Abstract
Mycobacterium avium subsp. paratuberculosis causes Johne's disease (JD) in ruminants, with substantial economic impacts on the cattle industry. Johne's disease is known for its long latency period, and difficulties in diagnosis are due to insensitivities of current detection methods. Eradication is challenging as M. avium subsp. paratuberculosis can survive for extended periods within the environment, resulting in new infections in naïve animals (W. Xu et al., J. Environ. Qual. 38:437-450, 2009). This study explored the use of a biosecure, static composting structure to inactivate M. avium subsp. paratuberculosis. Mycobacterium smegmatis was also assessed as a surrogate for M. avium subsp. paratuberculosis. Two structures were constructed to hold three cattle carcasses each. Naturally infected tissues and ground beef inoculated with laboratory-cultured M. avium subsp. paratuberculosis and M. smegmatis were placed in nylon and plastic bags to determine effects of temperature and compost environment on viability over 250 days. After removal, samples were cultured and growth of both organisms was assessed after 12 weeks. After 250 days, M. avium subsp. paratuberculosis was still detectable by PCR, while M. smegmatis was not detected after 67 days of composting. Furthermore, M. avium subsp. paratuberculosis remained viable in both implanted nylon and plastic bags over the composting period. As the compost never reached a homogenous thermophilic (55 to 65°C) state throughout each structure, an in vitro experiment was conducted to examine viability of M. avium subsp. paratuberculosis after exposure to 80°C for 90 days. Naturally infected lymph tissues were mixed with and without compost. After 90 days, M. avium subsp. paratuberculosis remained viable despite exposure to temperatures typically higher than that achieved in compost. In conclusion, it is unlikely composting can be used as a means of inactivating M. avium subsp. paratuberculosis associated with cattle mortalities.
INTRODUCTION
Composting can be used as a means to inactivate pathogenic bacteria (1–3). Most research regarding pathogen inactivation has examined composting of municipal waste, manure, and garden refuse, with considerably less information available regarding the composting of livestock mortalities (4). Compared to other carcass disposal methods, such as burial or incineration, composting has proven to be an inexpensive, safe, and an environmentally sustainable way to eliminate pathogens associated with livestock mortalities (3, 5). Several studies have evaluated the survival of Escherichia coli (O157:H7) and Salmonella spp. during composting (1, 5), but to date, no studies have been conducted to assess the survival of Mycobacterium avium subsp. paratuberculosis during composting.
M. avium subsp. paratuberculosis causes Johne's disease (JD) in cattle, a condition that is difficult to diagnose due to the asymptomatic nature of the disease, fastidious nature of the causative agent, an incubation period of 2 to 10 years, and the requirement for up to 16 weeks to culture within a laboratory (6). Tests typically used to detect early infection, such as milk or serum enzyme-linked immunosorbent assay (ELISA) or fecal culture, do not have sufficient specificity or sensitivity to detect M. avium subsp. paratuberculosis in cattle shedding intermittently or at low levels in milk or feces (7). This bacterium can also survive for extended periods in the environment (3, 8, 9), with survival in the soil demonstrated for at least 385 days (3), thereby exposing cattle to this pathogen from their surroundings. The mechanism by which survival may be achieved includes dormancy (10) and sporulation (11). A recent review reported that 3% of dairy cattle across Canada are positive for M. avium subsp. paratuberculosis (12), but it is estimated that 50% of dairy herds have at least one M. avium subsp. paratuberculosis-infected animal (13).
It has been suggested that M. avium subsp. paratuberculosis may be linked to Crohn's disease in humans (14–16). Although there is no medical consensus regarding the causal association between M. avium subsp. paratuberculosis and Crohn's disease, several possible sources of M. avium subsp. paratuberculosis have been identified. Studies have shown that M. avium subsp. paratuberculosis can survive pasteurization in milk and milk products (17–20), which may serve as vectors for the transmission of M. avium subsp. paratuberculosis from cattle to humans. Meat products contaminated with M. avium subsp. paratuberculosis from infected cattle may also be an infectious source (21, 22), but M. avium subsp. paratuberculosis has not been detected in retail ground beef (23). Most M. avium subsp. paratuberculosis-infected cattle are culled as a result of decreased milk yield, fertility, or weight gain, all of which contribute to significant economic losses for the producer (24). The costs of direct losses are estimated to be approximately $50 Canadian (CAD) per head per year for the dairy industry, based on prevalence levels derived from serum ELISA results (24). Significantly greater losses of $441 U.S. per head per lactation have been estimated based on prevalence estimates obtained by direct fecal culture (25). As most cattle are culled as a result of decreased productivity prior to the onset of clinical symptoms, many are likely to enter the food chain. Voluntary control programs have already been implemented in the United States (26) and Australia (27) and proposed within Canada (28), but more stringent on-farm programs may become mandatory if a zoonotic connection with Crohn's disease is established.
Recently, a biosecure, static composting system for large-scale cattle carcass disposal was developed (3, 29). It has been demonstrated that Campylobacter jejuni, Escherichia coli (O157:H7), and Newcastle disease virus were rendered nonviable by composting using this system, but the survivability of M. avium subsp. paratuberculosis under these conditions has not been assessed. The purpose of this study was to determine if static composting of cattle carcasses inactivates M. avium subsp. paratuberculosis in a biosecure composting system and, furthermore, to determine whether Mycobacterium smegmatis could be used as a surrogate for M. avium subsp. paratuberculosis, as it is a fast-growing species that is much easier to culture (30) and has no association with human disease.
MATERIALS AND METHODS
Experiment 1: biosecure, static composting of M. avium subsp. paratuberculosis. (i) Biocontainment piles.
