Abstract
Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137+PD-1+CTLA4 7-15 days following tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remain healthy >150 days after later treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity and required CD4+ cells and involved CD8+ cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19+ cells at tumor sites, increased IFNγ and TNFα producing CD4+ and CD8+ T cells and mature CD86+ DC, and it increased the ratios of effector CD4+ and CD8+ T cells to CD4+Foxp3+ regulatory T cells and to CD11b+Gr-1+ myeloid suppressor cells. This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137+PD-1+CLA4+CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
Keywords: ovarian carcinoma, melanoma, CTLA4, PD-1, CD137, CD19, inflammation
Immunological mechanisms play a key role in many and perhaps all cancers1-3, and there is increasing support of Virchow’s postulate that inflammation promotes carcinogenesis and tumor progression4-8. We recently investigated the local immune response in HPV infected women with various degree of cervical neoplasia, a system that lends itself particularly well to study carcinogenesis and tumor progression because the causative agent is known and the transition from infected cervical epithelial cells to invasive cancer is well characterized histopathologically. There was both local and systemic Th2 inflammation during progression from HPV infected cervical cells to invasive carcinoma, including B cells, plasma cells and Th2 cytokines, and an increased frequency of Foxp3+ Treg cells and cells secreting IDO1 reflected local immunosuppression7.
We hypothesized that approaches shifting a Th2 type inflammation to a Th1 response at the tumor site will overcome immunosuppression and promote tumor destruction. To test this hypothesis, we injected tumor-bearing mice with a combination of mAbs to CD137 (a.k.a. 4-1BB), PD-1 (a.k.a. CD274) and CTLA4. The mAbs were selected because each of them has therapeutic activity in some mouse tumor models and also in some human patients. Anti-CD137 mAb9 activates Th1 type CD8 and CD4 lymphocytes10,11 and NK cells12 and inhibits T cell dependent antibody production13. Anti-PD-1 mAb14,15 counteracts immunosuppression induced by the B7-H1/PD-1 pathway16,17 and synergizes with anti-CD137 mAb18. Anti-CTLA4 mAb19,20 counteracts immunosuppression by Treg cells and synergizes with anti-PD-121 and anti-CD137 mAbs22. Two syngeneic tumor models were chosen, the MOSEC ovarian carcinoma clone ID8 which grows intraperitoneally (i.p.) and shares many characteristics with human epithelial ovarian cancer, and the K1735 melanoma clone SW123 which grows subcutaneously (s.c.) and facilitates studies on intratumoral (i.t.) versus systemic (i.p.) delivery of the mAbs. SW1 is a demanding model which responds poorly to several forms of immunotherapy24,25. In the 1D8 model, administration of the mAbs commenced 10 or 15 days after tumor transplantation, and in the SW1 model mice were treated when their s.c. melanoma had 4-6 mm mean diameter which was around 7 days after the SW1 cells had been transplanted.
Dramatically improved survivals and likely cures were observed in both tumor models and were associated with a shift of the tumor microenvironment from a Th2 to a Th1 type immune profile with significantly decreased number of tumor-associated CD19+ B cells. In view of the latter finding and because B cells are involved in Th2 type tumor inflammation5,7,8,26,27, we did exploratory experiments in the SW1 model which showed that addition of an anti-CD19 mAb to the 3 mAb combination further improved the therapeutic efficacy. To indicate that our observations have general validity, we include, as Supplemental Information, data from ongoing experiments with the B16 melanoma and TC1 carcinoma which demonstrate dramatically prolonged survivals also in those two models.
MATERIALS AND METHODS
Tumor Lines and Mice
ID8 is a clone of the MOSEC ovarian carcinoma of C57BL/6 origin28, SW1 is a clone derived from the K1735 melanoma of C3H origin23,24, B16 is clone derived from a mouse spontaneous melanoma in C57BL/6 mouse29 and TC130 is a clone derived from primary epithelial cells of C57BL/6 mice co-transformed with HPV-16 E6 and E7. Tumor cells were cultured in IMEM supplemented with 10% FBS (Atlanta Biological, Norcross, GA), penicillin and streptomycin before cell suspensions were prepared and transplanted to mice.
Six to eight-week female C57BL and C3H mice were purchased (Charles River Laboratories, Wilmington, MA). The animal facilities are certified by the Association for Assessment and Accreditation of Laboratory Animal Care, and our protocols are approved by the institution (University of Washington).
Monoclonal antibodies for immunotherapy
The following mAbs were purchased from BioXcell: anti-CD137 (LOB12.3; Cat. #BE0169), anti-PD-1 (RMP1-14; Cat. #BE0146), anti-CTLA4 (9D9; Cat. #BE0164), anti-CD19 (1D3; Cat. #BE0150), anti-NK1.1 (PK-136; Cat. #BE0036), anti-CD8 (YTS 169.4; Cat. #BE0117), anti-CD4 (GK1.5; Cat. #BE0003-1) and control (2A3; Cat. #BE0089).
Animal studies
In experiments with the ID8 ovarian carcinoma, mice (5 or 10/group) were transplanted i.p. with 3×106 cells. Either 10 or 15 days later, they were injected i.p. with mAbs (as shown in the figures), which was repeated weekly for a total of 3 times (shown by arrows in the Figs), 0.5 mg of each mAb/mouse was given on each occasion. Controls received 0.5 mg of the 2A3 mAb referred to above. The mice were monitored daily for tumor growth, including swollen bellies indicating that they had developed ascites, and for evidence of toxicity. The survival of each mouse was recorded and overall survival was calculated as mean±standard error of mean (M±SEM).
In experiments with the SW1 melanoma, 5×105 cells were transplanted s.c. on the right flank, When the mice had tumors of about 4-5 mm mean diameter, they were randomized into treatment groups and injected with mAb combinations, 0.5 mg of each mAb; the mAbs were injected i.t. or i.p. at weekly intervals for a total of 3 times which was followed by 3 biweekly intervals (shown by arrows in the Figs). Mice were monitored daily for evidence of toxicity, and two perpendicular tumor diameters were measured twice/week and tumor surfaces were calculated. Overall survival was recorded.
We also performed an experiment in which mice were transplanted with 5×105 SW1 cells s.c. on their right and 1.5×105 cells on their left flank. When the right tumors were approx. 5-6 mm and those on the left side approx. 2-3 mm mean diameter, we commenced treatment by i.t. injecting a combination of mAbs to the right tumors while leaving the left tumors untreated; this treatment was repeated as for the other experiments with the SW1 tumor (shown by arrows in the Figs).
For in vivo depletion of CD4+, CD8+ T lymphocytes, or NK cells in the ID8 and SW1 tumor models, an anti-CD4 (0.5mg/mouse), anti-CD8 (0.5 mg/mouse) or anti-NK1.1 (0.1mg/mouse) mAb was injected i.p. concomitantly with the first 3 mAb-treatment and repeated 3 and 7 days later.
