Abstract
Comparisons of animals bearing and lacking microorganisms can offer valuable insight into the interactions between animal hosts and their resident microbiota. Most hosts are naturally infected, and therefore, these comparisons require specific procedures (e.g., antibiotic treatment or physical exclusion of microorganisms) to disrupt the microbiota, but the potential for confounding nonspecific effects of the procedure on the traits of the host exists. Microbe-dependent and nonspecific effects can be discriminated by using multiple procedures: microbe-dependent effects are evident in hosts made microbe free by different procedures, but nonspecific effects are unique to individual procedures. As a demonstration, two procedures, oral administration of chlortetracycline (50 μg ml−1 diet) and microbiota removal by egg dechorionation, were applied to Drosophila melanogaster in a 2-by-2 factorial design. Microorganisms were undetectable in flies from dechorionated eggs and reduced by >99% in chlortetracycline-treated flies. Drosophila flies subjected to both protocols displayed an extended preadult development time, suggesting that the microbiota promotes the development rate. Female chlortetracycline-treated flies, whether from untreated or dechorionated eggs, displayed reduced protein content and egg fecundity, which could be attributed to the nonspecific effect of the antibiotic. We recommend that procedures used to disrupt the microbiota of animals should be selected, following systematic analysis of alternative mechanistically distinct procedures, on the basis of two criteria: those that achieve the greatest reduction (ideally, elimination) of the microbiota and those that achieve minimal nonspecific effects.
INTRODUCTION
There is now overwhelming evidence that insects, like other animals, bear a substantial resident microbiota and that multiple aspects of the insect phenotype are strongly influenced by the activities of these microorganisms (1, 2). Resident microorganisms in the gut, cells, or specialized organs contribute to the nutrition of various insect groups, e.g., termites, various xylophagous beetles, tsetse flies and other blood feeders, and plant sap feeders, such as aphids and cicadas (3). Some microorganisms contribute to insect defense against natural enemies, often by the production of specific antibiotics or stimulation of the insect immune system (4–8). Other insect traits reported to be affected by the microbiota include dispersal behavior, insecticide resistance, food choice, thermal resistance, mate choice, virus vector competence, reproductive traits (including sex ratio), and body color (9–16).
Experimentally generated microbe-free insects play a pivotal role in many studies investigating microbial effects on insect traits. Multiple methods are available to disrupt the microbiota of insects, including thermal treatment, antibiotic treatment, and mechanical exclusion (17, 18). Unfortunately, all these manipulations have the potential to cause nonspecific deleterious effects on the animal host. Very commonly, a single procedure is applied without due consideration of these nonspecific effects, and this can result in spurious claims for microbial roles in animal function.
The purpose of this paper is to recommend and illustrate an experimental approach that aids discrimination of the microbiota-dependent and nonspecific effects of procedures that disrupt the microbiota. Specifically, it is recommended that two (or more) mechanistically distinct procedures be applied, with the expectation that microbiota-dependent effects are obtained by all the procedures but nonspecific effects are unique to individual procedures. Here, we describe the application of this experimental method to the fruit fly Drosophila melanogaster with two treatments that have been used in previous studies: dechorionation of Drosophila eggs with bleach (which eliminates surface microorganisms), followed by rearing on sterile food (19–22), and feeding of the insects with food supplemented with the antibiotic chlortetracycline (CT) (12, 23). CT and other tetracyclines are broad-spectrum antibiotics that inhibit bacterial protein synthesis (24), and they are widely used to disrupt the gut microbiota in various insects and other animals (18, 25, 26).
The experiments in this study determined the impact of dietary CT and egg dechorionation on the resident microbiota and the development time, fecundity, and nutritional status (protein and free glucose contents) of D. melanogaster. The experiments had a 2-by-2 factorial design, with antibiotic treatment and egg dechorionation being the factors. We applied this experimental design with the aim to discriminate the specific effects of microorganisms (where the response between the antibiotic treatments differed in flies derived from untreated eggs but not in those derived from dechorionated eggs) from the nonspecific effects of either procedure (where dechorionation or antibiotic treatment affected the trait of interest, yielding a significant main factor in the analysis). We demonstrated that some effects of dietary CT can be explained to be a consequence of the effect on the gut microbiota and others can be explained to be a direct effect of the procedure on insect function.
MATERIALS AND METHODS
Insect culture and manipulations.
A Wolbachia-free line of Drosophila melanogaster strain CantonS was reared in sterile Falcon tubes (BD Biosciences, San Jose, CA) at 25°C with a 12-h light and 12-h dark cycle on an autoclaved diet containing 96 g glucose (Sigma), 48 g inactive dry Saccharomyces cerevisiae, and 14 g agar (both from Genesee Scientific) per liter. To generate the CT-supplemented (+CT) diet, a filtered solution of CT (Sigma) was dispensed at a 1/100 dilution into autoclaved food at 50°C and mixed thoroughly before the food solidified. The concentration (50 μg ml−1) used in the +CT diet was selected by use of the criterion of the lowest concentration yielding a >90% reduction in the number of CFU from Drosophila homogenates (27). For egg dechorionation, eggs deposited overnight by mated females were washed in sterile water and then immersed in 10% sodium hypochlorite solution for 5 min, followed by two rinses in sterile water, and the eggs were then transferred to an autoclaved diet. All insect manipulations were conducted in a laminar-flow cabinet with aseptic technique.