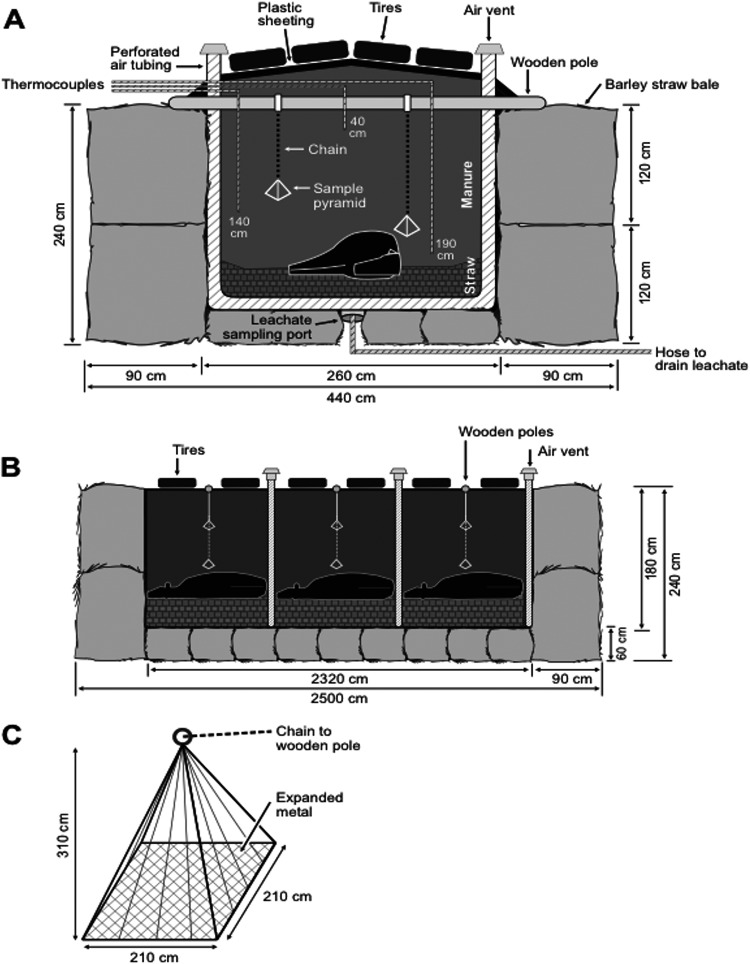
In March 2008, two biocontainment composting structures (Fig. 1), pile 1 and pile 2, were constructed on a concrete pad at the Glenlea Research Facility at the University of Manitoba (Winnipeg, Manitoba, Canada). The structures were 25 m long, 4.4 m wide, and 2.4 m high (Fig. 1A and B). The outer walls were made of large barley straw bales, with a dimension of 2.0 m by 0.9 m by 1.2 m. Small straw bales were used to create the floor in each structure. The inside of each structure was lined with heavy black plastic sheeting. Perforated air tubing was used to drain leachate into a stainless steel basin with a drain that was positioned in the middle of the straw floor. Wire mesh was placed on top of the basin to prevent large particles from blocking the drain. A hose attached to the bottom of the basin ran from underneath the pile so that it was accessible as a leachate sampling port (Fig. 1A). Within the structure, loose straw was spread on the bottom of the plastic sheeting to form a 60-cm layer. Three cull JD-negative Holstein cows were euthanized on site and laid head to tail on top of the straw layer. Next, a 120-cm layer of mixed cattle manure was laid over the carcasses. Flexible perforated plastic pipes were embedded between each carcass in the straw with air vents at the ends of the pipes passing through the plastic to enable passive aeration of the compost (Fig. 1A and B). The plastic was overlapped, and old tires were placed on top to limit inward seepage of moisture (Fig. 1A and B). On day 194, both piles were opened to turn and aerate the compost.
Fig 1.
Biocontainment structures used for composting cattle mortalities in experiment 1. The structures include straw outer walls, three carcasses per pile, perforated air tubing, leachate sampling ports, and inner thermocouple wires. The cross-section and dimensions are indicated along the width (A) and length (B) of each structure. Baker retrieval pyramids (BRPs) (32) used for sampling M. avium subsp. paratuberculosis were inserted in the vicinity of each carcass. The dimensions of the BRP are shown in panel C. The diagrams were adapted from Xu et al. (3), reprinted by permission from the American Society of Agronomy (ASA), the Crop Science Society of America (CSSA), and the Soil Science Society of America (SSSA).
(ii) Sample preparation.
The four treatments examined included (i) a control using extra-lean ground beef, (ii) lab-cultured M. avium subsp. paratuberculosis (ATCC 19698), grown in Middlebrook 7H9 broth with albumin-dextrose-catalase (ADC) enrichment, inoculated onto ground beef, (iii) lab-cultured M. smegmatis (mc2 155; ATCC 700084), grown in the same type of culture medium as M. avium subsp. paratuberculosis, also inoculated onto ground beef, and (iv) lymph tissues (ileal, mesenteric, and ileocecal) collected from six cows infected with M. avium subsp. paratuberculosis as determined by serum ELISA (31). For each treatment, tissues (15 g) were mixed and placed into either a heat-sealed nylon bag (pore size, 50 μm; Ankom rumen in situ bags; Ankom Technology, Macedon, NY), to assess the biochemical and microbial effects of composting on M. avium subsp. paratuberculosis viability, or into a sterile plastic bag to test the effects of temperature only.
Subsamples were cultured to ensure M. avium subsp. paratuberculosis and M. smegmatis were present in all tissues (day 1) prior to exposure to compost (culture method described below). To further ensure mycobacterial presence, a suspension was created with 2 g of tissue from each bag and 30 ml of Middlebrook 7H9 broth (Becton, Dickinson, and Company [BD], Franklin Lakes, NJ) with ADC enrichment (BD). The suspension was centrifuged at 1,700 × g for 20 min, the supernatant was decanted, and 500 μl of the resuspended pellet was added to Mycobacteria growth indicator (MGIT) tubes (BD) with oleic acid-albumin-dextrose-catalase (OADC) enrichment (BD) and a mixture of antibiotics consisting of polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) (BD). A pure culture of M. smegmatis was used as the positive control. Tubes were allowed to incubate at 37°C for 7 days, and mycobacterial presence was determined by fluorescing MGIT tubes under UV light.
Survivability of M. avium subsp. paratuberculosis in the nylon and plastic bags was investigated using Baker retrieval pyramids (BRPs) (Fig. 1C) (32). A mixture of manure and straw was used to pack eight samples (each treatment within each bag type) into a BRP in a manner so that there was no contact between bags. Two BRPs were suspended within the vicinity of each cow at 100- and 180-cm depths from a stainless steel cable anchored to wooden poles at 1.5-m intervals along the length of the pile (Fig. 1A and B). A total of five pyramids in pile 1 and six pyramids in pile 2 were embedded as each pile was constructed. One BRP per pile was removed after 35, 67, 96, 131, and 250 days of composting. For removal, the stainless steel cable holding the suspended BRP was unclamped from the suspension pole and attached to the front-end loader of a tractor and pulled from the pile, leaving the remainder of the plastic sheet intact.
After the BRPs were extracted, they were transported from the Glenlea Research Facility to a biosafety level 2 microbiology lab at the University of Manitoba. Sample bags were recovered from the manure in the BRP, and one-half of the tissue in each bag was stored at −20°C, while the remaining half was used for culturing purposes. Compost was collected by sampling 10 different areas from each pile on days 1, 96, 131, 194, 203, and 250. The compost samples from each day were composited, mixed, and stored at −20°C until further analysis.
(iii) Temperature monitoring.
Ambient and internal composting temperatures were monitored for each compost pile. At each carcass within each structure, three type-T thermocouples were embedded at depths of 40, 140, and 190 cm (Fig. 1A), resulting in nine locations at which temperature was measured. Temperature was measured once daily with a hand-held digital thermometer (Digi-Sense thermometer; Cole-Parmer Canada, Inc., Montreal, Quebec, Canada) for the first 150 days and then once weekly for the remainder of the composting experiment (total experimental period of 250 days). The thermocouple temperature within the piles was averaged at each site on each measurement day throughout the 250-day composting period. Temperature monitors (Hobo U12-015 stainless temperature data loggers; Onset Computer Corporation, Bourne, MA) were also attached to the outside of each composting structure and to each BRP to measure ambient and inner temperatures hourly and averaged for each day.
(iv) Chemical analysis.
