Abstract
Background. Erythrocebus patas (patas) monkeys were used to model antiretroviral (ARV) drug in human immunodeficiency virus type 1–infected pregnant women.
Methods. Pregnant patas dams were given human-equivalent doses of ARVs daily during 50% of gestation. Mesenchymal cells, cultured from bone marrow of patas offspring obtained at birth and at 1 and 3 years of age, were examined for genotoxicity, including centrosomal amplification, micronuclei, and micronuclei containing whole chromosomes.
Results. Compared with controls, statistically significant increases (P < .05) in centrosomal amplification, micronuclei, and micronuclei containing whole chromosomes were found in mesenchymal cells from most groups of offspring at the 3 time points.
Conclusions. Transplacental nucleoside reverse-transcriptase inhibitor exposures induced fetal genotoxicity that was persistent for 3 years.
Keywords: zidovudine, lamivudine, abacavir, nevirapine, Erythrocebus patas, mesenchymal fibroblasts, micronuclei, centrosomal amplification, aneuploidy
(See the major article by André-Schmutz et al on pages 235–43)
Antiretroviral (ARV) drug combinations prevent the transmission of human immunodeficiency virus type 1 (HIV-1) [1] in approximately 99% of the 10 000 HIV-1-infected women who become pregnant yearly in the United States. The nucleoside reverse-transcriptase inhibitors (NRTIs) are major components of the ARV combinations in clinical use. In addition, they become incorporated into nuclear and mitochondrial DNA of the host, resulting in the arrest of viral DNA replication [2], chromosome damage [3–5], and genomic instability [6].
Transplacental studies in mice showed incorporation of zidovudine (AZT) in fetal organ DNA, mutagenesis, and tumor induction in offspring [4, 7, 8]. Patas monkeys born to pregnant dams receiving AZT had AZT-DNA incorporation in nuclear and mitochondrial DNA of multiple organs [5] that was similar in magnitude to that observed in cord blood and umbilical cord DNA of human fetuses [2]. Furthermore, NRTI-induced mutagenicity was reported in human infants exposed in utero and during the perinatal period [2, 9], suggesting that these infants may be at risk for cancer induction [10].
To observe the fetal consequences of NRTI use in human pregnancy, we used pregnant patas dams exposed to clinically relevant ARV drug combinations in human-equivalent protocols. Some of the offspring were examined at birth and others were given the same drugs for the first 6 weeks of life and examined at 1 and 3 years of age. The patas placentation and NRTI pharmacokinetics are similar to those in humans [11], and because the patas cannot be infected with simian immunodeficiency virus, drug effects can be examined in the absence of virus.
MATERIALS AND METHODS
Patas Maintenance, Drug Sources, and Exposure Protocols
Maintenance of the patas has been previously detailed [12, 13]. Monkeys were housed and treated under conditions approved by the American Association for the Assessment and Accreditation of Laboratory Animal Care International, using protocols reviewed by the Institutional Animal Care and Use Committee of Bioqual or the National Cancer Institute Animal Care and Use Committee. Euthanasia was performed in accordance with the 2007 American Veterinary Medical Association Guidelines on Euthanasia. Monkeys were housed in groups of 1 male with 3 females, and pregnancy was assessed by ultrasonography. Pregnant dams (2–3/group) were given NRTIs during the final 50% (10 weeks) of gestation. Exposed and unexposed patas offspring were either taken near term by cesarean section [13] or born naturally and permitted to grow for 1 or 3 years. To obtain unexposed 3-year-old patas controls, cells were aspirated from bone marrow of 3–4-year-old patas.
AZT was from Sigma Chemical (St. Louis, MO), and the pediatric liquid clinical formulation of lamivudine (3TC) was from Glaxo-Wellcome (Raleigh, NC). Abacavir (ABC) and nevirapine (NVP) clinical formulations came from the National Institutes of Health Veterinary Pharmacy (Bethesda, MD). Each drug was dissolved in syrup that was placed inside of a banana or mixed into pudding and given to the pregnant patas, under supervision, as a treat. AZT and ABC were given for the last 10 weeks of gestation, in two 20-mg doses, for a total of 40 mg/day 5 days/week. 3TC and NVP were given for the last 4 weeks of gestation in two 12-mg doses, for a total of 24 mg/day 5 days/week. Unexposed monkeys received bananas containing syrup twice daily for 10 weeks. To model human clinical ARV use, in which infants receive NRTI therapy for 6 weeks after birth, neonates to be observed for 1 and 3 years received a dose of liquid formulation twice daily orally by syringe for the first 6 weeks of life [12].
