Abstract
Meniscus degeneration due to age or injury can lead to osteoarthritis. Though promising, current cell-based approaches show limited success. Here we present three-dimensional methacrylated gelatin (GelMA) scaffolds patterned via projection stereolithography to emulate the circumferential alignment of cells in native meniscus tissue. Cultured human avascular zone meniscus cells from normal meniscus were seeded on the scaffolds. Cell viability was monitored, and neo-tissue formation was assessed by gene expression analysis and histology after two weeks in serum free culture with TGFβ1 (10ng/ml). Light, confocal and scanning electron microscopy was used to observe cell/GelMA interactions. Tensile mechanical testing was performed on unseeded, fresh scaffolds and two-week old cell-seeded and unseeded scaffolds. Two-week old cell/GelMA constructs were implanted into surgically created meniscus defects in an explant organ culture model. No cytotoxic effects were observed three weeks after implantation, and cells grew and aligned to the patterned GelMA strands. Gene expression profiles and histology indicated promotion of a fibrocartilage-like meniscus phenotype, and scaffold integration with repair tissue was observed in the explant model. We show that micropatterned GelMA scaffolds are non-toxic, produce organized cellular alignment, and promote meniscus-like tissue formation. Prefabrication of GelMA scaffolds with architectures mimicking meniscus collagen bundle organization shows promise for meniscal repair. Furthermore, the technique presented may be scaled to repair larger defects.
Keywords: Meniscus, GelMA, projection stereolithography, Digital-Micromirror-Device Projection Printing System
1. Introduction
The meniscus has a role in stabilizing the knee joint and functions as a shock absorber which protects articular cartilage during walking and sporting activities. A meniscal tear is the most frequently recorded orthopedic diagnosis, and partial or total meniscectomy remains the most common orthopedic procedure [1]. The annual incidence of meniscal injuries in the US is estimated to be between 600,000 to 850,000 with 90% resulting in meniscal surgery. The vast majority of these procedures involve partial, sub-total, or total meniscectomy [2, 3, 4]. Untreated damaged or degenerated meniscus can lead to the development of osteoarthritis (OA). OA is the number one cause of disability in the United States, affecting over 27 million people [5]. Despite substantial developments in surgical techniques, instrumentation, and orthopedic devices, long-term clinical outcomes are not satisfactory.
Partial meniscectomy, in which only the torn and damaged portions of the menisci are removed [6], is now the treatment of choice for meniscal tears that cannot be repaired. Partial meniscectomy effectively relieves the acute symptoms, such as pain, swelling, and locking of the knee. However, partial meniscectomy fails to prevent the onset of severe osteoarthritis, which occurs on average 14 years after the original procedure [7, 8].
Surgical attempts to repair the torn tissues are ineffective in the avascular zone and are associated with a re-rupture rate of 30% even in the vascular zone [4]. Furthermore, repairs deemed successful in the short term do not mitigate long term degenerative changes and the onset of osteoarthritis. The sequelae of meniscal injury and the clinical outcomes of meniscectomy or repair are significantly worse in patients over the age of 40 [9]. Despite major advances in surgical techniques and biomedical device development, a meta-analysis of 42 clinical studies found no difference in the incidence of radiographic osteoarthritis after meniscal repair when compared to partial or total meniscectomy [8].