The moisture content of the compost was determined by drying at 60°C to a constant weight for 50 h (method 942.05 in reference 33). Oven-dried samples were then ground to pass through a 1-mm screen for analysis of total carbon (C), nitrogen (N), and sulfur (S) (method 968.06 in reference 33). Ash content was determined by placing the dried compost in a muffle furnace at 600°C for 2 h (method 942.05 in reference 33). Macromineral (Na, K, P, Mg, and Ca) content was determined by digestion of the ash with 1% HNO3·5 N HCl and analyzed using inductively coupled plasma (ICP) mass spectrophotometry (Vista-MPX charge-coupled device [CCD] for simultaneous ICP-optical emission spectrometry [OES]; Agilent Technologies Canada, Inc., Mississauga, ON, Canada). Organic matter (OM) was determined using the equation OM (g/kg) = 31.1 + 1.797 (total C g/kg) (34). The pH and electrical conductivity (EC) of the compost (12.5 g) were determined after compost was allowed to stand in 50 ml of distilled water for 2 h and measured with an Accumet Research AR2 dual-channel pH/ion meter (Fisher Scientific, Pittsburgh, PA). The volatile fatty acid (VFA) profile was determined by shaking a 10-g sample of compost with 0.1 N HCl for 12 h, mixing it with 1 ml of meta-phosphoric acid, and centrifuging it at 3,000 rpm for 20 min. The supernatant (2 ml) was analyzed using gas chromatography following the column conditioning instructions for the 80/100 Chromosorb WAW (Supelco, Bellefonte, PA). Analysis of chloride (section 04.05-CL in reference 35), ammonia (section 04.02-C in reference 35), and nitrate (section 04.02-B in reference 35) were conducted at A & L Canada Laboratories, Inc. (London, Ontario, Canada). Recommended conditions for optimal composting (mortality and nonmortality) were compared with the biochemical results from this study (Table 1).
Table 1.
Literature data for rapid composting in comparison with conditions from the present study over 250 days of composting of livestock mortalities
| Condition | Range from present study | Optimal rangea | References |
|---|---|---|---|
| C/N ratio | 10:1–22:1 | 13:1–50:1 | 3, 4, 38, 40 |
| Moisture content (%) | 48–80 | 40–65 | 4, 38, 47 |
| pH | 7.5–9.1 | 5.5–9.5 | 3, 37, 38, 48 |
| Temp (°C) | 10–64 | 40–65 | 4, 37, 38 |
Recommended conditions for effective mortality composting during the biooxidative phase.
(v) DNA analysis of bacteria.
To identify M. avium subsp. paratuberculosis from composted tissues, a digestion/decontamination step was performed following the manufacturer's protocol in the BBL MycoPrep specimen digestion/decontamination kit (BD). Samples were then centrifuged at 3,000 × g for 20 min, and the pellet was resuspended in BBL MycoPrep phosphate buffer (BD). Next, 200 μl of the resuspension was inoculated onto BBL Herrold's egg yolk agar slants with mycobactin J, amphotericin, nalidixic acid, and vancomycin (HEYA; BD) and incubated at 37°C for 12 weeks, with growth recorded weekly. At the end of 12 weeks, all growth from the surface of the slants was removed and DNA was extracted.
The DNA from the colonial growth on the surface of the slants was extracted using a fungal/bacterial DNA kit (Zymo Research Corp., Orange, CA) following the manufacturer's protocol. Survival of the cultured M. avium subsp. paratuberculosis and M. smegmatis strains in samples was verified with PCR. Primers used for verification are outlined in Table 2. A final volume of 25 μl containing 12.5 μl of PCR master mix (Promega Corp., Madison, WI), 10.5 μl of nuclease-free water (Promega Corp.), 0.5 μl of the forward primer (25 μM), 0.5 μl of the reverse primer (25 μM), and 1 μl of genomic DNA was used to perform PCR. Tubes were placed in a thermocycler (C1000 thermal cycler; Bio-Rad Laboratories, Inc., Hercules, CA) and amplified as follows: one cycle of denaturation at 94°C for 4 min followed by 40 cycles of denaturation at 94°C for 45 s, annealing at 61°C for 45 s, and extension at 72°C for 45 s and a final extension at 72°C for 10 min (36). Bacterial DNAs isolated from both M. avium subsp. paratuberculosis and M. smegmatis were used as positive controls to differentiate these closely related species based on the sizes of the amplicons. Water served as the negative control. The final PCR products were electrophoresed and visualized on a 1% (wt/vol) agarose gel stained with 2 μl ethidium bromide.
Table 2.
Primer sets for PCR amplification and differential identification of M. avium subsp. paratuberculosis and M. smegmatis
| Species | Primer orientation | Sequence (5′→3′) | Size (bp) |
|---|---|---|---|
| M. avium subsp. paratuberculosisa | Forward | GGGTTGATCTGGACAATGACGGTTA | 569 |
| Reverse | AGCGCGGCACGGCTCTTGTT | ||
| M. smegmatisb | Forward | GTGCGCTACCTCGTCATGATG | 624 |
| Reverse | CTAGTTCATGTTCCAGGGCTCG |
Primer set from Vansnike et al. (36) targeting the IS900 gene in M. avium subsp. paratuberculosis.
Primer set designed using NCBI BLAST targeting the MSMEI_5897 gene in M. smegmatis (http://www.ncbi.nlm.nih.gov/tools/primer-blast/; 2008).
Experiment 2: in vitro incubation of M. avium subsp. paratuberculosis.
The in vitro incubation of M. avium subsp. paratuberculosis included the following three treatments: (i) a control consisting of compost not mixed with M. avium subsp. paratuberculosis-infected tissues, (ii) lymph tissues (ileal, mesenteric, and ileocecal) from cattle positive for M. avium subsp. paratuberculosis determined by serum ELISA (31), and (iii) a combination of M. avium subsp. paratuberculosis-positive tissues and compost mixed 1:4, respectively. Cured compost sampled at the completion of experiment 1 was provided as the compost substrate for the control and 1:4 mixtures. Tissues were mixed together, and 100 g of homogenate was placed into eight plastic bags and mixed by hand for 5 min. Two bags of each treatment were then placed in separate incubators at 4, 45, 60, and 80°C. Samples were collected after 1, 2, 4, 8, 17, 30, 60, and 90 days and analyzed for viable M. avium subsp. paratuberculosis as outlined for experiment 1. Again, to ensure M. avium subsp. paratuberculosis was present in all treatments, tissue from each bag was cultured prior to incubation.
RESULTS
Experiment 1: M. avium subsp. paratuberculosis survival in composting piles.
During construction of the two composting piles, the daily average ambient temperature was −25°C (Fig. 2), with a windchill averaging −36°C (www.climate.weatheroffice.gc.ca). Despite these cold temperatures, the composting piles did not freeze as the internal temperatures of both piles remained above 0°C (Fig. 2) throughout the trial. Nonetheless, thermophilic conditions were not achieved when considered a homogeneous system, as the temperatures throughout either pile did not exceed 55°C. However, as the ambient temperature increased, so did the interior temperature of the piles. On day 194, both piles were turned and aerated, but the temperature of the compost did not notably increase in either pile (Fig. 2). During this mixing event, the carcasses were not readily identifiable, but some soft tissues and bones were observed. On day 250, large bones, such as the skull, pelvis, and long bones were still present, but all soft tissues had decomposed.
Fig 2.