Bone Marrow Culture
Using sterile technique, we collected bone marrow from the femur, and cultured the cells in T75 flasks (BD Falcon, Bedford, MA) with Roswell Park Memorial Institute 1640 medium and 10% or 20% inactivated fetal bovine serum (American Type Culture Collection, Manassas, VA). Harvested cells were incubated undisturbed for 48 hours, at which time the medium was changed, and colonies of adherent mesenchyme-derived cells were allowed to expand. At confluence, cells were removed by 0.05% Trypsin (Sigma-Aldrich, St. Louis, MO), and 5000 or 10 000 cells/chamber were transferred to 4- or 8-well chamber slides (BD Falcon), respectively. Quadruplicate wells were used per treatment group.
Pericentrin and γ-Tubulin Staining for Centrosomal Amplification
The pericentrin immunostaining has been previously described [14]. Anti-pericentrin (Covance, Emeryville, CA; 1:300 dilution) or anti-γ-tubulin (Sigma-Aldrich, 1:200 dilution) were incubated for 2 hours at room temperature. Secondary antibodies, anti-rabbit Alexa 488 (Invitrogen, Carlsbad, CA) for pericentrin and anti-mouse rhodamine red (Invitrogen) for γ-tubulin, were added for 30 minutes. DNA was stained blue with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen). Cells stained with γ-tubulin were red, and those stained with pericentrin antibody showed punctate green signals throughout the cytoplasm. A total of 1000 cells with visible centrosomes was scored for each treatment.
Micronuclei With and Without Whole Chromosomes
Cell monolayers were stained using DAPI to localize nuclei and micronuclei. To visualize entire chromosomes in micronuclei, an anti-kinetochore antibody was used to stain centromeres. Cells were fixed with methanol, permeabilized for 4 minutes with 0.1% Triton-x100 in phosphate-buffered saline (PBS; 8.1 mM Na2HPO4, 1.9 mM KH2PO4, 137.0 mM NaCl, and 2.7 mM KCl), and incubated overnight at 4°C with human anti-kinetochore calcinosis, Raynaud phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia (CREST) antibody (Antibodies, Davis, CA) at a dilution of 1:40 in blocking solution. Slides were washed as described above and incubated for 90 minutes at room temperature with an anti-human Alexa 488 (Invitrogen; dilution, 1:500) secondary antiserum in blocking solution (10% goat serum, 0.01% sodium azide, and 1% bovine serum albumin in PBS). DAPI was used to visualize nuclei and micronuclei. Scoring of 5000 cells per treatment allowed identification of micronuclei with or without CREST-positive green signals.
Fluorescent and Confocal Microscopy
Cells were observed using a Nikon Eclipse E-400 microscope (Nikon, Melville, NY) fitted with a Plan Apo 100× objective with a 1.40 numerical aperture. Stained cells were photographed on a Zeiss Axiovert 100 M microscope equipped with a Zeiss Plan Apochromat 100 × /1.4 oil dichromatic objective. Confocal images were captured using a Zeiss LSM 510 scanning laser microscope. LSM 510 zoom software was used to achieve a final magnification of 2000 × . Images shown are 3-dimensional maximal projections, which were generated from a series of images through the z-plane.
Statistical Evaluation
For each animal, 1000–5000 cells were examined per end point, and the averages from each animal were grouped by treatment (2–3 monkeys/group). Comparisons among treatment groups were performed using the Student t test.