The experimental design was 2-by-2 factorial, with egg treatment (dechorionation or no treatment of eggs) and diet (CT-free diet and +CT diet) being the experimental factors. Each of the four treatments comprised 10 eggs in each of 10 replicate vials containing ca. 8 ml diet. The vials were monitored daily, and the time to development to adulthood was scored. To quantify the protein and glucose contents of the flies, at 7 to 10 days of age after eclosion to adulthood, individual flies were homogenized in 80 μl ice-cold buffer comprising 10 mM Tris, 1 mM EDTA, pH 8.0, and 0.1% (vol/vol) Triton X-100 and centrifuged at 7,000 × g at 4°C for 1 min. The protein content of the supernatant was determined by the Coomassie brilliant blue microassay method (500-0201; Bio-Rad) with bovine serum albumin as the standard (40 to 480 mg protein ml−1). The glucose assay kit of Sigma (GAGO20) was used for glucose assays.
To administer Drosophila microbiota to flies, vials (diameter, 0.9 in.) of sterile diet were pretreated with 40 adult males for 24 h. The deposited feces were washed from each vial with 500 μl sterile phosphate-buffered saline, and 50-μl fecal washings were added to each test diet. The fecal washings contained viable bacteria, including Acetobacter and Lactobacillus species, which dominate the gut microbiota (28), as revealed by plating onto nutrient agar (as below).
Identification and quantification of bacteria.
The culturable bacterial load per insect was assessed by a previously described method (23). Ten replicate 7- to-10-day-old adult flies were individually hand homogenized in 250 μl sterile phosphate buffer (pH 7.4) until pieces of tissue were no longer visible. Homogenate samples (100 μl) in a 10-fold dilution series from 1× to 1/1,000× were spread onto nutrient agar plates (28 g liter−1; Oxoid) using sterile technique, and the number of CFU was scored after 7 days at 25°C. Colonies were sampled for identification by Sanger sequencing of 16S rRNA gene sequences. Briefly, 16S rRNA gene sequences were amplified from DNA extracted from single colonies by PCR with general primers 16SA1 (5′-AGAGTTTGATCMTGGCTCAG-3′) and 16SB1 (5′-TACGGYTACCTTGTTACGACTT-3′) (29) by a previously described procedure (30). The PCR products were purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA) and sequenced on an Applied Biosystems automated 3730 DNA analyzer using BigDye Terminator chemistry and AmpliTaq-FS DNA polymerase. Sequences were trimmed using Sequencher (version 4.10.1) software and identified by NCBI nucleotide BLAST analysis.
Statistical analysis.
Analysis of variance (ANOVA) was applied to data sets that conformed to normal distributions with homogeneity of variances (as determined by the Anderson-Darling test and Levene's test, respectively). Two-sample comparisons were conducted by the t test for normally distributed data sets or by the Mann-Whitney U test. Where multiple tests were conducted in parallel, the Bonferroni correction of the critical probability (P = 0.05) was applied.
Nucleotide sequence accession numbers.
The sequences of the bacteria recovered from the flies were deposited in the GenBank database with accession numbers KC485818 to KC485880 (Table 1).
Table 1.
Composition of resident microbiota in D. melanogaster reared on a CT-free diet and a +CT diet, determined by 16S rRNA gene sequence analysis of bacterial colonies cultured on nutrient agar
| NCBI accession no. of sequences recovered | Accession no. of NCBI sequence with identity | Taxonomic identity | % sequence identity | No. of bacterial colonies |
|
|---|---|---|---|---|---|
| CT-free diet (n = 33) | +CT diet (n = 30) | ||||
| KC485830–KC485840, KC485851–KC485862 | GQ359863.1 | Acetobacter sp. strain 6-C-2 16S rRNA gene, partial sequence | 98–99 | 11 | 12 |
| KC485818–KC485819 | NR_025512.1 | Acetobacter cerevisiae strain LMG 1625 16S rRNA, partial sequence | 97–99 | 2 | 0 |
| KC485872 | HM218620.1 | Acetobacter malorum strain NM156-4 16S rRNA gene, partial sequence | 98–99 | 0 | 1 |
| KC485841–KC485842, KC485871 | FJ227313.1 | Acetobacter pasteurianus strain bh12 16S rRNA gene, partial sequence | 96–98 | 2 | 1 |
| KC485843–KC485849, KC485873–KC485874 | FN429068.1 | Acetobacter pasteurianus strain SX461 16S rRNA gene, partial sequence | 96–99 | 7 | 2 |
| KC485850 | FN429074.1 | Acetobacter pasteurianus strain ZJ362 16S rRNA gene, partial sequence | 97 | 1 | 0 |
| KC485821–KC485829, KC485863–KC485870 | EU096229.1 | Acetobacter pomorum strain EW816 16S rRNA gene, partial sequence | 97–99 | 9 | 8 |
| KC485878 | GU369767.1 | Lactobacillus brevis strain JS-7-2 16S rRNA gene, partial sequence | 99 | 0 | 1 |
| KC485879 | FJ227317.1 | Lactobacillus brevis strain b4 16S rRNA gene, partial sequence | 98 | 0 | 1 |
| KC485875, KC485876 | GU253891.1 | Lactobacillus pentosus strain N3 16S rRNA gene, partial sequence | 98 | 0 | 2 |
| KC485880 | AB494721.1 | Lactobacillus plantarum strain KL23 16S rRNA gene, partial sequence | 99 | 0 | 1 |
| KC485877 | HM449702.1 | Micrococcus luteus strain PCSB6 16S rRNA gene, partial sequence | 97 | 0 | 1 |
| KC485820 | DQ981281.1 | Uncultured bacterium clone thom_c06 16S rRNA gene, partial sequence | 98 | 1 | 0 |
RESULTS
Impact of CT and egg dechorionation on bacterial complement of Drosophila.