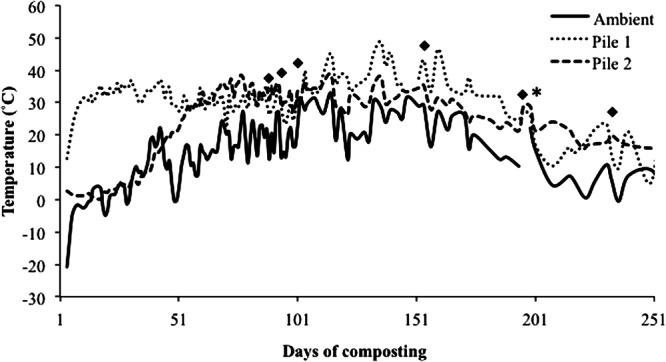
Ambient and internal composting temperature profiles of pile 1 and pile 2 over 250 days of composting. The asterisk indicates a turning event occurring at both compost piles; black diamonds designate major rain events throughout the composting experiment (>25 mm/day; http://climate.weatheroffice.gc.ca/climateData).
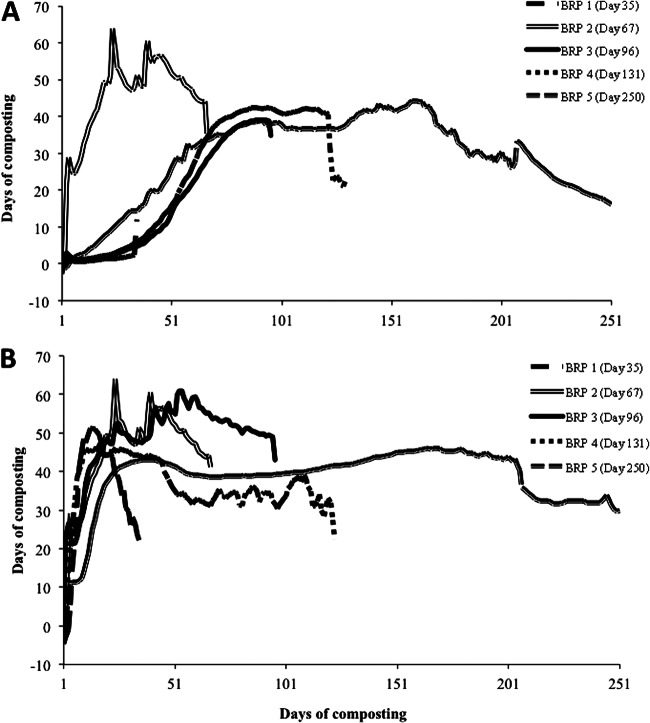
Temperature data collected from the Hobo data loggers (Onset Computer Corporation) attached to each BRP indicated that spatial variability did occur, and a thermophilic state was achieved at several locations. Within pile 1, the temperature of BRP 2 (extracted on day 67) was >50°C for 35 days, with a peak temperature of 64°C on day 25 (Fig. 3A). All other BRP locations within pile 1 remained between 35 and 40°C for the entire 250-day composting period, except for BRP 1 (extracted on day 35), which only reached up to 15°C (Fig. 3A). Within pile 2, the temperature at all BRP locations was >40°C by day 25 (Fig. 3B). In pile 2, BRP 1 increased to 52°C by day 15 and then declined to 20°C and remained at that temperature until extraction on day 35 (Fig. 3B), BRP 2, extracted on day 67, increased to 65°C on day 25 and remained at >50°C for approximately 40 days (Fig. 3B), BRP 3, extracted on day 96, remained between 50 and 62°C for about 60 days (Fig. 3B), BRP 4 increased to 47°C by day 20 and then remained between 25 and 40°C until removal on day 131 (Fig. 3B), and BRP 5 remained between 35 and 45°C for the entire 250-day composting period (Fig. 3B).
Fig 3.
Internal composting temperature profiles of pile 1 (A) and pile 2 (B) for each chamber location over 250 days of composting.
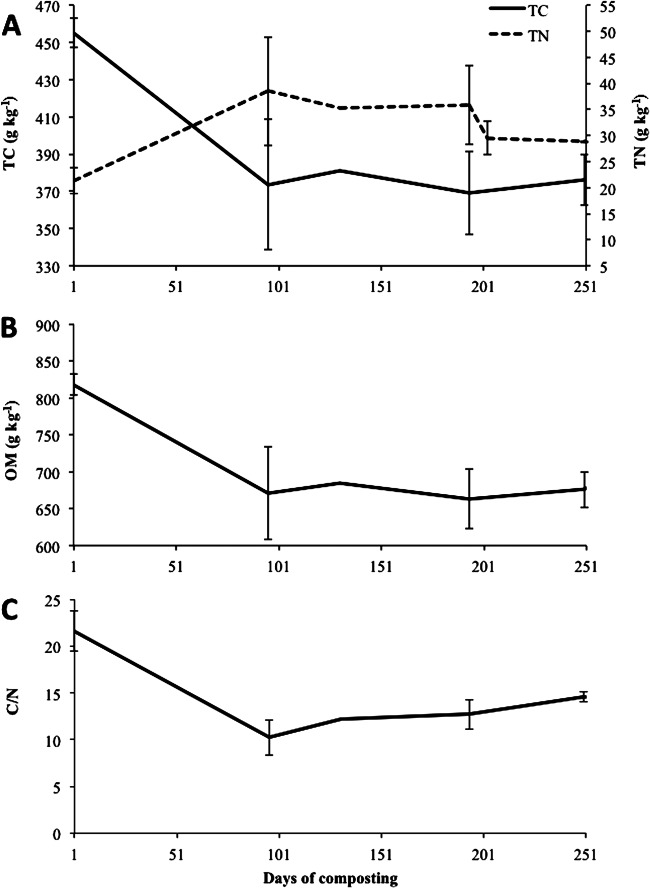
Moisture content ranged between 480 and 800 g/kg and was relatively constant throughout the experiment, except between days 101 and 201 (Fig. 4A [major rain events indicated in Fig. 2]). This period coincided with the summer months, where warmer ambient temperatures (between 10 and 30°C) and fewer rain events resulted in desiccation of the piles. Large volumes of leachate were collected from both piles (data not shown) prior to day 101 and after day 201. Prior to day 101, leachate volumes were attributed to the high moisture losses associated with the decomposition of carcasses. After day 201, an association between rain events and volume of leachate collected was observed (data not shown), which increased moisture content to 800 g/kg (Fig. 4A). The pH of the compost ranged from 7.5 to 9.0 (Fig. 4B). Total C rapidly declined from 450 to 370 g/kg until day 100 and plateaued thereafter to day 250 (Fig. 5A). As expected, organic matter (OM) exhibited the same slight decline observed for total C (Fig. 5B). Nitrogen increased until day 194 (Fig. 5A), when the compost was turned. Following an initial decline until day 94, the C/N ratio remained relatively stable at 12:1 over the 250-day composting period (Fig. 5C). Compost temperatures in the present study had a range of 10 to 64°C (Fig. 2), a much broader range than the optima of 40 to 65°C for the biooxidative phase (Table 1). However, according to the temperature readings from the data loggers attached to each BRP, several locations were within acceptable levels, indicating heterogeneity of the compost.
Fig 4.
