11 Theoretically, the prodrug of 5-FU–TPGS should alter p53 transcriptional and translational regulation as efficiently as 5-FU, leading to the upregulation of the protein. The expression of p53 in response to treatments was clearly upregulated by both 5-FU–TPGS and free 5-FU in both KB-3-1 (human epidermoid carcinoma cells, a 5-FU-sensitive cell line) and H460/TaxR cells (Figure 2b). Additionally, the cytotoxicity of 5-FU–TPGS was examined to confirm its ability to kill cancer cells. Using MTS in KB-3-1 cells, 5-FU–TPGS was demonstrated to possess the same anticancer efficacy as free 5-FU (data not shown).
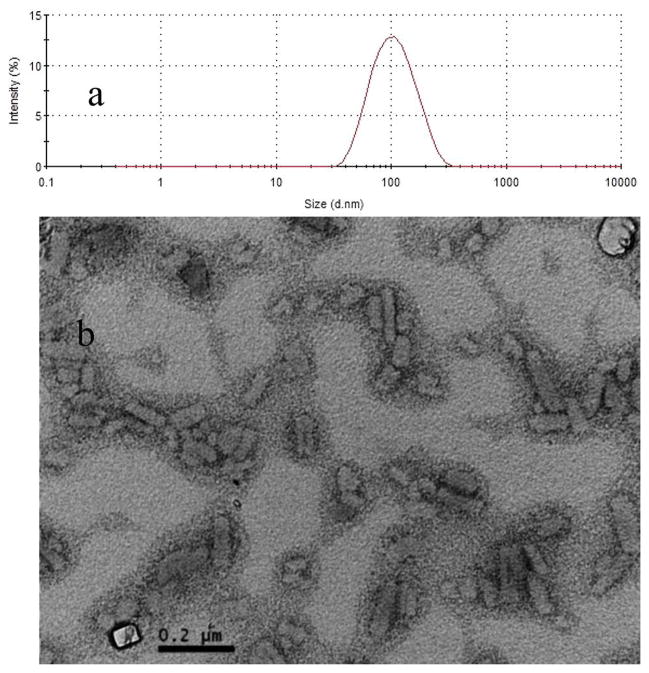
Previously published methods of preparation were used to synthesize MFNPs.12 PTX and 5-FU–TPGS were dissolved in chloroform and coprecipitated by evaporation of the chloroform with a steady flow of nitrogen gas. A trace amount of chloroform was removed by keeping the precipitates under a vacuum in a desiccator for 2–4 h. After being hydrated and vortexed in water for 30 min, suspensions were sonicated for 15 min in a bath-type sonicator (output of 80 kC, 80 W) to form MFNPs. As shown in Figure 3a, the final MFNPs with a PTX:5-FU–TPGS ratio of 1:6 (w:w) exhibit an average hydrodynamic diameter of 105 nm and a polydispersity index (PDI) of 0.142 (Figure 3a). The size and morphology of MFNPs were also determined using a transmission electron microscope; results illustrated that the NPs were rod-shaped (Figure 3b). The release of 5-FU from the MDNPs was confirmed using high-performance liquid chromatography. When the MFNPs were incubated at 37 °C in PBS for 12, 24, and 36 h, 16.4, 23.4, and 34.2% of released 5-FU were detected, respectively. It has been reported that 5-FU was released from its prodrug using CH2O as a linker after hydrolysis (two steps).12 As the 5-FU is conjugated to TPGS using the CH2O linker, the release of 5-FU may also occur through the two-step hydrolysis process.
Figure 3.
Characterization of MFNPs. (a) Particle size distribution of MFNPs, measured using dynamic light scattering. (b) Transmission electron microscopy imaging of MFNPs. The PTX:5-FU–TPGS ratio in MFNPs was 1:6 (w:w).