For in vitro mechanistic studies, mice which had been transplanted i.p. with ID8 cells or s.c. with SW1 cells (as for the therapy experiments) were euthanized 7 days after they had been injected with the 3 mAb combination (or control) as in the therapy experiments. Tissues were dissected and prepared for flow cytometry. In experiments with the ID8 tumor we also assayed for an antigen-specific immune response and in experiments with the SW1 melanoma we also performed studies with PCR arrays and quantitative PCR.
Preliminary studies were performed to investigate whether our approach would be effective also in two additional models. Mice were transplanted s.c. on the right side of the back with either 5×105 TC1 or 1×105 B16 cells. Treatment in the form of weekly i.t. injection of a combination of mAbs to CD137+PD-1+CTLA4+CD19, (0.5 mg of each mAb weekly for a total of 6 weeks) commenced when the tumors had a mean diameter of 4-6 mm which was 7 days after TC1 transplantation and 14 days after B16 transplantation. Tumor growth and overall survival was recorded. Both experiments were repeated once.
Flow cytometry
Single cell suspensions from spleens and lymph nodes were prepared as described before25,31. To obtain peritoneal lymphocytes in the ID8 model, 3 ml PBS was injected into the peritoneal cavity of mice with ID8 tumors immediately after euthanasia, their belly was massaged and the fluid was removed, filtered through a 70 μM cell strainer (BD Biosciences, San Jose, CA), washed and cells were isolated by using a mouse lymphocyte isolation buffer. TIL were isolated from pooled SW1 tumors (2-3 mice) as described24 and statistical significance was calculated. All flow cytometry experiments were performed at least 3 times.
Single cell suspensions were washed with FACS staining buffer and incubated with mouse Fc receptor binding inhibitor for 10 minutes before staining with antibodies of CD45 (clone 30-F11), CD3 (clone 145-2C11), CD4 (clone GK1.5), CD8 (clone 53-6.7), CD19 (clone eBio1D3), CD86 (clone GL1), CD11b (clone M1/70), Gr-1 (clone RB6-8C5) and CD11c (clone N418; all from eBioscience, San Diego, CA) for 30 minutes. For intracellular staining of Foxp3 (clone FJK-16s; eBioscience), IFNγ (clone XMG1.2 eBioscience) and TNFα (clone MP6-XT22; eBioscience), cells were fixed, permeabilized and stained following the instruction of Cytofix/Cytoperm kit (BD Bioscience, San Jose, CA). Flow cytometry was performed using FACSCalibur (BD Biosciences) and the lymphocyte population was selected by gating CD45+ cells. The data were analyzed using Flow Jo software (Tree Star, Ashland, OR).
Antigen-specific immune response assay
Isolated splenocytes from mice with ID8 tumor were cultured in the presence of H-2Db-restricted mesothelin-derived peptides (mesothelin amino acid 406-414; Anaspec, Fremont, CA32) or control HPV-E7-derived peptide (HPV-E7 49-57, Anaspec, Fremont, CA) for 3 days. Subsequently, IFNγ in the supernatants was detected by Mouse IFNγ Quantikine ELISA Kit (R&D systems, Minneapolis, MN). We determined the frequency of CD3+ cells in the experimental and control samples which did not differ significantly (43.7±3.5% vs 47.9±2.5%). The results were analyzed after normalization according to the T cell numbers from the two groups.
PCR arrays
Total RNA was extracted from pooled TLN or tumor tissues from 2-3 mice of each group with SW1 tumors using Qiagen RNeasy Mini Kit, followed by cDNA synthesis using RT2 First Strand kit; 320 ng RNA was used as starting material for each treatment condition. Two RT2 Profiler PCR Array Systems (PAMM-011Z and PAMM-034Z) from SABiosciences (Frederick, MD, USA) were used to analyze expression of Th1 and Th2 type immune response genes. The template combined with the RT2 SYBR green/ROX qPCR mix (10 μl/well) was loaded into a 384-well array plate coated with predispensed gene-specific primer sets and processed on a TaqMan ABI Prism 7900 Sequence Detector System (Applied Biosystems, Foster City, CA, USA) according to the RT2 Profiler PCR Array manufacturer’s protocol. The data analysis was based on the ΔΔCt method with normalization to the housekeeping gene on the array, according to the RT2 Profiler PCR Array manufacturer’s protocol.
Quantitative PCR
Quantitative PCR assays were performed on 4 mice per treatment group to assess the transcription levels of gene encoding the inflammation related cytokines CXCL9, CXCL10 and CXCL11, which were overexpressed based on the PCR array data. Total RNA was extracted from tumors and TLNs and reverse transcribed to cDNA using the First Strand cDNA synthesis kit (Fischer Scientific, IL). Subsequently, cDNA was measured by qPCR assays on ABI Prism 7900 Sequence Detector System (Applied Biosystems, Foster City, CA, USA). The relative quantification was performed using the ΔΔCt method described by the manufacturer.
Statistics
Results were expressed as mean ± SEM. Student’s t test was used to compare the statistical difference between two groups and one-way ANOVA was used to compare three or more groups. Kaplan-Meier survival analyses were performed using GraphPad Prism 5, and the Gehan-Breslow-Wilcoxon test was used to determine significance. p < 0.05 was considered to be statistically significant.