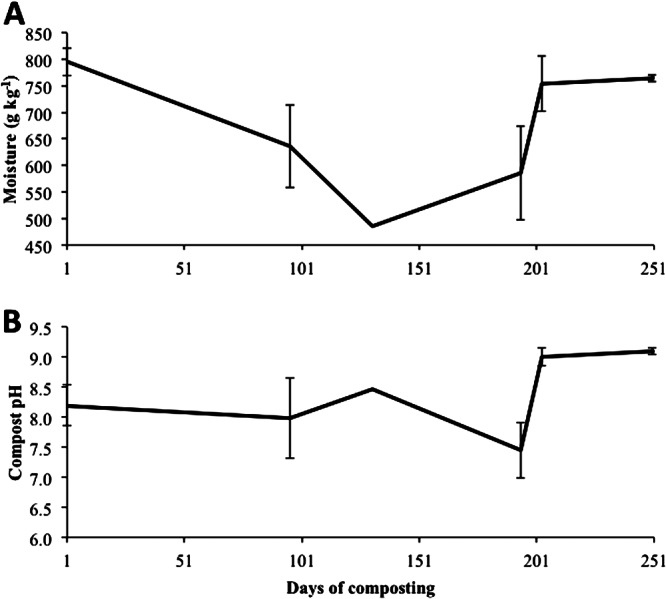
Moisture (A) and compost pH (B) measured over 250 days of composting.
Fig 5.
Total carbon (TC) and total nitrogen (TN) (A), organic matter (OM) (B), and the C/N ratio (C) over 250 days of composting.
Survival rates of M. avium subsp. paratuberculosis and M. smegmatis from both piles after 1, 35, 67, 96, and 250 days of composting are presented in Table 3. After day 67, M. smegmatis was not detected; however, M. avium subsp. paratuberculosis, irrespective of the source or sample bag, was isolated over the entire 250 days of composting.
Table 3.
Viability of M. avium subsp. paratuberculosis and M. smegmatis in samples from piles 1 and 2 after 250 days of composting
| Sample typeb | Survival in sample from pile 1/pile 2 at composting day showna |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
35 |
67 |
96 |
250 |
||||||
| M. aviumc | M. smegmatis | M. avium | M. smegmatis | M. avium | M. smegmatis | M. avium | M. smegmatis | M. avium | M. smegmatis | |
| Nylon bag | ||||||||||
| Control | −/− | −/− | −/NAd | −/NA | −/− | −/− | −/− | −/− | −/− | −/− |
| M. smegmatis | −/− | +/+ | −/NA | +/NA | −/− | −/+ | −/− | −/− | −/− | −/− |
| M. avium | +/+ | −/− | +/NA | −/NA | +/+ | −/− | +/+ | −/− | +/+ | −/− |
| LN | +/+ | −/− | +/NA | −/NA | +/+ | −/− | −/+ | −/− | +/+ | −/− |
| Plastic bag | ||||||||||
| Control | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| M. smegmatis | −/− | +/+ | −/− | +/− | −/− | −/+ | −/− | −/− | −/− | −/− |
| M. avium | +/+ | −/− | +/+ | −/− | +/+ | −/− | −/+ | −/− | +/+ | −/− |
| LN | +/+ | −/− | +/+ | −/− | +/+ | −/− | −/+ | −/− | +/+ | −/− |
Viability in all samples was detected by PCR with the primer sets for M. avium subsp. paratuberculosis and M. smegmatis shown in Table 1.
The control samples represent ground beef not inoculated with M. avium subsp. paratuberculosis. The M. smegmatis samples represent lab-cultured M. smegmatis inoculated into ground beef. The M. avium samples represent lab-cultured M. avium subsp. paratuberculosis inoculated into ground beef. LN, M. avium subsp. paratuberculosis-positive mesenteric lymph tissue.
M. avium = M. avium subsp. paratuberculosis throughout.
NA, no samples available for analysis from pile 1 at 35 days of composting.
Experiment 2: in vitro survival of M. avium subsp. paratuberculosis.
The in vitro incubation experiment revealed that M. avium subsp. paratuberculosis was recoverable from compost mixed with infected tissues held at 80°C for 90 days (Table 4). Although M. avium subsp. paratuberculosis was not consistently identified in compost samples collected on all days (i.e., it was not identified in the 1:4 mixture on days 8 and 60 at 60°C), we attribute this to the inability of PCR to amplify DNA due to sampling variability as M. avium subsp. paratuberculosis-spiked tissues were mixed with compost in a 1:4 ratio (Table 4).
Table 4.
Viability of M. avium subsp. paratuberculosis in vitro over 90 days of incubation
| Sample type and temp(s) (°C) | Viability on sample day: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 30 | 60 | 90 | |
| Compost (control)a | ||||||||
| 4, 45, 60, and 80 | − | − | − | − | − | − | − | − |
| Lymph tissueb | ||||||||
| 4, 45, and 60 | + | + | + | + | + | + | + | + |
| 80 | + | + | + | + | + | + | + | − |
| Compost mixed with lymph tissuec | ||||||||
| 4 | − | + | + | + | + | + | + | + |
| 45 | + | + | + | − | + | + | + | + |
| 60 | + | − | + | − | + | + | − | + |
| 80 | − | − | + | + | + | + | + | + |
M. avium subsp. paratuberculosis-negative compost was used as the control.
M. avium subsp. paratuberculosis-positive mesenteric lymph tissue.
M. avium subsp. paratuberculosis-positive mesenteric lymph tissue and compost in a 1:4 ratio, respectively.
DISCUSSION
A biosecure, static structure for composting livestock mortalities was constructed to assess inactivation of M. avium subsp. paratuberculosis and M. smegmatis during the decomposition of organic matter. Throughout the 250-day experimental period, temperature and various environmental conditions were monitored in an attempt to characterize those factors that may influence bacterial survival.
During composting, temperature changes associated with aerobic metabolic activity of the composting microorganisms can be described by two phases: the biooxidative phase and the maturation phase (4, 37). The biooxidative phase generally begins within the first few days of composting, when primarily mesophilic organisms (with optimum growth at temperatures between 20 and 45°C [8]) decompose OM, resulting in an increase in temperature to over 40°C (4, 37). As the temperature increases, thermophilic organisms proliferate (optimum growth at temperatures between 50 and 70°C [8]) and decompose OM and inactivate many pathogens (4, 37). Optimal composting during this phase should result in temperatures between 40 and 65°C (38). Temperatures between 52 and 60°C are optimal for OM decomposition and temperatures of >55°C inactivate a number of pathogens (4, 39). As the remaining available OM is degraded, the temperature declines due to reduced microbial activity (37) and the compost enters the maturation phase. During this phase, the compost temperature remains between 10 and 40°C, while the remaining OM, which includes recalcitrant cellulose and lignin, undergoes humification (37).
Although internal temperatures measured with the thermocouples averaged 30°C over the 250 days of composting in both piles (Fig. 2), temperatures measured with Hobo data loggers (Onset Computer Corporation) at the site of sample implantation were far more variable, ranging from 0°C to 60°C (Fig. 3A and B). Xu et al. (3) used a similar composting system and observed rapid heating of the compost, with temperatures reaching 55 to 65°C within 20 days and remaining in this range for 35 days in both structures. However, these piles were exposed to ambient temperatures that remained around 20°C over the 147-day composting period. As mentioned previously, the average ambient temperature was as low as −25°C (Fig. 2), with the windchill resulting in average ambient temperatures of −36°C throughout construction of the compost structures. An initial temperature spike was not observed in this study, and due to the low ambient temperature, the average internal temperatures (20°C) for both piles over the first 35 days (Fig. 2) were below the recommended range (Table 1). Construction of the composting system at subzero temperatures (−20°C) precluded the rapid increase in compost temperature and impeded microbial activity at a key point in the composting process. However, upon closer evaluation of the internal temperatures, each BRP was subject to a unique temperature profile (Fig. 3A and B) as a consequence of the heterogeneous nature that is characteristic of a carcass composting matrix. It is plausible that the composting pile temperatures observed were only in part a consequence of the low ambient temperature.