The first experiments tested for the presence of bacteria in Drosophila. The flies derived from untreated eggs on a CT-free diet yielded 3.2 × 104 CFU per fly (median; range, 520 to 2.9 × 105 CFU per fly; n = 10). The equivalent value for flies from the +CT diet was 118 CFU per fly (range, 5 to 9 × 103 CFU per fly; n = 10), demonstrating that, on average, >99% of the culturable bacteria were eliminated from flies reared on a +CT diet. A subset of bacterial colonies was sampled for identification by 16S rRNA gene sequencing. The most abundant bacteria were Acetobacter (Alphaproteobacteria), accounting for 97% and 80% of the colonies from untreated and CT-treated flies, respectively (Table 1). A parallel pyrosequencing analysis of PCR-generated 16S rRNA gene amplicons from flies reared on the CT-free diet yielded only Acetobacter species, with Acetobacter cerevisiae accounting for 98% of the 29,858 reads (data not shown), indicating that the bacterial community in CT-treated flies was drastically depleted and not dominated by unculturable forms.
The great majority of the culturable bacteria in flies reared on the +CT diet were susceptible to CT, as indicated by the very limited recovery of CFU from parallel fly samples reared on plates supplemented with 50 μg CT ml−1 (7/10 flies yielded no CFU, and the remaining 3 flies yielded 38, 260, and 420 CFU, respectively, giving a median number of CFU per fly of 0).
Every fly tested that developed from dechorionated eggs yielded no bacterial colonies on nutrient agar plates. Parallel PCR assays with general bacterial 16S rRNA gene primers also yielded no product, indicating that dechorionation eliminates all bacteria.
Fitness indices of Drosophila.
The two indices of fitness assayed yielded different patterns of response to CT and egg dechorionation.
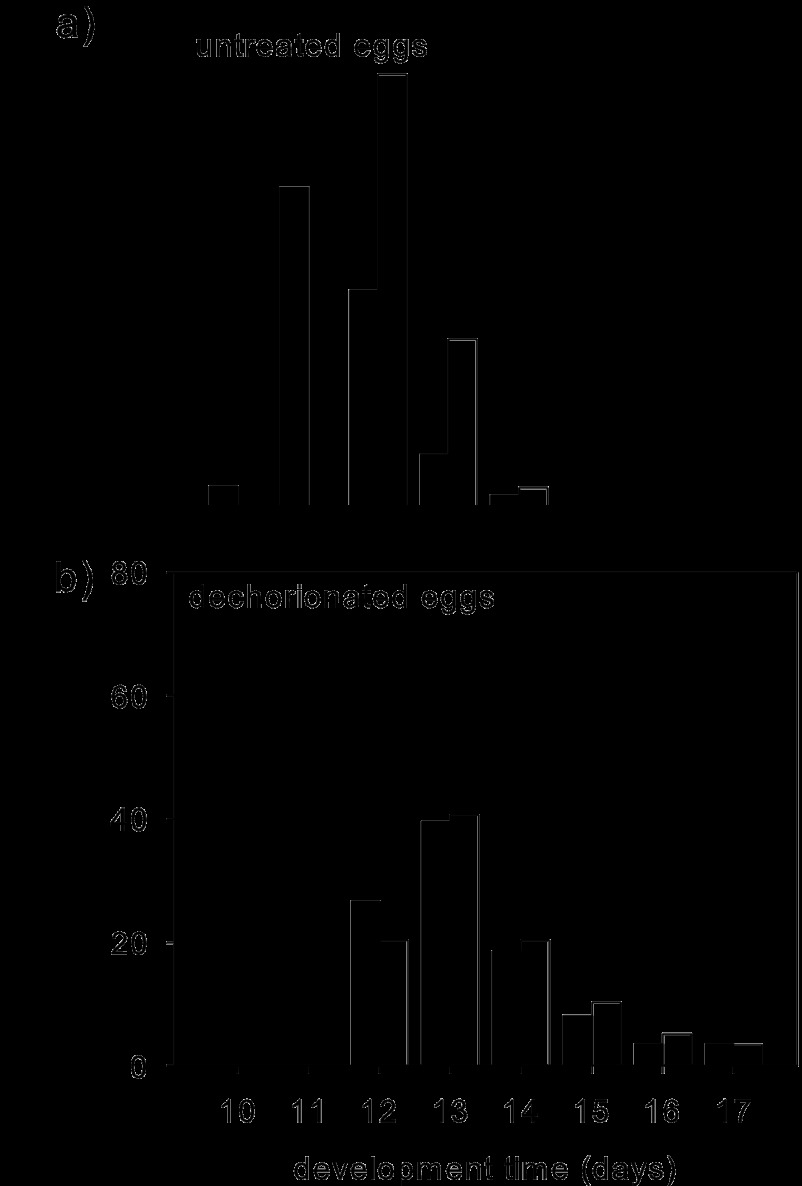