RESULTS
Combining mAbs to CD137+PD-1+CTLA4 significantly prolonged survival of mice with established ID8 or SW1 tumors
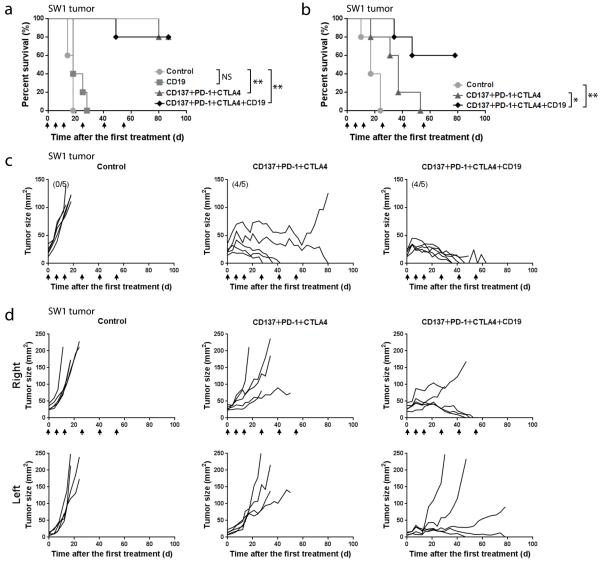
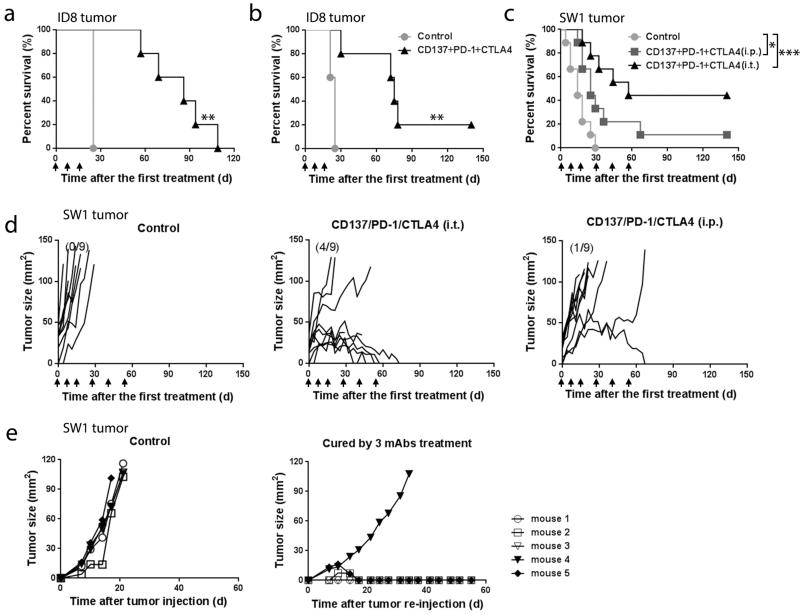
In the ID8 ovarian carcinoma model, mice were injected i.p. with mAbs to CD137+PD-1+CTLA4 either 10 (Fig. 1a) or 15 days (Fig. 1b) after tumor inoculation; unless stated otherwise, mice receiving a combination of these 3 mAbs are referred to below as ‘treated’. Administration of the 3 mAb combination prolonged survival (mean ± SEM) from 24.2 ± 1.7 to 74.4 ± 24.4 days (n=15, p < 0.01) and one of the treated mice was healthy and, according to autopsy, tumor-free when euthanized 140 days after the first treatment. None of the 3 mAbs prolonged survival when used alone and neither did combinations of mAbs to CD137+CTLA4 or to PD-1+CTLA4 (n=5, p>0.05; Supplementary Fig. 1a), while mAbs to CD137+PD-1 increased survival (mean ± SEM) to 54.5 ± 15.2 days (n=5, p<0.01; Supplementary Fig. 1a). Similar results were obtained in a repeat experiment.
FIGURE 1.
Survival of mice with ID8 or SW1 tumors. Mice transplanted i.p. with 3×106 ID8 cells 10 (10 mice/group, Panel a) or 15 (5 mice/group, Panel b) days previously were injected i.p. with the indicated mAbs; mAb injections were repeated as shown by arrows. Panel c depicts the survival of mice with SW1 tumors (9 mice/group); arrows show when the mAbs were injected. Panel d shows tumor growth curves for individual mice from the SW1 experiment in Panel c; numbers in parentheses indicate the fraction of tumor-free mice at 140 days post first therapy. Panel e depicts an experiment in which 5 mice whose SW1 tumors had completely regressed were transplanted s.c. with 5×106 SW1 cells and followed for tumor growth, as were 5 age matched naïve mice. * p<0.05, **p<0.01, *** p<0.001.
To investigate the role of CD4+, CD8+ and NK cells in the anti-tumor activity, treated mice were injected with mAbs to the respective cells as stated in Methods. Depletion of CD4+ (n=5, p<0.01) or CD8+ cells (n=5, p<0.05) abrogated the anti-tumor effect, while depletion of NK cells only had a marginal effect (n=5, p>0.05). The data are presented in Supplementary Fig. 1b.
In the SW1 melanoma model, injection of the 3 mAb combination i.t. induced complete tumor regression (CR) in 4 of 9 mice (Fig. 1d, right panel), and i.p. injection induced CR in 1 of 9 mice (Fig. 1d, middle panel). Two of the 9 mice in the i.t. group with regressing tumors died while still being treated and one of the mice with CR in that group temporarily lost its hair, most likely due to autoimmunity.
Experiments similar to those in the ID8 model were performed to investigate the role of CD4+, CD8+ and NK cells in the anti-tumor activity by injecting treated mice with mAbs to the respective cells as stated in Methods (Supplementary Fig. 1 c). In this model, CD4+ cells (n=5, p<0.01) were needed for therapeutic efficacy. Removal of CD8+ cells or NK cells also decreased the therapeutic response, although the differences were not statistically significant (n=5, p>0.05).
The 5 mice with CR were transplanted s.c. with 5 × 105 SW1 cells 140 days after the first treatment (which was 80 days after the last one), as were 5 age matched naïve controls. Tumors were rejected by 4 of the mice whose original SW1 tumors had regressed. These mice remain tumor free 190 days after initial treatment and are most likely cured; tumor growth was significantly delayed in the fifth mouse (Fig. 1e). The 5 controls died with progressing tumors. Except for the two mice in one experiment with the SW1 melanoma which died with regressing tumors and one mouse which lost its hair in that experiment (as described above), there was no weight loss or other evidence of toxicity in any other treated mice with SW1 or ID8 tumors.