Biochemical compost parameters from this study were within the recommended ranges for effective composting (Table 1). Mortality compost is a heterogeneous mixture of animal carcasses with high moisture (leading to lowered porosity) and high N and low C mixed with materials of low to medium moisture (leading to higher porosity) and low N and high C (4). Such mixtures tend to become imbalanced in their C/N ratio as C is oxidized and N is concentrated. This leads to decreased microbial activity and a transition from the biooxidative to the mature composting phase (4, 40). However, in the present study, one location in pile 1 and three locations in pile 2 had temperatures of >50°C, but heating was not homogeneous throughout each pile. The ambient temperature during construction was likely the limiting factor for uniform heating throughout the compost piles.
Under the described conditions, M. avium subsp. paratuberculosis remained viable after 250 days of composting in large-scale composters designed for the disposal of cattle mortalities despite internal composting temperatures adequate for pathogen inactivation (3, 4, 37, 38) being reached in specific locations within each pile for several weeks (Fig. 3A and B). Several sites in both piles achieved temperatures between 50 and 65°C for at least 3 weeks, with M. avium subsp. paratuberculosis recoverable at all sites, regardless of the temperature. Furthermore, M. avium subsp. paratuberculosis was still recovered in an in vitro study after sustained temperatures of 80°C over 90 days, suggesting compost conditions are not sufficient to inactivate M. avium subsp. paratuberculosis from infected carcasses. As indicated in Table 3, M. avium subsp. paratuberculosis remained viable after 90 days at this temperature, suggesting that even effective composting conditions are unlikely to be a suitable method for disposal of cattle infected with M. avium subsp. paratuberculosis. First, it is unlikely that temperatures during carcass composting will be homogeneous throughout the pile, as demonstrated in this study, or that temperatures as high as 80°C will be achieved during the composting process (4). Second, if such temperatures were attained, they would have a deleterious effect on composting, by inactivating many beneficial organisms essential to the process, as temperatures greater than 80°C, are not within the optimal range of 40 to 65°C for composting (38). Temperatures greater than 72°C have been shown to markedly reduce microbial activity during composting (37).
It has been observed that M. avium subsp. paratuberculosis possesses several characteristics that may account for its environmental fitness under extreme conditions. The thick, lipid-rich cell wall is responsible for its extreme tolerance to heat (17, 20, 41, 42) and resistance to desiccation (9, 10) and UV radiation (43). The thermal tolerance of M. avium subsp. paratuberculosis demonstrated in this study has been observed elsewhere. Studies examining resistance concluded that although numbers of M. avium subsp. paratuberculosis were reduced by pasteurization (72°C), chlorination of water (2 ppm) (44), and exposure to 24 min of UV radiation in milk (43), viable cells could still be isolated after these treatments. Turbulent-flow pasteurization has also been shown to significantly reduce but not eradicate M. avium subsp. paratuberculosis in milk (20, 41, 45). In the present study, viability of M. avium subsp. paratuberculosis was not observed on days 8 and 60 at 60°C (Table 4). It was speculated that sampling variability rather than thermal intolerance was responsible as M. avium subsp. paratuberculosis was detected on day 90 at 60°C and 80°C. However, if previous studies did not completely eliminate M. avium subsp. paratuberculosis, it is possible that viable cells (cultured on HEYA but not quantified in this study) were still present, but at numbers too low to be detected by PCR.
Survival of M. avium subsp. paratuberculosis has been demonstrated for extended periods of time in various environments (1, 9, 10, 44). As early as 1944, Lovell et al. (9) demonstrated that feces infected with M. avium subsp. paratuberculosis placed in an open bowl exposed to atmospheric conditions survived for 246 days after exposure to freezing and desiccation at temperatures between −3°C and 23°C. Whittington et al. (10) recovered viable M. avium subsp. paratuberculosis cells after 385 days in feces applied to soil in a fully shaded environment. In addition, the survival of M. avium subsp. paratuberculosis was not influenced by soil pH (5.7 to 7.4) (10). Similarly, compost pH did not influence M. avium subsp. paratuberculosis survival in the present study (Fig. 4B). Whittington et al. (10) concluded that dormancy, defined as a non-spore-forming state permitting survival, was the most likely survival mechanism as M. avium subsp. paratuberculosis was recovered after 24 weeks when previous attempts to culture fecal and soil samples at 18 weeks were negative. However, those aforementioned studies did not examine viability in a composting environment. Grewal et al. (1) examined the persistence of M. avium subsp. paratuberculosis in 4-liter vessels designed to simulate liquid manure storage and a composting environment. Temperatures of 55°C were maintained in the liquid manure and composting environments throughout a 56-day period. Viable cells were isolated using standard culture techniques from the liquid storage treatment on all sample days (days 0, 3, 7, 14, 28, and 56) and from the compost on days 14, 28, and 56 but not on days 3 and 7. Further, M. avium subsp. paratuberculosis DNA was detectable in all samples collected from both the liquid storage and compost. In addition, M. avium subsp. paratuberculosis DNA was also detectable in samples taken on day 175 in the liquid storage. Grewal et al. (1) speculated that since M. avium subsp. paratuberculosis was not observed in culture on day 3 or 7, the cells were either dead or below detectable levels at that time. Conversely, the large-scale composting piles in the present study demonstrated that M. avium subsp. paratuberculosis was not inactivated after exposure to both microbial and biochemical compost conditions. The observed differences regarding the presence of M. avium subsp. paratuberculosis may be attributed to differences in the methodologies used in the two studies. In the present study, M. avium subsp. paratuberculosis DNA was extracted directly from the bacterial cultures and confirmed positive with PCR. Thus, all samples positive for M. avium subsp. paratuberculosis were considered viable. Grewal et al. (1), however, analyzed M. avium subsp. paratuberculosis via culture and PCR separately, and therefore M. avium subsp. paratuberculosis was only considered viable if observed in culture.
Other adaptive functions of M. avium subsp. paratuberculosis have been suggested to explain why it can survive for extended periods in the environment. In an in silico analysis by Whittington et al. (10), the M. avium subsp. paratuberculosis genome contained dormancy genes similar to those coding for resistance to nutritional and oxidative stress in other species of mycobacteria (i.e., M. smegmatis, M. bovis, and M. tuberculosis). However, the exact physiological mechanism triggering dormancy was unclear (10). More recently, sporulation has been suggested as a means for M. avium subsp. paratuberculosis environmental persistence (11). An M. avium subsp. paratuberculosis spore-like morphotype was produced that possesses the M. avium subsp. paratuberculosis genotype with the ability to infect bovine macrophages and remained viable after exposure to heat treatment at 70°C and postexposure to lysozyme and proteinase K (11). Although sporulation was induced by nutrient deprivation in that study, the authors of this study speculated that temperature-induced sporulation may be the mechanism by which M. avium subsp. paratuberculosis survived at >55°C in the compost piles and at 80°C in vitro (11). It is apparent that more research is needed to further evaluate the M. avium subsp. paratuberculosis spore-like morphotype in soil, water, and postpasteurization (11). Intracellular growth and survival in environmental protozoa (44, 46), biofilm formation, or aerosolization (44) have also been proposed as survival tactics. However, all studies concluded that more research is required to determine the exact cause of M. avium subsp. paratuberculosis resilience in the environment.