The time of insect development from oviposition to adulthood was 11 to 17 days (Fig. 1) and varied significantly across the four insect groups (Kruskal-Wallis test, H = 125.77, P < 0.001). As previously reported (31), dechorionation of the eggs resulted in a significantly extended time of development to adulthood relative to that for untreated eggs on the CT-free diet (Mann-Whitney test, W = 2336.5, P < 0.001). The development time on the +CT diet was also significantly prolonged relative to that on the CT-free diet for insects derived from untreated eggs (median, 12 days versus 11 days; W = 3081; P < 0.001) but not for insects derived from dechorionated eggs (median, 13 days for both treatments; W = 5988.5; P = 0.75) (Fig. 1). These results are consistent with the interpretation from previous studies (19, 20, 31, 32) that the microbiota increases the rate of Drosophila development.
Fig 1.
Development time of flies from oviposition to adulthood. Closed bars, CT-free diet; open bars, +CT diet. Number of replicates: 60 on CT-free diet and 100 on the +CT diet (a) and 86 on the CT-free diet and 59 on the +CT diet (b).
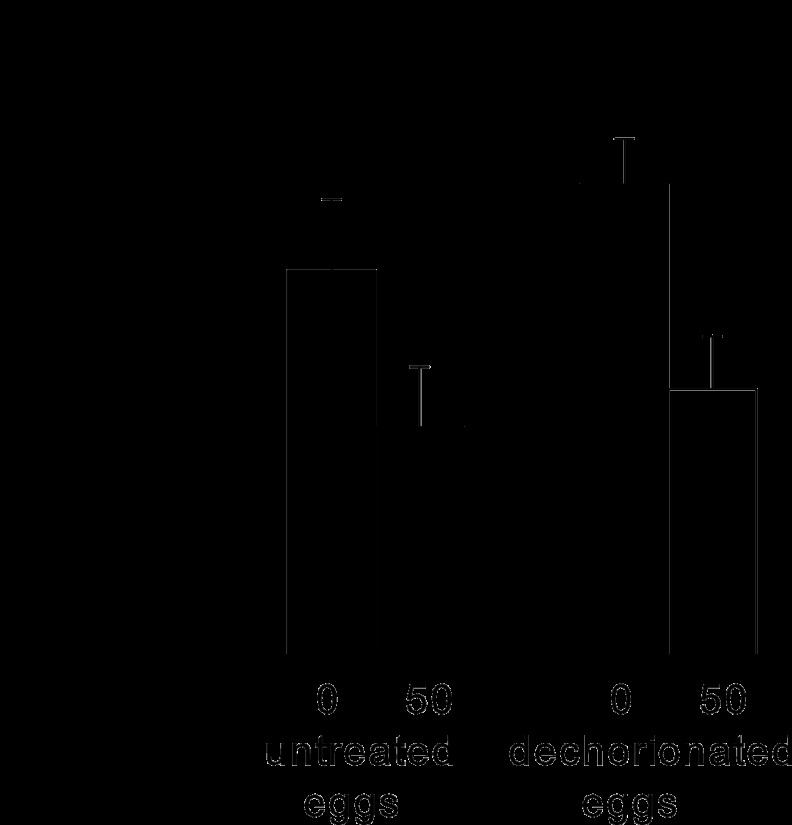
The fecundity of flies derived from both untreated and dechorionated eggs was negatively affected by dietary CT, with 40% fewer eggs being deposited by flies on the +CT diet than by those on the CT-free diet. In the ANOVA (Fig. 2), the interaction term was not statistically significant, indicating that the negative effect of CT on fecundity cannot be explained by the elimination of microbiota and is likely a consequence of the direct effect of the antibiotic on the insect.
Fig 2.
Median number of eggs deposited by 10 replicate flies over 7 days from days 3 to 10 posteclosion. Closed bars, CT-free diet; open bars, +CT diet. s.e., standard error. ANOVA results were as follows: for CT treatment, F1,36 = 9.58, P = 0.004; for egg treatment, F1,36 = 1.14, P > 0.05; for interaction, F1,36 = 0.17, P > 0.05. The x-axis values indicate the CT concentration in μg ml−1.
Nutritional and metabolic indices of Drosophila.