To address this medical challenge, an attempt to repair meniscus tears is the first logical step taken by many researchers. Biomaterials used to culture meniscus cells or stem cells for meniscus repair include bioresorbable collagen matrix [10], which have been implanted in human patients with varied outcomes [11, 12, 13]. Fibrin alone or fibrin with added growth factors has been used to heal horizontal tears in human patients [14]. A recent report describes human meniscus cells seeded on a resorbable combination of polyglycolic acid (PGA) and hyaluronic acid in fibrin [15]. Polyurethane scaffolds possess sufficient mechanical properties with optimal interconnective macro-porosity to facilitate cell in growth and differentiation [16]. Vicryl mesh scaffolds have also been used to repair bucket-handle lesions in porcine meniscus [17]. A combination of human bone marrow derived stem cells (hMSC) and a collagen scaffold was used in ovine meniscus explants showing promising integration [18]. Better integration was seen when the open/spongy scaffold structure was adjacent to the tissue. Devitalized meniscus also has been used to take advantage of the existing tissue architecture [19, 20]. Though using the natural tissue may seem more promising for meniscus tears, a mature tissue scaffold without cells may not be ideal, and availability of mensiscal tissue is also an issue.
A cell-seeded supportive scaffold system that emulates the structure and possess the mechanical properties of native meniscus may aid in integrating and stabilizing the repair site and promote seamless repair. In the short term, such an implanted cell-seeded scaffold should permit the cells to proliferate locally, migrate into the interface between the scaffold and native tissue, and secrete matrix components that integrate the scaffold.
Building structures mimicking native tissues can be accomplished using a number of nano- and micro-fabrication techniques including melt molding, porogen leaching, gas foaming, phase separation, lamination and fiber-based techniques [21, 22, 23, 24, 25, 26]. More recently, rapid-prototyping techniques have been applied to biomaterial scaffold fabrication to refine the spatial complexity with which complex 3D physiological architectures can be replicated in vitro using laser ablation, microfluidics and 3D printing [21, 27, 28, 29]. In particular, projection stereolithography (PSL) or digital micromirror device (DMD) microfabrication that uses an array of digitally-controlled micromirrors to fabricate 3D scaffolds, layer-by-layer, via a reflective photomask is a promising technique. Because photomasks can be changed easily on-demand, the PSL approach is attractive due to its relative speed and flexibility when compared to other photopatterning techniques [27, 30, 31, 32, 33, 34]. This technique allows the rapid assembly of cell-responsive hydrogels that feature highly-specified complex 3D geometries with micrometer resolution.
In this study we demonstrate the feasibility of combining cell therapy, photocrosslinkable hydrogels, and PSL microfabrication to produce graft tissue for implantation and integration into a meniscus tear. We fabricated scaffolds using a hydrolyzed form of collagen type I, the major structural component of the meniscal tissue, to provide structure and function similar to the surrounding native meniscus tissue. We confirmed cell compatibility (viability), observed cell interactions with the patterned scaffold (attachment and organization), characterized mechanical properties of the scaffold before and after cell seeding, demonstrated human meniscus neo-tissue formation, and explored the potential for cell-seeded GelMA scaffolds to integrate with native meniscus tissue in an ex vivo human meniscus defect.
2. Methods
2.1. Tissue procurement
Normal human meniscus (medial and lateral) was obtained from tissue banks (approved by Scripps institutional review board), from six donors (age range: 18–61 years, mean age: 37.2 ± 17.5, one female, five males). A previously reported macroscopic and histologic grading system was used to select normal menisci [35].
2.2. Cell isolation and monolayer culture
Meniscus tissue was cut to isolate the avascular (inner two thirds) and vascular (outer one third) regions. The separated tissues were subjected to collagenase digestion as previously described [36], except digested over 5–6 hours. The digested tissues were filtered through 100 μm cell strainers (BD Biosciences, San Jose, CA) and seeded in monolayer culture in DMEM (Mediatech Inc, Manassas, VA) supplemented with 10% calf serum (Omega Scientific Inc. Tarzana, CA) and Penicillin/Streptomycin/Gentamycin (Invitrogen, Carlsbad, CA).