Mycobacterium smegmatis possesses desirable characteristics as a surrogate for M. avium subsp. paratuberculosis. It is a fast-growing species of mycobacteria (30) with no linkage to human disease and possesses dormancy genes similar to those in M. avium subsp. paratuberculosis (10). These characteristics have led to its use as a surrogate for Mycobacterium tuberculosis (10). However, our data suggest that M. smegmatis is not a suitable surrogate for M. avium subsp. paratuberculosis, as it could not be detected after 67 days of composting. Furthermore, M. avium subsp. paratuberculosis may have a resistance mechanism, such as sporulation, that is not active or present in M. smegmatis under composting conditions.
In conclusion, M. avium subsp. paratuberculosis could not be inactivated in a biosecure, static composting system for cattle mortalities. Neither temperatures of 60°C at several sites nor the biochemical conditions that prevailed within the compost rendered M. avium subsp. paratuberculosis nonviable. An in vitro incubation experiment demonstrated M. avium subsp. paratuberculosis survival after 90 days at 80°C in the presence of a compost matrix, making it unlikely that M. avium subsp. paratuberculosis could ever be completely inactivated through composting. Furthermore, it was concluded that M. smegmatis is not a suitable surrogate for M. avium subsp. paratuberculosis. Given the possible role of M. avium subsp. paratuberculosis as a causative agent in Crohn's disease and its resistance to inactivation in many different environments, an alternative means for disposal of JD-infected cattle is necessary if biocontainment is to play a role in arresting the spread of this disease. Additional research into traits such as dormancy or sporulation is also required to further understand the M. avium subsp. paratuberculosis mechanism of survival within the environment.
ACKNOWLEDGMENTS
We gratefully acknowledge Colleen Wilson, Terri Garner, and Sanjiv Bhandari for all of their help with construction and maintenance of the composting structures. Denis Krause, who passed away shortly before completion of the manuscript, was responsible for funding procurement, project design, and implementation. Tim McAllister accepted and fulfilled the role of cosupervisor on this project, in lieu of D. Krause.
The majority of the financial support was provided by the Manitoba Rural Adaptation Council (MRAC). Additional contributions were provided by the Crohn's and Colitis Foundation and the Livestock Stewardship Initiative (LSI), with in-kind support from the University of Manitoba, Agriculture and Agri-Food Canada (AAFC), the University of Saskatchewan, and Manitoba Agriculture Food and Rural Initiatives (MAFRI).
Footnotes
Published ahead of print 15 March 2013
REFERENCES
- 1. Grewal SK, Rajeev S, Sreevatsan S, Michel FC. 2006. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 72: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Senne DA, Panigrahy B, Morgan RL. 1994. Effect of composting poultry carcasses on survival of exotic avian viruses: highly pathogenic avian influenza (HPAI) virus and adenovirus of egg drop syndrome-76. Avian Dis. 38: 733–737 [PubMed] [Google Scholar]
- 3. Xu W, Reuter T, Inglis GD, Larney FJ, Alexander TW, Guan J, Stanford K, Xu Y, McAllister TA. 2009. A biosecure composting system for disposal of cattle carcasses and manure following infectious disease outbreak. J. Environ. Qual. 38: 437–450 [DOI] [PubMed] [Google Scholar]
- 4. Kalbasi A, Mukhtar S, Hawkins SE, Auvermann BW. 2005. Carcass composting for management of farm mortalities: a review. Compost Sci. Util. 13: 180–193 [Google Scholar]
- 5. Larney FJ, Yanke LJ, Miller JJ, McAllister TA. 2003. Fate of coliform bacteria in composted beef cattle feedlot manure. J. Environ. Qual. 32: 1508–1515 [DOI] [PubMed] [Google Scholar]
- 6. Whitlock RH, Buergelt C. 1996. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. North Am. Food Anim. Pract. 12: 345–356 [DOI] [PubMed] [Google Scholar]
- 7. McKenna SLB, Keefe GP, Barkema HW, Sockett DC. 2005. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 110: 105–111 [DOI] [PubMed] [Google Scholar]
- 8. Misra RV, Roy RN, Hiraoka H. 2003. On farm composting methods. Land and Water Discussion Paper. Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 9. Lovell R, Levi M, Francis J. 1944. Studies on the survival of Johne's bacilli. J. Comp. Pathol. 54: 120–129 [Google Scholar]
- 10. Whittington RJ, Marshall DJ, Nicholls PJ, Marsh IB, Reddacliff LA. 2004. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 70: 2989–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamont EA, Bannantine JP, Armién A, Ariyakumar DS, Sreevatsan S. 2012. Identification and characterization of a spore-like morphotype in chronically starved Mycobacterium avium subsp. paratuberculosis cultures. PLoS One 7: e30648 doi:10.1371/journal.pone.0030648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiwari A, VanLeeuwen JA, McKenna SL, Keefe GP, Barkema HW. 2006. Johne's disease in Canada. Part I. Clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. 47: 874–882 [PMC free article] [PubMed] [Google Scholar]
- 13. VanLeeuwen JA, Tiwari A, Plaizier JC, Whiting TL. 2006. Seroprevalences of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in beef and dairy cattle in Manitoba. Can. Vet. J. 47: 783–786 [PMC free article] [PubMed] [Google Scholar]
- 14. Hermon-Taylor J, Barnes N, Clarke C, Finlayson C. 1998. Mycobacterium paratuberculosis cervical lymphadenitis, followed five years later by terminal ileitis similar to Crohn's disease. BMJ 316: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waddell LA, Rajic A, Sargeant J, Harris J, Amezcua R, Downey L, Read S, McEwen SA. 2008. The zoonotic potential of Mycobacterium avium spp. paratuberculosis: a systematic review. Can. J. Public Health 99: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiodini RJ, Van Kruiningen HJ, Merkal RS, Thayer WR, Jr, Coutu JA. 1984. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J. Clin. Microbiol. 20: 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cerf O, Griffiths M, Aziza F. 2007. Assessment of the prevalence of Mycobacterium avium subsp. paratuberculosis in commercially pasteurized milk. Foodborne Pathog. Dis. 4: 433–447 [DOI] [PubMed] [Google Scholar]
- 18. Ellingson JL, Anderson JL, Koziczkowski JJ, Radcliff RP, Sloan SJ, Allen SE, Sullivan NM. 2005. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food Prot. 68: 966–972 [DOI] [PubMed] [Google Scholar]