RESULTS
Centrosomal Amplification
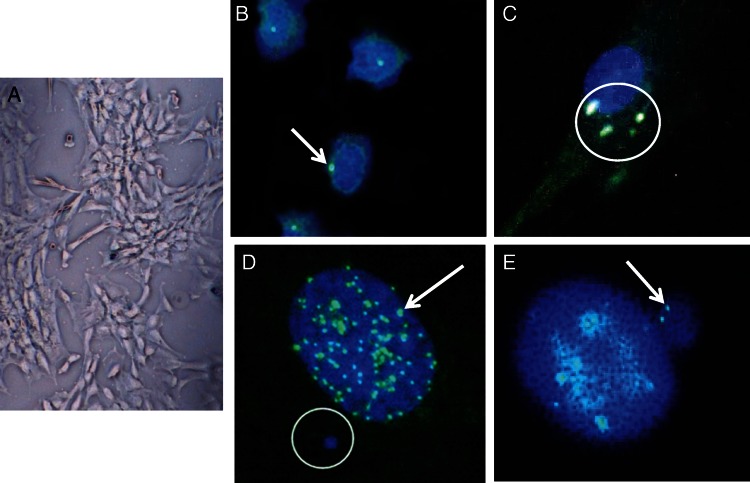
Mesenchymal fibroblasts (Figure 1A) were cultured from bone marrow, and centrosomes were visualized in fixed cells, using either a pericentrin antiserum with a fluorescent tag (Figure 1B and 1C) or a γ-tubulin antiserum with a rhodamine red tag (data not shown). Figure 1B shows representative cells from an unexposed patas infant with only 1 centrosome per cell. In contrast, Figure 1C shows multiple centrosomes in a cell from a patas infant exposed to AZT/3TC. Quantification of centrosomal amplification (Table 1) showed an 8-fold increase in the percentage of cells with centrosomal amplification for AZT/3TC-exposed patas at birth, compared with unexposed patas at birth (P = .0009). Similarly, in 1-year-old patas exposed to AZT/3TC/ABC, there was a 6-fold increase in the percentage of cells with centrosomal amplification, compared with unexposed controls aged 1 year (P = .0001; Table 1). Finally, in AZT/3TC/ABC-exposed patas aged 3 years, there was a 4-fold increase in the percentage of cells with centrosomal amplification, compared with unexposed controls (P = .0006; Table 1). In contrast, AZT/3TC/NVP-exposed patas at 3 years of age (Table 1) showed no significant increase in the percentage of cells with centrosomal amplification.
Figure 1.
Photos showing representative examples of mesenchymal fibroblasts grown from patas bone marrow (A); unexposed patas fibroblasts with centrosomes (pericentrin staining), shown in green (arrow; B); a fibroblast from a zidovudine/lamivudine–exposed patas showing centrosomal amplification (circle; C); a large nucleus stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue) showing centromeres stained with calcinosis, Raynaud phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia (CREST) antiserum in green (arrow) and micronucleus stained with DAPI (circle; D); and a large nucleus from a nucleoside reverse-transcriptase inhibitor–exposed animal with an adjacent micronucleus containing whole chromosomes (CREST-positive green dots, arrow; E).
Table 1.
Percentages of Cells With Centrosomal Amplification (CA), Micronuclei (MN), and MN Containing Whole Chromosomes (MN + C) Among Patas Treatment Groups
| Age, Exposure (No.) | Animals, No. | Percentage of Cells |
|||||
|---|---|---|---|---|---|---|---|
| With CA | P | With MN | P | With MN + C | P | ||
| Birth | |||||||
| Control | 3 | 0.57 ± 0.35 | .0009 | 1.14 ± 0.16 | .04 | 0.28 ± 0.09 | .009 |
| AZT/3TC | 3 | 4.50 ± 0.27 | 2.34 ± 0.36 | 0.83 ± 0.07 | |||
| 1 y | |||||||
| Control | 3 | 0.74 ± 0.05 | .0001 | 0.58 ± 0.07 | < .0001 | 0.11 ± 0.06 | .0005 |
| AZT/3TC/ABC | 2 | 4.40 ± 0.18a | 6.23 ± 0.13a | 3.02 ± 0.22a | |||
| 3 y | |||||||
| Control | 3 | 0.49 ± 0.11 | .0006 | 0.70 ± 0.21 | .13 | 0.18 ± 0.15 | .05 |
| AZT/3TC/ABC | 3 | 1.90 ± 0.09a | 1.47 ± 0.36a,b | 0.72 ± 0.19a | |||
| 3 y | |||||||
| Control | 3 | 0.49 ± 0.11 | .07 | 0.70 ± 0.21 | .0009 | 0.18 ± 0.15 | .003 |
| AZT/3TC/NVP | 3c | 3.75 ± 1.55c | 3.11 ± 0.18b | 1.09 ± 0.14 | |||
Data are mean ± standard error.
Abbreviations: ABC, abacavir; AZT, zidovudine; NVP, nevirapine; 3TC, lamivudine.
a Differences for AZT/3TC/ABC at 1 year vs 3 years are significant for CA values (P = .0008), MN values (P = .002), and MN+C values (P = .0004), demonstrating reduction in AZT/3TC/ABC genotoxicity between 1 and 3 years of age.
b At 3 years of age, values for MN in cultured bone marrow cells from monkeys exposed to AZT/3TC/ABC were significantly lower than for monkeys exposed to ABC/3TC/NVP (P = .01).
c Only 2 monkeys had CA values.