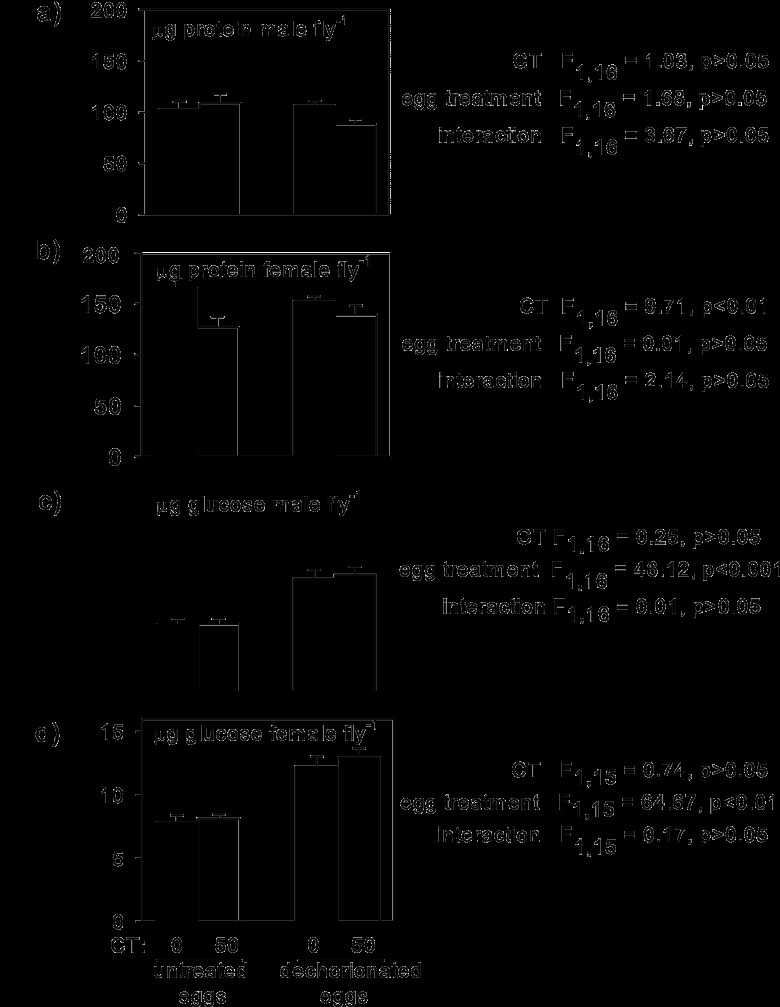
It has previously been shown that Drosophila flies derived from dechorionated eggs have an elevated glucose content, but their protein content is comparable to that in untreated flies (31). In this study, we investigated how these nutritional indices responded to CT treatment. (The protein and glucose contents of flies reared on a CT-free diet contributing to this analysis have been published previously [31].)
The protein content of males did not differ significantly between flies reared on the CT-free diet and those reared on the +CT diet, but that of females was reduced by 17% when they were on the +CT diet, independently of the egg treatment, and this effect was statistically significant (Fig. 3a and b). As with fecundity (see above), these data are indicative of a direct effect of the antibiotic on the female fly. To check whether the differential effect of CT on the protein content of the two sexes was concentration dependent, males were reared on a diet containing 300 μg CT ml−1. The protein content of these flies (104 ± 6.8 μg per fly, mean ± standard error, 5 replicates) also did not differ significantly from that of flies reared on a CT-free diet (103 ± 7.3 μg per fly, 5 replicates) (t7 = 0.887, P > 0.05).
Fig 3.
Nutritional indices of 5- to 7-day-old adult Drosophila flies derived from untreated and dechorionated eggs and reared on a CT-free diet (closed bars) or a +CT diet (open bars). Five replicates per treatment (except for 4 replicates for the glucose content of females from dechorionated eggs on the CT-free diet). The critical probability was 0.0125 after use of the Bonferroni correction for four tests.