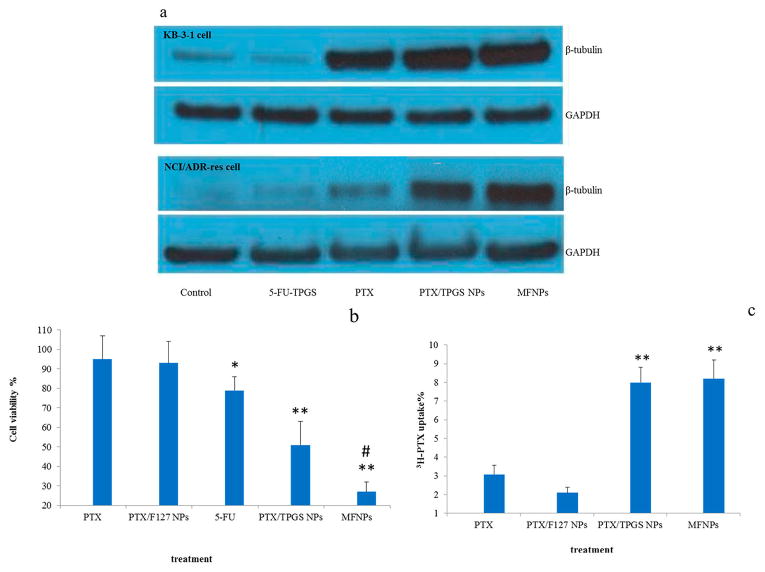
PTX exerts cytotoxic effects by binding tubulin, promoting the assembly of tubulin, and stabilizing microtubules, ultimately leading to cell death by apoptosis. Therefore, we measured the levels of β-tubulin in KB-3-1 and NCI/ADR-RES (MDR human breast cancer) cell lines after these cells were treated with PTX delivered in different formulations. Western blot results showed that β-tubulin assembly was enhanced in KB-3-1 cells by PTX treatments, including PTX alone, PTX/TPGS nanoparticles (PTX/TPGS NPs), and MFNPs (Figure 4a). Importantly, MDR cell lines of NCI/ADR-RES that were treated with both PTX/TPGS NPs and MFNPs displayed enhanced assembly of β-tubulin, but cells treated with PTX alone did not. These results suggest (1) 5-FU–TPGS encapsulated in MFNPs does not interfere with the PTX-mediated binding of β-tubulin and (2) 5-FU–TPGS can efficiently inhibit the ability of P-gp to pump the released PTX out of cells.
Figure 4.
Bioactivity assay of MFNPs. (a) Enhanced tubulin assembly detected by Western blot analysis in KB-3-1 and NCI/ADR-RES cells (the MDR cell line, H460/TaxR, was not used here because it was induced by PTX, resulting in a high background of β-tubulin) after these cells were treated with PTX, PTX/TPGS NPs, or MFNPs. All formulations were normalized to equivalent levels of PTX (5 μM), and PTX/TPGS NPs contained the same molar weight of TPGS as MFNPs loaded with 5-FU–TPGS. (b) Cytotoxicity assay in H460/TaxR cells (n = 6). One asterisk indicates a significant difference compared to PTX (p < 0.05). Two asterisks indicate a highly significant difference compared to PTX (p < 0.01). A number sign indicates a significant difference compared to PTX/TPGS NPs (p < 0.05). (c) Cellular uptake of [3H]PTX in different formulations. Two asterisks indicate a highly significant difference compared to PTX (p < 0.01).
Next, the ability of MFNPs (5 μM PTX) to overcome MDR and achieve antitumor effects in H460/TaxR cells was evaluated. PTX/F127 (Pluronic F-127) NPs developed in our lab were selected as a control because of the similarities in geometry to that of the MFNPs and the fact that F127 is an ineffective inhibitor of P-gp.13 As shown in Figure 4b, PTX alone and PTX/F127 NPs [1:5 (w:w) PTX:F127 ratio] did not induce significant toxicity. However, compared to PTX alone, 5-FU and PTX/TPGS NPs exhibited 79% (p < 0.05) and 55% (p < 0.01) toxicity to cell viability, respectively. On the other hand, the cytotoxicity was increased to 27% viability in cells treated with MFNPs (compared to PTX/TPGS NPs, p < 0.05), eluding to the synergistic effect of the combined delivery of PTX with 5-FU.
We assumed that the level of cellular accumulation of PTX would increase as TPGS was released during the hydrolysis of 5-FU–TPGS in the acidic environment of the endosomal compartment. The inhibition of P-gp prevents the removal of PTX from cells, causing an increase in the concentration of the drug. This pattern was illustrated through the use of 3H-labeled PTX, which was added to the preparations of PTX/5-FU–TPGS MFNPs, PTX/TPGS NPs, and PTX (in DMSO). After incubation with the 3H-labeled samples for 5 h at 37 °C, the cells were harvested with trypsin-EDTA, washed with PBS to ensure the removal of free PTX or NPs, and lysed with Triton X-100. The radioactivity of 3H-labeled PTX was measured using a liquid scintillation analyzer. The cellular accumulation of PTX was significantly enhanced in the cells treated with either MFNPs or PTX/TPGS NPs compared with free PTX or PTX/F127 NPs (Figure 4c). The liquid scintillation analyzer results also support our conclusion that 5-FU–TPGS can inhibit P-gp activity as efficiently as TPGS, which is consistent with the data illustrating the uptake of RH123 (Figure 2a).
Currently, all nanoparticles that have been approved by the U.S. Food and Drug Administration for cancer therapy or in advanced stages of clinical testing rely on the enhanced permeability and retention (EPR) mechanism.14 The EPR mechanism allows passive targeting through the disorganized and leaky vasculature, as well as the reduced lymphatic drainage of tumor tissues. The newly developed MFNPs containing both hydrophobic PTX and covalently conjugated hydrophilic 5-FU, with a particle size of ~100 nm, are also able to accumulate in tumor tissues through the EPR effect. The MFNPs reported here can be further improved through the addition of a tumor-targeting ligand that selectively targets MDR cancer cells without affecting the health of other cells throughout the body.