Micronuclei
Micronuclei, portions of genetic material separated from the nuclei, are revealed as small, blue, extranuclear circles by staining with DAPI (Figure 1D). The percentage of cells with micronuclei in AZT/3TC-exposed patas at birth was significantly higher than in unexposed controls (P = .04; Table 1). At 1 year of age, after in utero exposure to AZT/3TC/ABC, there was a 10-fold increase in the percentage of cells with micronuclei, compared with the unexposed controls (P < .0001; Table 1). Cells from AZT/3TC/ABC-exposed patas aged 3 years did not show a significant increase in micronuclei (Table 1). In contrast, a 4.4-fold increase in the percentage of cells with micronuclei was observed in AZT/3TC/NVP-exposed patas at 3 years of age, compared with age-matched controls (P = .0009; Table 1).
Micronuclei Containing Whole Chromosomes
Positive staining with the CREST antiserum is indicative of the presence of kinetochore or centromeric material. Because the centromere is required for chromosomal replication, positive CREST staining within a micronuclei corresponds to the presence of a whole (or functional) chromosome. Figure 1D shows CREST-positive regions, or centromeres, within a nucleus. Aneuploidy results in the corresponding nucleus when micronuclei with a CREST signal (Figure 1E) contain a whole chromosome.
For cells cultured from unexposed and AZT/3TC-exposed patas taken at birth, the percentage of cells with micronuclei containing whole chromosomes (Table 1) was 3-fold higher in NRTI-exposed patas, compared with unexposed patas (P = .009). Patas taken at 1 year of age after in utero exposure to AZT/3TC/ABC (Table 1) showed a 27-fold increase in percentage of cells with micronuclei containing whole chromosomes, compared with age-matched unexposed controls (P = .0005). In addition, cells from AZT/3TC/ABC-exposed patas aged 3 years (P = .05; Table 1) and from AZT/3TC/NVP-exposed patas aged 3 years (P = .003; Table 1) both showed significant increases in the percentage of cells with micronuclei containing whole chromosomes, compared with the unexposed controls.
Additional Comparisons and Statistical Differences
The statistical relationships in this study, summarized in Table 1, show some additional informative comparisons. For example, values for the percentage of cells with centrosomal amplification, the percentages of cells with micronuclei, and the percentage of cells with micronuclei containing whole chromosomes among cells from unexposed patas were similar at all time points, suggesting that there is no increase in these biomarkers during the first 3 years of life. However, patas offspring exposed to ARVs had significant increases in almost all of these biomarkers at the times investigated, with the exception of the percentage of cells with micronuclei at 3 years in infants exposed to AZT/3TC/ABC and the percentage of cells with centrosomal amplification at 3 years in infants exposed to AZT/3TC/NVP. Significantly lower values for the percentage of cells with centrosomal amplification, with micronuclei, and with micronuclei containing whole chromosomes were observed in 3-year-old offspring treated with AZT/3TC/ABC, compared with their 1-year-old counterparts (Table 1), showing a reduction in the number of damaged cells over time. Also, at 3 years of age monkeys exposed to AZT/3TC/ABC had fewer micronuclei than those exposed to AZT/3TC/NVP (P = .01), suggesting that AZT/3TC/NVP is the more persistently genotoxic combination. Finally, the overall values for the percentage of cells with micronuclei containing whole chromosomes were lower than those for the percentage of cells with micronuclei because the loss of a whole chromosome is a rarer event than the partial loss of genetic material.
DISCUSSION
We used an ex vivo approach to show that genomic instability and chromosome loss induced in patas monkey bone marrow by in utero and perinatal exposure to human-equivalent protocols of ARV drugs is persistent up to 3 years of age and can be revealed by measuring biomarkers of genotoxicity. The data showed significantly increased percentages of cells with centrosomal amplification, with micronuclei, and with micronuclei containing whole chromosomes in most ARV-exposed groups, compared with unexposed animals, at birth, 1 year of age, and 3 years of age. The damage in patas aged 1 and 3 years, to which the drug combinations had been given in utero and for the first 6 weeks of life, revealed the persistence of this damage over time. Overall, these experiments reveal that, in addition to the direct mutagenic effects caused by NRTI-DNA incorporation, NRTIs damage DNA indirectly by producing centrosomal and spindle abnormalities resulting in aneuploidy.
These patas transplacental studies have been designed to model human clinical protocols, and the daily doses given are close to those used in humans, although for 3TC and NVP the duration of exposure was shortened to 4 weeks instead of 10 weeks. Interestingly, the companion article to this study, by Andre-Schmutz et al [15], describes results of karyotyping of cord blood CD34─ cells from ARV-exposed and -unexposed infants, which showed significantly more aneuploid cells in ARV-exposed infants born to HIV-1-infected mothers than in unexposed infants from uninfected pregnancies. Taken together, the patas and human studies provide a powerful statement for induction of fetal hematopoietic genotoxicity by in utero and perinatal ARV exposures, especially since a 3-year-old patas monkey is developmentally similar to a 14-year-old human. It may be important to follow HIV-1-uninfected children born to HIV-1-infected mothers over a long duration to evaluate the potential for persistent genotoxicity.