Combination of mAbs to CD137+PD-1+CTLA4 shifted the local immune response from a Th2 to a Th1 phenotype
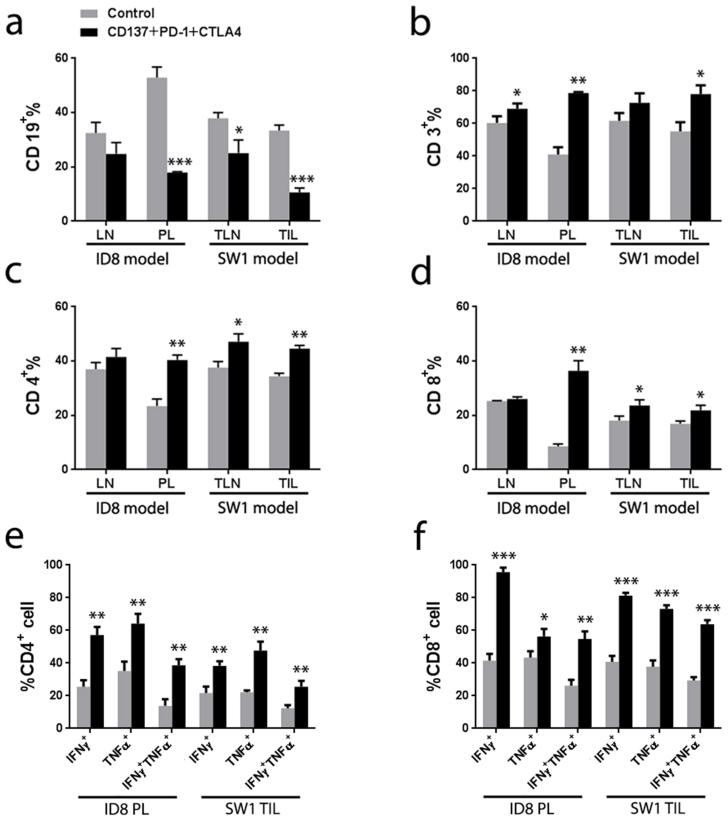
We applied flow cytometry to analyze the composition of lymphoid cells collected from the peritoneal lavage of ID8-bearing mice, TIL from SW1 bearing mice and TLN in both models, studying treated and control mice in parallel. The frequency of CD19+ cells decreased dramatically in the peritoneal lavage from treated mice with ID8 i.p. (n=5, p < 0.001 vs control, Fig. 2a & Supplementary Fig. 2a) as well as among tumor infiltrating lymphocytes (TIL) in the SW1 model (n=5, p < 0.001 vs control, Fig. 2a & Supplementary Fig. 2b) and cells from tumor-draining lymph nodes (n=5, p < 0.01, Fig. 2a & Supplementary Fig. 2b). There was an increased frequency of tumor-associated CD3+ (n=5, p < 0.05; Fig. 2b), CD4+ (n=5, p < 0.05; Fig. 2c) and CD8+ cells (n=5, p < 0.01; Fig. 2d) in treated mice from both models. The frequency of IFNγ+ TNFα+ CD4+ lymphocytes in the peritoneal lavage of treated mice with ID8 tumors tripled (n=5, p<0.01; Fig.2e) and IFNγ+ TNFα+ CD8+ cells doubled (n=5, p<0.01; Fig.2f) as compared to controls. Similarly, the frequency of IFNγ+TNFα+ CD4+ and IFNγ+ TNFα+ CD8+ cells among TIL doubled in treated SW1 tumors (n=5, p<0.01; Fig.2e & 2f).
FIGURE 2.
Flow cytometry analysis of cells in peritoneal lavages (PL) and peritoneal lymph nodes (LN) from treated and control mice with ID8 tumors (2-3 mice/group) and of cells from tumor-draining lymph nodes (TLN) and tumor infiltrating lymphocytes (TIL) from mice with SW1 tumors (2-3 mice/group). Administration of the 3 mAb combination decreased the frequency of CD19+ cells (Panel a) and increased CD3+ (Panel b), CD4+ (Panel c) and CD8+ (Panel d) cells in both tumor models. Intracellular cytokine staining shows that TNFα and IFNγ expression increased significantly in CD4+ (Panel e) and CD8+ (Panel f) cells. Representative dot plots from 3 similar experiments are shown in Supplemental Figure S2. n=5, * p<0.05, ** p<0.01, *** p <0.001.
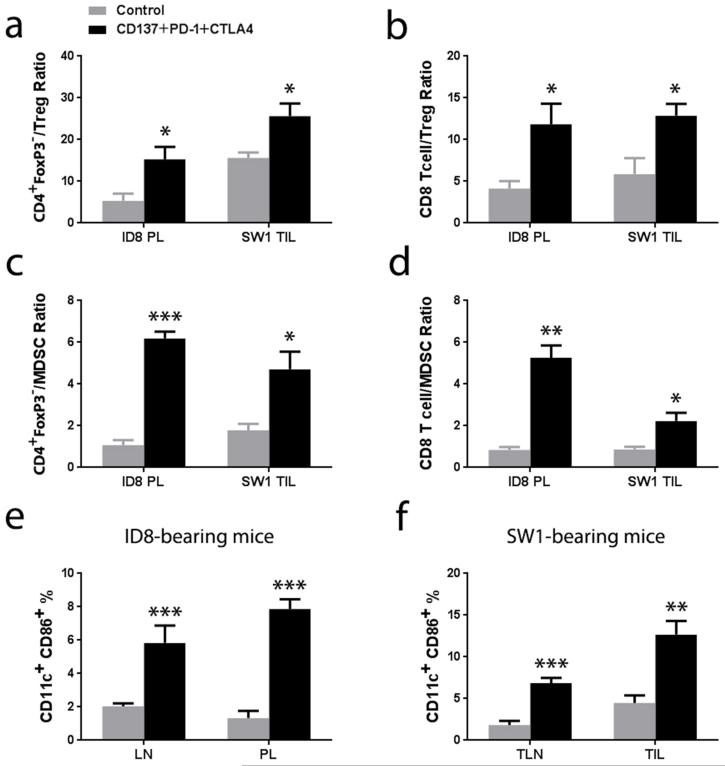
The ratios between CD8+ and CD4+Foxp3− cells to CD4+Foxp3+ Treg cells were increased in treated vs control mice which had ID8 (n=3, p<0.05; Fig. 3a & 3b) or SW1 tumors (n=3, p<0.05; Fig. 3a & 3b) as did the ratios between Foxp3− CD4 and CD8 cells to CD11b+Gr-1+ myeloid derived suppressor cells (MDSC) (n=3, p < 0.01 in ID8 tumors, Fig.3c & 3d; n=3, p < 0.05 in SW1 tumors, Fig. 3c & 3d). The frequency of dendritic cells (DC) expressing the maturation marker CD86 was analyzed by gating the CD11c+ population from the peritoneal lavage of mice with ID8 tumors, among TIL from mice with SW1 tumors and among cells in tumor draining lymph nodes from mice with either ID8 or SW1 tumors; samples from treated and control mice were analyzed in parallel. The 3 mAb combination upregulated the expression of CD86 in the CD11c+ cells (Supplementary Fig. 3) increasing the frequency of mature DC up to 5 times (n=5, p < 0.01; Fig. 3e & 3f).
FIGURE 3.
Panel a shows the ratio of CD4+FoxP3−/Treg for cells from the peritoneal lavage (PL) of mice with ID8 tumors and tumor infiltrating lymphocytes (TIL) from mice with SW1 tumors as in the experiment in Fig. 2. Panel b shows the ratio CD8+ T cell/Treg for the cells from PL and TIL. Panel c and panel d shows CD4+FoxP3−/MDSC and CD8+ T cell/MDSC for the cells from PL and TIL separately. Panel e shows ratios of the frequency of CD11c+CD86+ cells among lymphoid cells from the peritoneal lymph nodes (LN) and PL from mice with ID8 tumors. Panel d shows the frequency of CD11c+CD86+ cells in tumor-draining lymph nodes (TLN) and among TIL from mice with SW1 tumor. The Tregs are defined as CD4+ cells expressing FoxP3+ and the CD11b+Gr-1+ cells are defined as MDSCs. n=3, * p<0.05, **p<0.01, *** p<0.001.