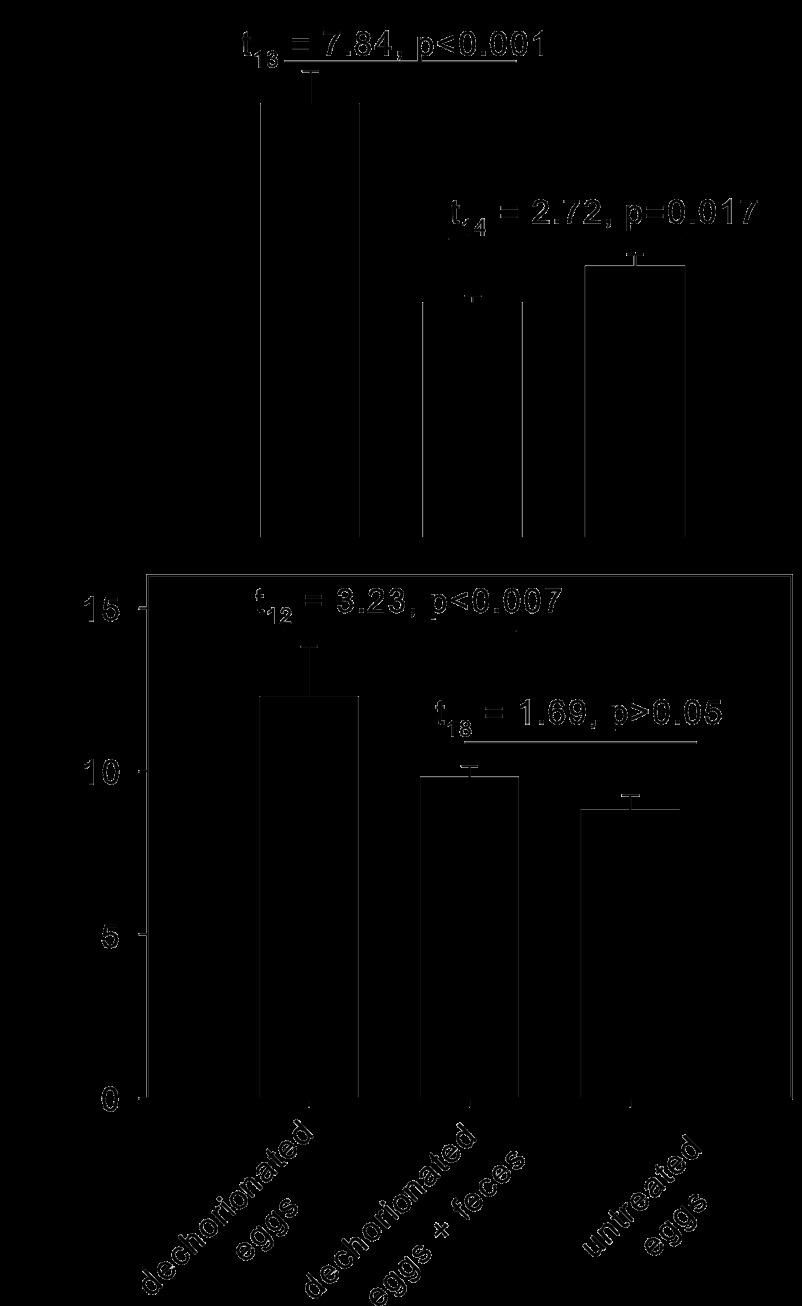
The glucose content of both male and female flies reared from untreated eggs on the +CT diet did not differ significantly from that of the equivalent flies reared on the CT-free diet and was significantly lower than that of flies derived from dechorionated eggs (Fig. 3c and d). These data are open to two alternative interpretations: (i) the elevated glucose content of flies from dechorionated eggs is a nonspecific effect of the egg treatment, or (ii) the small numbers of bacteria associated with the flies on the +CT diet is sufficient to reduce the glucose content to values comparable to those for conventionally reared flies. To discriminate between these possibilities, dechorionated eggs were transferred to a sterile diet supplemented with Drosophila feces, which contain live bacteria. This treatment resulted in a significant reduction in the glucose content of the flies for both males and females to levels that did not differ significantly from those for flies derived from untreated eggs (Fig. 4).
Fig 4.
Glucose content of Drosophila flies derived from dechorionated and untreated eggs. (Top) Males; (bottom) females. Ten replicates were used for untreated eggs and dechorionated eggs plus feces; 5 replicates were used for dechorionated eggs. The critical probability was 0.0125 after use of the Bonferroni correction for four t tests.
DISCUSSION
Any intervention to disrupt the resident microbiota of insects has the potential to cause nonspecific effects, and interpretation of results is critically dependent on discrimination between these nonspecific effects and effects attributable to the microbiota. This study demonstrates how the application of two mechanistically different procedures in a factorial design can be useful to make this discrimination.
Insect eggs can be sensitive to physical manipulations, especially removal of the egg shell (17). Nevertheless, multiple lines of evidence indicate that dechorionation of Drosophila eggs has no discernible nonspecific effect on preadult development. Specifically, untreated and dechorionated eggs develop to hatching at the same rates (31); the extended development time of insects derived from dechorionated eggs was also displayed by flies reared from untreated eggs on a +CT diet, in which the microbiota was greatly depleted (Fig. 1), and administration of bacteria to insects derived from dechorionated eggs rescued both the low glucose content (this study) and the rapid development rate (31) of untreated insects. Intriguingly, the glucose levels, but not the development rates, in CT-treated flies with a much-depleted bacterial content were comparable to those in untreated flies, suggesting that these two indices differ in their responsiveness to the abundance of bacteria. Further research is required to investigate the basis of this effect.
The application of two mechanistically different methods to disrupt the microbiota has revealed that CT treatment is a less satisfactory method than egg dechorionation for elimination of the microbiota of Drosophila. Importantly, direct effects of the antibiotic treatment on the insect are obtained at a concentration (50 μg ml−1) that fails to eliminate the bacteria, indicating that no CT concentration would achieve bacterial elimination without side effects. Although greater bacterial depletion can be achieved by the use of CT concentrations higher than those used in this study (27), treatment with tetracycline antibiotics at concentrations of ≥100 μg CT ml−1 diet is well-known to have substantial and transgenerational effects on mitochondria (33), particularly affecting systems strongly dependent on mitochondrial function, e.g., embryo development (17) and sperm viability (34). For this reason, the use of protocols using antibiotics to eliminate specific bacteria, e.g., Wolbachia, delays the ability to perform experiments on insects for multiple generations after antibiotic treatment (33). It has been assumed that the nonspecific deleterious effects of tetracyclines and other antibiotics are insignificant at concentrations of ≤100 μg ml−1, and 50 μg ml−1 is widely used to remove bacteria from insects used for study within a single generation (18, 35). This study demonstrates that, for Drosophila, this supposition is invalid. Both protein content and fecundity are significantly depressed in female Drosophila flies feeding on a +CT diet (50 μg ml−1) relative to that in flies derived from untreated and dechorionated eggs on a CT-free diet.