In conclusion, we have established MFNPs that are able to deliver a hydrophobic drug, PTX, and a hydrophilic drug, 5-FU, for the purpose of overcoming MDR in cancer. MFNPs are now being actively investigated and highlight the promising future of nanotechnology.15,16 Our data show for the first time that MFNPs with a high efficiency of drug loading can be formulated through a single-molecule modification, i.e., conjugation of 5-FU to TPGS. The yield of loading of the combined drugs in MFNPs can be as high as 20% (PTX with 5FU/excipient), demonstrating the high drug carrying capacity of the NPs. Combined drugs have been routinely applied as cotherapies to achieve optimal therapeutic benefits. The new MFNPs codelivering PTX with 5-FU are ideally suited to achieve maximal therapeutic activity, as each drug simultaneously targets different mechanisms and/or different signaling pathways in cancer killing, particularly in MDR cancer cells.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute Grant 5R01CA149387.
Footnotes
Experimental details, 1H NMR spectrum of the 5-FU–TPGS conjugate, ESI mass spectra of 5-FU–TPGS and TPGS, cytotoxicity of 5-FU–TPGS detected by MTS, flow cytometry detection of the uptake of RH123, microtubule assembly assay, and Western blot analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: Implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR) Curr Drug Targets. 2006;7(7):893–909. doi: 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- 3.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16(10):1550–1556. doi: 10.1023/a:1015000503629. [DOI] [PubMed] [Google Scholar]
- 4.Wempe MF, Wright C, Little JL, Lightner JW, Large SE, Caflisch GB, Buchanan CM, Rice PJ, Wacher VJ, Ruble KM, Edgar KJ. Inhibiting efflux with novel non-ionic surfactants: Rational design based on vitamin E TPGS. Int J Pharm. 2009;370(1–2):93–102. doi: 10.1016/j.ijpharm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad S, Ozaki S, Nagase T, Iigo M, Tokuzen R, Hoshi A. A facile method for synthesis of N-acyloxymethyl-5-fluorouracils, as a class of antitumor agents. Chem Pharm Bull. 1987;35(10):4137–4143. doi: 10.1248/cpb.35.4137. [DOI] [PubMed] [Google Scholar]
- 7.Anbharasi V, Cao N, Feng SS. Doxorubicin conjugated to D-α-tocopheryl polyethylene glycol succinate and folic acid as a prodrug for targeted chemotherapy. J Biomed Mater Res, Part A. 2010;94(3):730–743. doi: 10.1002/jbm.a.32734. [DOI] [PubMed] [Google Scholar]
- 8.Takemura Y, Kobayashi H, Miyachi H. Mechanisms of multidrug resistance in tumor cells and analytical methods for its detection. Jpn J Clin Pharmacol. 1998;46(8):745–758. [PubMed] [Google Scholar]
- 9.Teraishi F, Wu S, Sasaki J, Zhang L, Zhu HB, Davis JJ, Fang B. P-glycoprotein-independent apoptosis induction by a novel synthetic compound, MMPT [5-[(4-methylphenyl)methylene]-2-(phenylamino)-4(5H)-thiazolone] J Pharmacol Exp Ther. 2005;314(1):355–362. doi: 10.1124/jpet.105.085654. [DOI] [PubMed] [Google Scholar]
- 10.Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Orntoft TF, Andersen CL, Dobbelstein M. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68(24):10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seong HA, Ha H. Murine protein serine-threonine kinase 38 activates p53 function through Ser15 phosphorylation. J Biol Chem. 2012;287(25):20797–20810. doi: 10.1074/jbc.M112.347757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor HE, Sloan KB. 1-Alkylcarbonyloxymethyl prodrugs of 5-fluorouracil (5-FU): Synthesis, physicochemical properties, and topical delivery of 5-FU. J Pharm Sci. 1998;87(1):15–20. doi: 10.1021/js9702574. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Park JY, Zhang Y, Conwell C, Liu Y, Bathula SR, Huang L. Targeted cancer therapy with novel high drug-loading nanocrystals. J Pharm Sci. 2010;99(8):3542–3551. doi: 10.1002/jps.22112. [DOI] [PubMed] [Google Scholar]
- 14.Murphy EA, Majeti BK, Mukthavaram R, Acevedo LM, Barnes LA, Cheresh DA. Targeted nanogels: A versatile platform for drug delivery to tumors. Mol Cancer Ther. 2011;10(6):972–982. doi: 10.1158/1535-7163.MCT-10-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, Xing L, Holland Cheng R, Liu GY, Lam KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjugate Chem. 2010;21(7):1216–1224. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed AO, Magnusson JP, Moradi E, Soliman M, Wang W, Stolnik S, Thurecht KJ, Howdle SM, Alexander C. Modular construction of multifunctional bioresponsive cell-targeted nano-particles for gene delivery. Bioconjugate Chem. 2011;22(2):156–168. doi: 10.1021/bc100149g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.