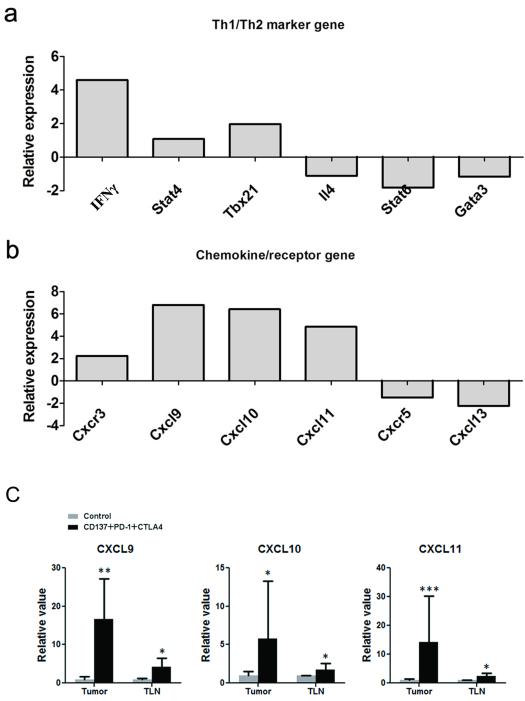
The 3 mAb combination shifted the gene expression signature from a Th2 into a Th1 type
To further investigate the immune response in treated mice, we performed PCR array analysis on tumor-draining lymph nodes from mice with SW1 tumors. As shown in the Fig.4 panel A, the 3 mAb combination upregulated transcription of the Th1-related molecules IFNγ, Stat4 and TBX21 and downreguled transcription of the Th2 molecules IL-4, Stat6 and GATA3, which is consistent with the increased percentage of IFNγ producing T cells according to intracellular cytokine staining (Fig.2e & 2f). There was also an increase of the Th1-associated chemokine/receptor CXCL9-11/CXCR3 and a decrease of the B cell chemotactic chemokine/receptor CXCL13/CXCR5 (Fig. 4, panel B), which is concordant with the increase of Th1 type CD4+ and CD8+ cells and decrease of B cells in tumor-draining lymph nodes (Fig. 2). Upregulation of CXCL9 (n=4, p < 0.01 in tumor, p < 0.05 in TLN), CXCL10 (n=4, p < 0.05 in tumor, p < 0.05 in TLN) and CXCL11(n=4, p < 0.001 in tumor, p < 0.05 in TLN) was confirmed by quantitative real-time PCR (Fig 4, panel C) in both tumors and tumor draining lymph nodes from treated mice in a separate experiment, supporting a shift of the immune response toward Th1 type by the 3 mAb treatment.
FIGURE 4.
Gene expression in tumor-draining lymph nodes (TLN) from treated and control mice with SW1 tumors. Panel a shows PCR array data demonstrating upregulated transcription of the Th1-related molecules IFNγ, Stat4 and TBX21 and downregulated transcription of the Th2 molecules IL-4, Stat6 and GATA3 in treated mice. Panel b shows PCR array data demonstrating elevation of the Th1-associated chemokine/receptor CXCL9-11/CXCR3 and decrease of the B cell-chemotatic chemokine/receptor CXCL13/CXCR5 in TLN from treated mice. Panel C shows results from quantitative PCR demonstrating that mRNA for CXCL9, CXCL10, CXCL11 was significantly induced in tumor-draining lymph nodes and tumors following i.t. injection of SW1 tumors with a combination of mAbs to CD137+PD-1+CTLA4. n = 4, * p < 0.05, ** p < 0.01, *** p < 0.001.
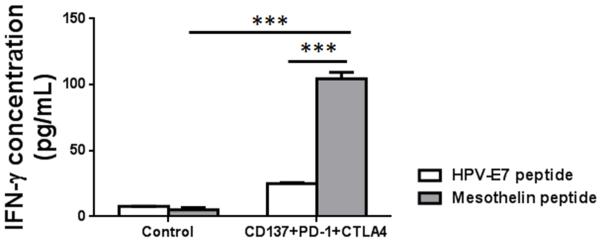
The 3 mAb combination induced an antigen specific immune response
Since ID8 cells express a mouse homologue of human mesothelin32, we harvested splenocytes from treated mice and controls, and the same number of splenocytes was cultured in the presence of 10 μg/mL of H-2Db-restricted mesothelin specific peptide (amino acid 406-414) or control HPV-E7 peptide (amino acids 49-57) for 3 days and assayed the culture supernatants for IFNγ by ELISA. Since the frequency of CD3+ cells in spleens from control (43.7 ± 3.5%) and 3 mAb treated mice (47.9 ± 2.5%) were slightly different, the IFNγ level was normalized according to the of CD3+ cells between 3 mAb treated and control mice. Splenocytes from treated mice, as compared to controls, secreted increased levels of IFNγ when stimulated with the mesothelin peptide as compared to stimulation by the HPV peptide (n=3, p<0.001; Fig. 5).
FIGURE 5.
Antigen-specific immune response of treated and control ID8-bearing mice to mesothelin. Mice with ID8 tumors were injected with mAbs to CD137+PD-1+CTLA4 as in the therapy experiments. Spleen cells were harvested 7 days later and cultured in the presence of H-2Db-restricted mesothelin- or HPV-E7-derived peptide as described in Methods, after which IFNγ was measured in supernatant. The results were represented after normalization according to the T cell numbers from the two groups. n=3, ***p<0.001.
Depletion of CD19+ cells increased the anti-tumor efficacy of the 3 mAb combination
Since the frequency of CD19+ cells among tumor associated lymphocytes decreased in treated mice and in view of the association of B cells with Th2 type inflammation, we explored in the SW1 model whether addition of an anti-CD19 mAb to the 3 mAbs combination improved the therapeutic efficacy. Injection of the 4 mAb combination i.t. depleted 95% of B cells in tumors, lymph nodes and spleens as observed 7 days post-treatment (Supplementary Fig.4). The 3 or 4 mAb combinations both induced complete tumor regression in 4 of 5 mice which remain tumor free, 90 days after onset of therapy (Fig 6a, n = 5, p <0.01 VS control) and were probably cured; however, addition of an anti-CD19 mAb induced regression more quickly (Fig 6c). Injection of the anti-CD19 mAb by itself prolonged survival (mean ± SEM) from 16.4 ± 2.2 to 21.4 ± 4.8 days (n=5, NS; Fig 6a).
FIGURE 6.