A related issue is the physiological condition of the residual bacteria in the CT-treated flies. The culturable bacteria were largely CT susceptible, as revealed by the minimal growth of bacteria from CT-treated flies on CT-supplemented medium. Taken with other data indicating that the bacteria associated with Drosophila are generally culturable (23), these data suggest that bacterial protein synthesis and linked processes (metabolism, growth, division, etc.) are largely inactive in flies on a +CT diet. They contrast with data for some insects which are known to bear antibiotic-resistant bacteria (36–39), whose interactions with the insect host would presumably be unaffected by the antibiotic treatment.
These considerations lead to two methodological recommendations. First, dechorionation is preferable over CT treatment for elimination of the gut microbiota of Drosophila, because CT has microbe-independent deleterious effects on Drosophila function at concentrations that are insufficient to achieve complete bacterial elimination. Second, control experiments with insects derived from dechorionated eggs should be conducted in studies where other antibiotics, e.g., erythromycin and rifampin (40, 41), are used to manipulate the microbiota of Drosophila. The microbe-independent effects of tetracycline and possibly other antibiotics used to disrupt the microbiota likely apply to other insects, and the tolerance of insect eggs to dechorionation procedures may vary among insect species. The factorial design (e.g., antibiotic and dechorionation) used in this study has general value to tease apart the microbe-dependent and microbe-independent effects of treatments to eliminate the microbiota.
An important caveat to these considerations relates to Wolbachia, present in 20 to 70% of all insect species (21, 42), including many laboratory lines and field isolates of D. melanogaster (43–46); the strain of D. melanogaster used in this study was Wolbachia free. As well as being a reproductive parasite (16), Wolbachia can confer virus resistance and nutritional benefits (47–49). Wolbachia can be eliminated by antibiotics but not surface sterilization/dechorionation of eggs, because it is transmitted vertically in the egg cytoplasm (16). Although the interactions between the gut microbiota and Wolbachia have received little study, elimination of the gut microbiota and the resultant changes in the insect signaling networks and immune function (5, 20, 32) could lead to changes to the population size, tissue tropism, and activities of Wolbachia. Consequently, differences between Wolbachia-positive insects derived from untreated and dechorionated eggs may be caused by gut microbe-dependent effects on both the insect and Wolbachia.
In conclusion, experimentally generated insects in which the microbiota is depleted or eliminated offer a vitally important tool to investigate insect-microbe interactions. Access to alternative methods with different modes of action is particularly valuable, to provide independent confirmation of proposed interactions, to identify microbe-independent effects (e.g., depressed protein content of CT-treated Drosophila flies), and to select the most appropriate method to manipulate the microbiota for different purposes.
ACKNOWLEDGMENTS
We thank Mariana Wolfner, who provided the Drosophila flies used in this study.
This work was supported by studentship awards from the Biotechnology and Biological Sciences Research Council (to E.V.R.), a graduate student fellowship from The Sarkaria Institute of Insect Physiology and Toxicology (to A.C.N.W), and NIH grant 1R01GM095372 (to A.E.D.).