- 19. Foddai A, Elliott CT, Grant IR. 2010. Rapid assessment of the viability of Mycobacterium avium subsp. paratuberculosis cells after heat treatment, using an optimized phage amplification assay. Appl. Environ. Microbiol. 76: 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rademaker JL, Vissers MM, Te Giffel MC. 2007. Effective heat inactivation of Mycobacterium avium subsp. paratuberculosis in raw milk contaminated with naturally infected feces. Appl. Environ. Microbiol. 73: 4185–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alonso-Hearn M, Molina E, Geijo M, Vazquez P, Sevilla I, Garrido JM, Juste RA. 2009. Isolation of Mycobacterium avium subsp. paratuberculosis from muscle tissue of naturally infected cattle. Foodborne Pathog. Dis. 6: 513–518 [DOI] [PubMed] [Google Scholar]
- 22. Brady C, O'Grady D, O'Meara F, Egan J, Bassett H. 2008. Relationships between clinical signs, pathological changes and tissue distribution of Mycobacterium avium subspecies paratuberculosis in 21 cows from herds affected by Johne's disease. Vet. Rec. 162: 147–152 [DOI] [PubMed] [Google Scholar]
- 23. Jaravata CV, Smith WL, Rensen GJ, Ruzante J, Cullor JS. 2007. Survey of ground beef for the detection of Mycobacterium avium paratuberculosis. Foodborne Pathog. Dis. 4: 103–106 [DOI] [PubMed] [Google Scholar]
- 24. Chi J, VanLeeuwen JA, Weersink A, Keefe GP. 2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 55: 137–153 [DOI] [PubMed] [Google Scholar]
- 25. Raizman EA, Fetrow JP, Wells SJ. 2009. Loss of income from cows shedding Mycobacterium avium subspecies paratuberculosis prior to calving compared with cows not shedding the organism on two Minnesota dairy farms. J. Dairy Sci. 92: 4929–4936 [DOI] [PubMed] [Google Scholar]
- 26. US Department of Agriculture 2010. Uniform program standards for the Voluntary Bovine Johne's Disease Control Program. USDA. APHIS 91-45-016. US Department of Agriculture, Washington, DC [Google Scholar]
- 27. Kennedy DJ, Allworth MB. 2000. Progress in national control and assurance programs for bovine Johne's disease in Australia. Vet. Microbiol. 77: 443–451 [DOI] [PubMed] [Google Scholar]
- 28. McKenna SL, Vanleeuwen JA, Barkema HW, Jansen JT, Hauer G, Hendrick SH, Cote G, Salsberg EB, Empringham RE. 2006. Proposed Canadian voluntary national Johne's disease prevention and control program. Can. Vet. J. 47: 539–541 [PMC free article] [PubMed] [Google Scholar]
- 29. Xu W, Xu Y, Reuter T, Gilroyed B, Jin L, Stanford K, Larney FJ, McAllister TA. 2010. An improved design for biocontained composting of cattle mortalities. Compost Sci. Util. 18: 32–41 [Google Scholar]
- 30. Chaturvedi V, Dwivedi N, Tripathi RP, Sinha S. 2007. Evaluation of Mycobacterium smegmatis as a possible surrogate screen for selecting molecules active against multi-drug resistant Mycobacterium tuberculosis. J. Gen. Appl. Microbiol. 53: 333–337 [DOI] [PubMed] [Google Scholar]
- 31. Hendrick SH, Duffield TE, Kelton DE, Leslie KE, Lissemore KD, Archambault M. 2005. Evaluation of enzyme-linked immunosorbent assays performed on milk and serum samples for detection of paratuberculosis in lactating dairy cows. J. Am. Vet. Med. Assoc. 226: 424–428 [DOI] [PubMed] [Google Scholar]
- 32. Reuter T, Xu W, Alexander TW, Baker BC, Larney FJ, Stanford K, McAllister TA. 2008. A simple method for temporal collection of tissue and microbial samples from static composting systems. Can. Biosyst. Eng. 50: 6.17–6.20 [Google Scholar]
- 33. Association of Official Analytical Chemists 1995. Official methods of analysis of the Association of Official Analytical Chemists. AOAC, Washington, DC [Google Scholar]
- 34. Larney FJ, Ellert BH, Olson AF. 2005. Carbon, ash and organic matter relationships for feedlot manures and composts. Can. J. Soil Sci. 85: 261–264 [Google Scholar]
- 35. Thompson W. 2002. Test methods for the examination of composting and compost. US Composting Council, Bethesda, MD: http://compostingcouncil.org/tmecc/ Accessed 20 July 2011 [Google Scholar]
- 36. Vansnick E, de Rijk P, Vercammen F, Geysen D, Rigouts L, Portaels F. 2004. Newly developed primers for the detection of Mycobacterium avium subspecies paratuberculosis. Vet. Microbiol. 100: 197–204 [DOI] [PubMed] [Google Scholar]
- 37. Bernal MP, Alburquerque JA, Moral R. 2009. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 100: 5444–5453 [DOI] [PubMed] [Google Scholar]
- 38. Rynk R. (ed). 1992. On-farm composting handbook. Northeast Regional Agricultural Engineering Service, Ithaca, NY [Google Scholar]
- 39. Miller FC. 1993. Composting as a process based on the control of ecologically selective factors, p 515–544 In Metting FB., Jr (ed), Soil microbial ecology: applications in agricultural and environmental management. Marcel Dekker, Inc, New York, NY [Google Scholar]
- 40. Bishop PL, Godfrey C. 1983. Nitrogen transformations during sludge composting. Biocycle 24: 34–39 [Google Scholar]
- 41. Whittington RJ, Waldron A, Warne D. 2010. Thermal inactivation profiles of Mycobacterium avium subsp. paratuberculosis in lamb skeletal muscle homogenate fluid. Int. J. Food Microbiol. 137: 32–39 [DOI] [PubMed] [Google Scholar]
- 42. Gao A, Mutharia L, Chen S, Rahn K, Odumeru J. 2002. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. J. Dairy Sci. 85: 3198–3205 [DOI] [PubMed] [Google Scholar]
- 43. Donaghy J, Keyser M, Johnston J, Cilliers FP, Gouws PA, Rowe MT. 2009. Inactivation of Mycobacterium avium ssp paratuberculosis in milk by UV treatment. Lett. Appl. Microbiol. 49: 217–221 [DOI] [PubMed] [Google Scholar]
- 44. Rowe MT, Grant IR. 2006. Mycobacterium avium ssp. paratuberculosis and its potential survival tactics. Lett. Appl. Microbiol. 42: 305–311 [DOI] [PubMed] [Google Scholar]
- 45. Hammer P, Kiesner C, Walte HG, Knappstein K, Teufel P. 2004. Heat resistance of Mycobacterium avium ssp paratuberculosis in raw milk tested in a pilot-plant pasteurizer. Kiel. Milchwirtsch. Forschungsber. 54: 275–303 [Google Scholar]
- 46. Mura M, Bull TJ, Evans H, Sidi-Boumedine K, McMinn L, Rhodes G, Pickup R, Hermon-Taylor J. 2006. Replication and long-term persistence of bovine and human strains of Mycobacterium avium subsp paratuberculosis within Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72: 854–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keener HM, Elwell DL, Monnin MJ. 2000. Procedures and equations for sizing of structures and windrows for composting animal mortalities. Appl. Eng. Agric. 16: 681–692 [Google Scholar]
- 48. Langston J, Carman D, VanDevender K, Boles JC., Jr 2002. Disposal of swine carcasses in Arkansas. Cooperative Extension Service, University of Arkansas, Little Rock, AR [Google Scholar]