Therapeutic effects of the indicated mAb combinations versus control. Panel a shows survival curves for an experiment (5 mice/group) where the mAb combinations were given i.t. when the mice had SW1 tumors of 4-5 mm mean diameter. Panel b shows survival curves for an experiment (5 mice/group) with mice transplanted s.c. on both sides of the back with SW1 cells as stated in Methods. The mAb combinations were injected into the larger (right) while the smaller (left) tumor was not treated. Panel c shows tumor growth curves for individual mice whose survival is depicted in Panel a and Panel d shows tumor growth curves for individual mice whose survival is shown in Panel b; the mAbs were injected at times shown by arrows. n=5, * p<0.05, **p<0.01.
As described in Methods, mice which had a 5-6 mm SW1 tumor on the right side of the back and a 2-3 mm SW1 tumor on the left side (i.e. a larger tumor load than in the preceding experiments) were injected i.t. into the right tumor while the left tumor remained untreated. I.t. injection of the 3 mAb combination impeded the growth of both the treated and untreated tumors (Fig. 6d), indicating that it induced a systemic immune response. As shown by the tumor growth curves (Fig. 6d), addition of an anti-CD19 mAb to the 3 mAb combination more effectively inhibited growth of both treated and untreated tumors, prolonging survival (mean ± SEM) to 59 ± 17.5 days as compared to the 3 mAb combination (35 ± 12.9 days, n=5, p < 0.05) and control (18.4 ± 5.6 days, n = 5, P<0.01) (Fig. 6b); 3 of 5 mice receiving the 4 mAb combination are alive with small regressing tumors 80 days after the first treatment. There was a difference in survival between the 3 and 4 mAb combinations at the p<0.05 level.
Preliminary experiments in the TC1 carcinoma and B16 melanoma models
Two experiments were performed in each of the TC1 carcinoma and B16/F10 melanoma models, injecting s.c. tumors of 4-6 mm diameter with either the 3 mAb or 4 mAb combination similarly to what was done with the SW1 melanoma. In the TC1 model (Supplementary Fig.5a), the 3 mAb treatment prolonged survival to 49.4 ± 22.9 days as compared to controls (13.2 ± 3.3 days, n = 5, p < 0.01) and the 4 mAb combination (i.e. the one also comprising a mAb to CD19) further prolonged survival to 61 ± 18.5 days; 2 mice remain healthy and tumor free in the 3 mAb group as compared to 3 mice in the 4 mAb group for an observation period > 60 days. As shown in Supplementary Fig.5 b, B16-bearing mice treated with 4 mAb combination had a survival of 26.2 ± 26.1 days as compared to 6 ± 2.7 days in the control (n = 5, p < 0.05) and 2 of 5 mice remain healthy and tumor-free >60 days after onset of therapy, while the 3 mAb combination did not significantly prolong survival in the B16 model. Both experiments have been repeated, confirming strong therapeutic efficacy of the 4 mAb combination for both established TC1 and B16 tumors.
DISCUSSION
There is an emerging interest in the tumor microenvironment33. It displays features of a Th2 type inflammation with many B lymphocytes5,7,26 and plasma cells7,34, and there is a strong local immunosuppression30,35 which is reflected by infiltration of tumors by Treg cells36 and MDSC37 as well as by cells which contain high levels of molecules that can suppress or modify a Th1 response7. This presents an obstacle to T cell-based therapies, including therapeutic cancer vaccination.
We show that a combination of mAbs to CD137+PD-1+CTLA4 tripled survival of mice with the ID8 ovarian carcinoma growing i.p. and prolonged survival (most likely curing) >40% of mice with established SW1 melanoma. Local delivery of the mAb combination to the tumor area appeared to be more effective than systemic administration, but the difference was not statistically significant and needs to be validated. It is noteworthy that local delivery of immunostimulating agents has been reported to have better efficacy and less systemic toxicity compared with systemic administration21. A combination of mAbs to CD137+PD-1 doubled survival in the ID8 model but was ineffective for SW1 (data not shown). Under the conditions of our experiments, mAbs to CD137, PD-1, CTLA4 or CD19 were ineffective as single agents when tested in the ID8 model, as were combinations of mAbs to PD-1+CTLA4 and CD137+CTLA4, though both of these combinations can improve the efficacy of therapeutic tumor vaccination22,38. As shown for SW1, administration of the 3 mAb combination was therapeutically effective against an untreated tumor in the same mouse, and the response has immunological memory. It is noteworthy that CD4 cells were needed for tumor rejection in both models while the role of CD8 cells appeared to be less than that of CD4 cells, particularly in the SW1 model.
Both animal and human studies have indicated that immunotherapy39, including administration of mAbs to CTLA4, PD-1 or CD137, can induce serious autoimmune responses. Although no mice displayed evidence of toxicity such as weight loss in the ID8 model, two mice whose SW1 melanoma had regressed after i.t. injection died and one mouse which had long term complete regression temporarily lost its hair and displayed depigmentation. Major efforts are needed to learn more about the toxicity associated with the mAb combinations we have applied (although it has been relatively modest) and develop ways to overcome it.
According to flow cytometry, therapeutic efficacy was associated with a shift in the tumor microenvironment from a Th2 type to a Th1 type immunological profile with decrease in tumors and tumor-draining lymph nodes of cells expressing CD19, a B cell marker40. Concomitantly with the decrease of CD19+ cells, the frequency of CD3+, CD4+ and CD8+ cells in the peritoneal lavage from mice with ID8 tumors or infiltrating SW1 tumors increased in mice receiving the 3 mAb combination as did the frequency of tumor-associated mature DC, and the ratio increased between CD4+ and CD8+ effector cells to either Tregs or MDSCs. Importantly, an antigen-specific Th1 type response to mouse mesothelin was induced in treated mice with ID8 tumors. Further studies are needed to explore whether responses can be induced also to other tumor antigens, including individual tumor-specific epitopes that are expressed as a result of the high mutability of tumor cell populations.
The conclusion that the therapeutic efficacy is associated with a shift from a Th2 to a Th1 response is supportive by data from PCR assays and qPCR, including an increased transcription of the Th1-associated chemokine/receptor CXCL9-11/CXCR3 and a downregulation of the CXCL13/CXCR5 signaling pathway that is essential for B cell migration41,42. Recently, Messina, et al reported that upregulation of a 12-chemokine gene expression signature, including CXCL9-11, is characteristic for an intratumoral immune reaction which is associated with better overall survival among melanoma patients who underwent therapeutic vaccination43. Administration of a combination of mAbs to CD137+PD-1+CTLA4 upregulated most of these 12-chemokines in our study, although expression of CXCL13 was downregulated.