Notes
Acknowledgments. We thank Mr E. Davis and the National Institutes of Health Animal Center Staff, for primate assistance; and Dr Gene Shearer, for critical review of the manuscript.
Financial support. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother-to-child HIV-1 transmission. Lancet. 2000;355:2237–44. doi: 10.1016/S0140-6736(00)02415-6. [DOI] [PubMed] [Google Scholar]
- 2.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199:151–61. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Witt KL, Cunningham CK, Patterson KB, et al. Elevated frequencies of micronucleated erythrocytes in infants exposed to zidovudine in utero and postpartum to prevent mother-to-child transmission of HIV. Environ Mol Mutagen. 2007;48:322–9. doi: 10.1002/em.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivero OA, Anderson LM, Diwan BA, et al. Transplacental effects of 3′-azido-2′,3′-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst. 1997;89:1602–8. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 5.Olivero OA, Fernandez JJ, Antiochos BB, Wagner JL, St Claire ME, Poirier MC. Transplacental genotoxicity of combined antiretroviral nucleoside analogue therapy in Erythrocebus patas monkeys. J Acquir Immune Defic Syndr. 2002;29:323–9. doi: 10.1097/00126334-200204010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Borojerdi JP, Ming J, Cooch C, et al. Centrosomal amplification and aneuploidy induced by the antiretroviral drug AZT in hamster and human cells 1. Mutat Res. 2009;665:67–74. doi: 10.1016/j.mrfmmm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DM, Malarkey DE, Seilkop SK, et al. Transplacental carcinogenicity of 3′-azido-3′-deoxythymidine in B6C3F1 mice and F344 rats. Environ Mol Mutagen. 2007;48:283–98. doi: 10.1002/em.20297. [DOI] [PubMed] [Google Scholar]
- 8.Dobrovolsky VN, Shaddock JG, Mittelstaedt RA, et al. Frequency of Hprt mutant lymphocytes and micronucleated erythrocytes in p53-haplodeficient mice treated perinatally with AZT and AZT in combination with 3TC. Environ Mol Mutagen. 2007;48:270–82. doi: 10.1002/em.20280. [DOI] [PubMed] [Google Scholar]
- 9.Escobar PA, Olivero OA, Wade NA, et al. Genotoxicity assessed by the comet and GPA assays following in vitro exposure of human lymphoblastoid cells (H9) or perinatal exposure of mother-child pairs to AZT or AZT-3TC. Environ Mol Mutagen. 2007;48:330–43. doi: 10.1002/em.20285. [DOI] [PubMed] [Google Scholar]
- 10.Benhammou V, Warszawski J, Bellec S, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22:2165–77. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- 11.Divi RL, Doerge DR, Twaddle NC, et al. Metabolism and pharmacokinetics of the combination Zidovudine plus Lamivudine in the adult Erythrocebus patas monkey determined by liquid chromatography-tandem mass spectrometric analysis. Toxicol Appl Pharmacol. 2008;226:206–11. doi: 10.1016/j.taap.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Divi RL, Leonard SL, Kuo MM, et al. Cardiac mitochondrial compromise in 1-yr-old Erythrocebus patas monkeys perinatally-exposed to nucleoside reverse transcriptase inhibitors. Cardiovasc Toxicol. 2005;5:333–46. doi: 10.1385/ct:5:3:333. [DOI] [PubMed] [Google Scholar]
- 13.Divi RL, Einem TL, Fletcher SL, et al. Progressive mitochondrial compromise in brains and livers of primates exposed in utero to nucleoside reverse transcriptase inhibitors (NRTIs) Toxicol Sci. 2010;118:191–201. doi: 10.1093/toxsci/kfq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peloponese JM, Jr, Haller K, Miyazato A, Jeang KT. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc Natl Acad Sci USA. 2005;102:18974–9. doi: 10.1073/pnas.0506659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andre-Schmutz A, Dal-Cortivo L, Six E, et al. Genotoxic signature in cord blood cells of newborns exposed in utero to a zidovudine-based antiretroviral combination. J Infec Dis. 2013;208:235–43. doi: 10.1093/infdis/jit149. [DOI] [PubMed] [Google Scholar]