Footnotes
Published ahead of print 8 March 2013
REFERENCES
- 1. Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 36:533–543 [Google Scholar]
- 3. Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23:38–47 [Google Scholar]
- 4. Haine ER. 2008. Symbiont-mediated protection. Proc. Biol. Sci. 275:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. 2010. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215 [DOI] [PubMed] [Google Scholar]
- 6. Oliver KM, Moran NA, Hunter MS. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. U. S. A. 102:12795–12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kroiss J, Kaltenpoth M, Schneider B, Schwinger MG, Hertweck C, Muaddula RK, Strohm E, Svatos A. 2010. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6:261–263 [DOI] [PubMed] [Google Scholar]
- 8. Giordano R, Weber E, Waite J, Bencivenga N, Krogh PH. 2010. Effect of a high dose of three antibiotics on the reproduction of a parthenogenetic strain of Folsomia candida (Isotomidae: Collembola). Env. Entomol. 39:1170–1177 [DOI] [PubMed] [Google Scholar]
- 9. Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc. Biol. Sci. 274:1979–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330:1102–1104 [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T. 2012. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. U. S. A. 109:8618–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 107:20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodacre SL, Martin OY, Bonte D, Hutchings L, Woolley C, Ibrahim K, Thomas C, Hewitt GM. 2009. Microbial modification of host long-distance dispersal capacity. BMC Biol. 7:32 doi:10.1186/1741-7007-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, Sobol I, Czosnek H, Vavre F, Fleury F, Ghanim M. 2010. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 84:9310–9317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wernegreen JJ. 2012. Mutualism meltdown in insects: bacteria constrain thermal adaptation. Curr. Opin. Microbiol. 15:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Neill SL, Hoffmann AA, Werren JH. (ed). 1997. Influential passengers—inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 17. Douglas AE. 1989. Mycetocyte symbiosis in insects. Biol. Rev. 64:409–434 [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson TL. 1998. The elimination of intracellular microorganisms from insects: an analysis of antibiotic treatment in the pea aphid (Acyrthosiphon pisum). Comp. Biochem. Physiol. 119A:871–881 [Google Scholar]
- 19. Bakula M. 1969. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invertebr. Pathol. 14:365–374 [DOI] [PubMed] [Google Scholar]
- 20. Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674 [DOI] [PubMed] [Google Scholar]
- 21. Werren JH, Windsor DM, Guo L. 1995. Distribution of Wolbachia among neotropical arthropods. Proc. Biol. Sci. 262:197–204 [Google Scholar]
- 22. Brummel T, Ching A, Seroude L, Simon AF, Benzer S. 2004. Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:12974–12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ren C, Webster P, Finkel SE, Tower J. 2007. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 6:144–152 [DOI] [PubMed] [Google Scholar]
- 24. Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barr KL, Hearne LB, Briesacher S, Clark TL, Davis GE. 2010. Microbial symbionts in insects influence down-regulation of defense genes in maize. PLoS One 5:e11339 doi:10.1371/journal.pone.0011339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17–37 [DOI] [PubMed] [Google Scholar]
- 27. Ridley EV. 2011. The impact of chlortetracycline on Drosophila melanogaster and Aedes aegypti. Ph.D. thesis. University of York, York, United Kingdom [Google Scholar]
- 28. Wong C-N, Ng P, Douglas AE. 2011. Low diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 13:1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukatsu T, Nikoh N. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 64:3599–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douglas AE, Francois CLMJ, Minto LB. 2006. Facultative ‘secondary’ bacterial symbionts and the nutrition of the pea aphid, Acyrthosiphon pisum. Physiol. Entomol. 31:262–269 [Google Scholar]
- 31. Ridley EV, Wong AC, Westmiller S, Douglas AE. 2012. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS One 7:e36765 doi:10.1371/journal.pone.0036765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 14:403–414 [DOI] [PubMed] [Google Scholar]
- 33. Ballard JW, Melvin RG. 2007. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol. Biol. 16:799–802 [DOI] [PubMed] [Google Scholar]
- 34. Zeh JA, Bonilla MM, Adrian AJ, Mesfin S, Zeh DW. 2012. From father to son: transgenerational effect of tetracycline on sperm viability. Sci. Rep. 2:375 doi:10.1038/srep00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmed MZ, Ren SX, Xue X, Li XX, Jin GH, Qiu BL. 2010. Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr. Microbiol. 61:322–328 [DOI] [PubMed] [Google Scholar]
- 36. Channaiah LH, Subramanyam B, McKinney LJ, Zurek L. 2010. Stored-product insects carry antibiotic-resistant and potentially virulent enterococci. FEMS Microbiol. Ecol. 74:464–471 [DOI] [PubMed] [Google Scholar]
- 37. Macovei L, Zurek L. 2006. Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings. Appl. Environ. Microbiol. 72:4028–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen HK, Cloud-Hansen KA, Wolinski JM, Guan C, Greene S, Lu S, Beyink M, Broderick NA, Raffa KF, Handelsman J. 2009. Resident microbiota of the gypsy moth midgut harbors antibiotic resistance determinants. DNA Cell Biol. 28:109–117 [DOI] [PubMed] [Google Scholar]
- 39. Kadavy DR, Hornby JM, Haverkost T, Nickerson KW. 2000. Natural antibiotic resistance of bacteria isolated from larvae of the oil fly, Helaeomyia petrolei. Appl. Environ. Microbiol. 66:4615–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blatch SA, Meyer KW, Harrison JF. 2010. Effects of dietary folic acid level and symbiotic folate production on fitness and development in the fruit fly Drosophila melanogaster. Fly 4:1–8 [DOI] [PubMed] [Google Scholar]
- 41. Cox CR, Gilmore MS. 2007. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect. Immun. 75:1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeyaprakash A, Hoy MA. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9:393–405 [DOI] [PubMed] [Google Scholar]
- 43. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6:741–751 [DOI] [PubMed] [Google Scholar]
- 44. Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. 2007. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl. Environ. Microbiol. 73:3470–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clark ME, Anderson CL, Cande J, Karr TL. 2005. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 170:1667–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chandler SM, Wilkinson TL, Douglas AE. 2008. Impact of plant nutrients on the relationship between a herbivorous insect and its symbiotic bacteria. Proc. Biol. Sci. 275:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. [DOI] [PubMed] [Google Scholar]
- 48. Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O'Neill SL. 2009. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 5:e1000368 doi:10.1371/journal.ppat.1000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6:e1000833 doi:10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]