Exploratory experiments in the SW1 model indicate that addition of an anti-CD19 mAb to the anti-CD137+PD-1+CTLA4 combination can further improve the therapeutic efficacy, including the regression of a second, untreated tumor in the same mice. Our findings indicate an important role of B cells in the escape of tumors from immunological control. Data indicating that antibody forming cells and antibodies can promote the escape of tumors from immunological control were published several decades ago44-48. However, most evidence that B cells and antibodies promote tumor growth is of recent origin and relates to a Th2 type inflammation at tumor sites5,7,8,26,27. For example, development of squamous cell carcinomas is inhibited in B-cell deficient mice by a mechanism involving antibodies27, activating Fcγ receptors on myeloid cells in tumors can promote cancer progression49, the absence of B cells can enhance a Th1 immune response50 and reduce the number of Treg cells15, and depletion of B cells by using an anti-CD20 antibody can activate CD8+ T cells and retard tumor growth51. Furthermore, castration-resistant prostate cancer progression is promoted by tumor infiltrating B cells14. However, there are also contradictory data. For example, DiLillo et al reported that B cell depletion in mice impaired the induction of functional CD4+ and CD8+ T cell and enhanced B16 melanoma growth20, and in another study, B cells were found to play no role with respect to mammary carcinogenesis7. The controversial findings may reflect functional differences between different B cell populations and/or differences between tumor models and mouse strains (and between genetically different human subjects). The function of B cells in cancer immunity is complex, and further studies are needed.
We are encouraged by our findings with the ID8 ovarian carcinoma and SW1 melanoma and by similar, preliminary data from the B16 and TC1 models, i.e. a combination of mAbs to CD137+PD-1+CTLA4 displayed therapeutic efficacy in 3 of 4 models and a combination also comprising a mAb to CD19 caused tumor regression in the 3 models where it was tested (SW1, B16, TC1). However, one should remain cautious since we have no data for primary tumors and their metastases or for large, disseminated cancers. Nevertheless, our findings, as well as those of others16-18,21,22, indicate that shifting the tumor microenvironment from a Th2 to a Th1 type immune response may open new avenues for cancer therapy. The availability of, and clinical experience with, human versions of mAbs to CD137, PD-1, CTLA4, as well as mAbs to CD19 and CD20 facilitates ‘translation’ of the animal data to the clinic. We expect that future studies will identify even more effective ways to achieve tumor regression by modifying the immune response as well as simpler approaches than injecting a combination of 3 or 4 mAbs into tumors and ways to decrease side-effects. If local delivery to tumors proves to be more effective than systemic application, it may be improved, e.g. by using ‘functionalized’ nanoparticles52 or by targeting systemically applied therapeutic agents (e.g. in the form of bispecific antibodies or immunoconjugates if small molecules can be found with similar efficacy as the mAbs). Further studies may also show that a combination of therapeutic tumor vaccination with procedures that shift the tumor microenvironment from a Th2 type to a Th1 type response will improve therapy by acting additively or even synergistically.
Supplementary Material
Supplementary Fig. 1 Panel a shows survival for mice transplanted i.p. with 3×106 ID8 cells and injected weekly with the indicated mAb or mAb combinations for a total of 3 times (n=5, **p<0.01). Panel b shows survival of ID8 tumor-bearing mice which had been injected with anti-CD137+PD-1+CTLA4 mAbs as shown by arrows and subsequently injected 3 times i.p. with mAbs to deplete CD4, CD8 or NK1.1 cells as shown by asterisks (n=5, *p<0.05, **p<0.01). Panel c shows survival of SW1 tumor-bearing mice which had been injected with anti-CD137+PD-1+CTLA4 mAbs as shown by arrows and subsequently injected 3 times i.p. with mAbs to deplete CD4, CD8 or NK1.1 cells as shown by asterisks (n=5, *p<0.05, **p<0.01).
Supplementary Fig. 2 Panel a shows representative flow cytometry dot plots of cells from peritoneal lavages (PL) and peritoneal lymph nodes (LN) from mice (2-3 mice/group) which had been transplanted with ID8 cells and injected with anti-CD137+PD-1+CTLA4 mAbs as in the therapy experiments and euthanized 7 days later. Panel b shows representative dot plots of cells from tumor-draining lymph nodes (TLN) and tumor-infiltrating lymphoid cells (TIL) from mice (2-3 mice/group) whose SW1 tumors were injected with anti-CD137+PD-1+CTLA4 mAbs as in the therapy experiments and euthanized 7 days later. At least 3 repeated experiments had been performed and the statistical analysis was shown in the Figure 2.
Supplementary Fig. 3 Representative flow cytometry histograms showing that treatment with the 3 mAb combination increased the expression of CD86 among CD11c+ DC from peritoneal lymph nodes (LN) and peritoneal lavage (PL) from ID8-bearing mice and among cells from tumor-draining lymph nodes (TLN) and tumor-infiltrating lymph nodes (TIL) from treated SW1-bearing mice. Controls are depicted by red and treated groups by blue color.
Supplementary Fig. 4 Representative flow cytometry dot plots showing that addition of anti-CD19 mAb to the 3 mAb combination significantly decreased the frequency of CD19+ cells in spleens, tumor-draining lymph nodes (TLN) and tumors (TIL).
Supplementary Fig. 5 Therapeutic effects of the indicated mAb combinations versus control in TC1 tumor model and B16 tumor model. Panel a shows survival curves for an experiment (5 mice/group) where the mAb combinations were given i.t. when the mice had TC1 tumors of 4-5 mm mean diameter. Panel b shows survival curves for an experiment (5 mice/group) where the mAb combinations were given i.t. when the mice had B16 tumors of 5-6 mm mean diameter. *p<0.05, ** p<0.01.
ACKNOWLEDGMENTS
Our work was supported by grants R01-112073 and R01CA134487 from National Institutes of Health. We thank Dr. N. Kiviat for support. Huafeng Wei is supported by the National Natural Science Foundation of China (No. 30901380/C081501) and the Shanghai Morning Light Program (09CG36).
Footnotes
M.D. and H.W. contributed equally.
Financial Disclosure: All authors have declared there are no conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hellstrom KE, Hellstrom I. Cellular immunity against tumor specific antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Cerottini JC, Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Nardo D, Coussens L. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9 doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon BS, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 1997;272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Kim SH, Mantel P, et al. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses. Eur J Immunol. 1998;28:881–890. doi: 10.1002/(SICI)1521-4141(199803)28:03<881::AID-IMMU881>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Ye Z, Hellstrom I, Hayden-Ledbetter M, et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 12.Melero I, Johnston JV, Shufford WW, et al. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 13.Mittler RS, Bailey TS, Klussman K, et al. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammirante M, Luo JL, Grivennikov S, et al. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi S, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flies DB, Sandler B, Sznol M, et al. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol. Med. 2011;84:409–421. [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 18.Ascierto P, Simeone E, Sznol M, et al. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransen MF, Sluijter M, Morreau H, et al. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 22.Curran M, Kim MR, Montalvo W, et al. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation and cytokine production. PloS One. 2011;6 doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price JE, Tarin D, Fidler I. Influence of organ microenvironment on pigmentation of a metastatic murine melanoma. Cancer Res. 1988;48:2258–2264. [PubMed] [Google Scholar]
- 24.Yang SC, Yang Y, Raycraft J, et al. Melanoma cells transfected to express CD83 induce anti-tumor immunity that can be increased by also engaging CD137. Proc. Natl Acad. Sci. 2004;101:4990–4995. doi: 10.1073/pnas.0400880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Jaffar J, Zhou J, et al. Inhibition of TGFbeta 1 makes nonimmunogenic tumor cells effective for therapeutic vaccination. J. Immunother. 2009;32:232–239. doi: 10.1097/CJI.0b013e318197ac86. [DOI] [PubMed] [Google Scholar]
- 26.de Visser K, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 27.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Roby KF, Taylor CC, Sweetwood JP, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 29.Fidler IJ, Gersten DM, Budmen MB. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976;36:3160–3165. [PubMed] [Google Scholar]
- 30.Tuve S, Liu Y, Tragoolpua K, et al. In situ adenovirus vaccination engages T effector cells against cancer. Vaccine. 2009;27:4225–4239. doi: 10.1016/j.vaccine.2009.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Jaffar J, Hellstrom I, et al. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. 2010;33:53–59. doi: 10.1097/CJI.0b013e3181b56af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung CF, Tsai Y, He L, et al. Controll of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin-peptide specific CD8 T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Ovestad IT, Gudlaugsson E, Skaland I, et al. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol. 2010;23:1231–1240. doi: 10.1038/modpathol.2010.109. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Tuve S, Persson J, et al. Adenovirus-mediated intratumoral expression of immunostimulatory proteins in combination with systemic Treg inactivation induces tumor-destructive immune responses in mouse models. Cancer Gene Therapy. 2011:1–12. doi: 10.1038/cgt.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 37.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 38.Curran MA, Kim M, Montalvo W, et al. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilboa E. The risk fof autoimmunity associated with tumor immunotherapy. Nature Immunol. 2001;2:789–792. doi: 10.1038/ni0901-789. [DOI] [PubMed] [Google Scholar]
- 40.Rickert R, Rajewsky K, Roes J. Impairment of T-cell dependent B-cell responses and B-1 cell development in CD19 deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 41.Legler D, Loetscher M, Roos R, et al. B cell-attrracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attaracts b lymphocytes via aBLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammirante M, Luo J, Grivennikov S, et al. B-cell0-cerived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;11:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Scientific reports. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellstrom I, Hellstrom KE, Evans CA, et al. Serum-mediated protection of neoplastic cells from inhibition by lymphocytes immune to their tumor-specific antigens. Proc Natl Acad Sci U S A. 1969;62:362–368. doi: 10.1073/pnas.62.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sjogren HO, Hellstrom I, Bansal SC, et al. Suggestive evidence that the “blocking antibodies” of tumor-bearing individuals may be antigen--antibody complexes. Proc Natl Acad Sci U S A. 1971;68:1372–1375. doi: 10.1073/pnas.68.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldwin RW, Price MR, Robins RA. Blocking of lyimphocyte-mediated cytotoxicity for rat hepatoma cells by tumour-specific antigen-antibody complexes. Nature. 1972;238:185–187. doi: 10.1038/newbio238185a0. [DOI] [PubMed] [Google Scholar]
- 47.Hellstrom KE, Hellstrom I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- 48.Moller G. Effect on tumor growth in syngeneic recipients of antibodies against tumor-specific antigens in methylcholanthrene-induced mouse sarcomas. Nature. 1964;204:846–847. doi: 10.1038/204846a0. [DOI] [PubMed] [Google Scholar]
- 49.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, Fridlender ZG, Dunn R, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31:446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- 52.Lei C, Liu P, Chen B, et al. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am.Chem. Soc. 2010;132:6906–6907. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Panel a shows survival for mice transplanted i.p. with 3×106 ID8 cells and injected weekly with the indicated mAb or mAb combinations for a total of 3 times (n=5, **p<0.01). Panel b shows survival of ID8 tumor-bearing mice which had been injected with anti-CD137+PD-1+CTLA4 mAbs as shown by arrows and subsequently injected 3 times i.p. with mAbs to deplete CD4, CD8 or NK1.1 cells as shown by asterisks (n=5, *p<0.05, **p<0.01). Panel c shows survival of SW1 tumor-bearing mice which had been injected with anti-CD137+PD-1+CTLA4 mAbs as shown by arrows and subsequently injected 3 times i.p. with mAbs to deplete CD4, CD8 or NK1.1 cells as shown by asterisks (n=5, *p<0.05, **p<0.01).
Supplementary Fig. 2 Panel a shows representative flow cytometry dot plots of cells from peritoneal lavages (PL) and peritoneal lymph nodes (LN) from mice (2-3 mice/group) which had been transplanted with ID8 cells and injected with anti-CD137+PD-1+CTLA4 mAbs as in the therapy experiments and euthanized 7 days later. Panel b shows representative dot plots of cells from tumor-draining lymph nodes (TLN) and tumor-infiltrating lymphoid cells (TIL) from mice (2-3 mice/group) whose SW1 tumors were injected with anti-CD137+PD-1+CTLA4 mAbs as in the therapy experiments and euthanized 7 days later. At least 3 repeated experiments had been performed and the statistical analysis was shown in the Figure 2.
Supplementary Fig. 3 Representative flow cytometry histograms showing that treatment with the 3 mAb combination increased the expression of CD86 among CD11c+ DC from peritoneal lymph nodes (LN) and peritoneal lavage (PL) from ID8-bearing mice and among cells from tumor-draining lymph nodes (TLN) and tumor-infiltrating lymph nodes (TIL) from treated SW1-bearing mice. Controls are depicted by red and treated groups by blue color.
Supplementary Fig. 4 Representative flow cytometry dot plots showing that addition of anti-CD19 mAb to the 3 mAb combination significantly decreased the frequency of CD19+ cells in spleens, tumor-draining lymph nodes (TLN) and tumors (TIL).
Supplementary Fig. 5 Therapeutic effects of the indicated mAb combinations versus control in TC1 tumor model and B16 tumor model. Panel a shows survival curves for an experiment (5 mice/group) where the mAb combinations were given i.t. when the mice had TC1 tumors of 4-5 mm mean diameter. Panel b shows survival curves for an experiment (5 mice/group) where the mAb combinations were given i.t. when the mice had B16 tumors of 5-6 mm mean diameter. *p<0.05, ** p<0.01.