2.3. Synthesis of CaCO3 Particle Incorporated methacrylated gelatin (GelMA)
Gelatin methacrylate was prepared as described previously [37]. Briefly, porcine skin gelatin (Sigma-Aldrich) was dissolved at 10% w/v in phosphate buffered saline (PBS; Gibco/Life Technologies, Grand Island, NY) at 60°C and stirred for 1 hr. Methacrylic anhydride (Sigma-Aldrich, St. Louis, MO) was added at a rate of 0.5 mL/min at 50°C to achieve a final concentration of 7.5% v/v and allowed to react for 2 hrs. The product was dialyzed against dH2O at 40°C for 1 week using dialysis tubing (12–14 kDa MWCO; Spectrum Laboratories). Finally, the solution was filtered (0.2 μm), frozen overnight (−80°C), and lyophilized for 1 week. The final product was stored at −80°C until further preparation.
To aid in mechanical stability during fabrication, CaCO3 particles were incorporated into the final GelMA macromer solution. First, a 3% w/v macromer solution was prepared by adding GelMA to pre-warmed PBS (60°C) and stirring until fully dissolved. An equal volume of 1.65 M CaCl2 in PBS was added to the GelMA solution until thoroughly mixed. 1.65 M Na2CO3 in PBS (volume equivalent to the CaCl2 solution) was added dropwise, and the mixture was stirred at 40°C for 24 hrs. After allowing the mixture to settle, excess supernatant was removed to produce a 1.5% w/v GelMA concentration. Additional GelMA was then added to reach a 15% w/v concentration. Photoinitiator Irgacure 2959 (1% w/v; Ciba/BASF, Florham Park, NJ), UV absorber 2-hydroxy-4-methoxy-benzphenone-5-sulfonic acid (0.1% w/v), and UV quencher TEMPO (0.01% w/v; Sigma-Aldrich) were added sequentially until fully dissolved.
To determine the extent of methacrylate conversion (i.e. the degree of modification of epsilon amine groups on lysines in gelatin), the 2,4,6-Trinitrobenzenesulfonic acid solution (TNBS) assay as described by Habeeb was used [38]. The percentage degree of amine substitution was calculated using the following formula:
2.4. Digital-Micromirror-Device (DMD) Projection Stereolithography System
GelMA scaffolds were fabricated using a modified version of a DMD projection stereolithography (PSL) system described elsewhere [33]. The fabrication platform (Fig. 1A) comprises a DMD system (1920 × 1080 Discovery 4000, Texas Instruments, TX, USA), a servo-controlled stage (CMA-25-CCCL & ESP300, Newport), a UV light source (200 W, S2000, EXFO), a UV-grade projection lens (Edmond Optics), and a replaceable glass coverslip window (No. 1, 22×22mm, Fisher) onto which the UV light is focused. Using a computer, specified patterns for each layer of the scaffold are loaded onto the DMD, which consists of a 1920 × 1080 pixel array of micro-mirrors. These patterns provide a series of reflective photomasks that can be changed on demand. During fabrication, UV light is reflected off the DMD and focused via the projection lens onto the photo-curable GelMA solution, which is placed between the glass coverslip and the servo-controlled stage. The GelMA solution is selectively cured in the regions specified by the DMD array, with the height of each cured layer defined by the distance between the glass coverslip and the servo-controlled stage. The glass coverslip is coated with Krytox 157 FSH oil (DuPont, Wilmington, DE) to aid in release of the polymerized scaffold layer from the coverslip surface after UV exposure. The glass coverslip is replaced after fabrication of each layer.
Fig. 1.
A. Overview of the Digital-Micromirror-Device Projection Stereolithography (DMD-PSL) system used to make GelMA scaffolds and design of scaffold. B. Cartoon showing basic design of scaffold design pattern with dimensions C and D. Phase contrast micrograph images of GelMA scaffolds produced (Mag. C= 4x and D= 10x).
2.5. Layer-by-Layer Scaffold Fabrication
Figure 1A describes the scaffold fabrication process. The photocurable GelMA/CaCO3 mixture was stirred at 35°C during fabrication. CaCO3 aids in maintaining better structural integrity of the scaffold structure during fabrication and is removed after fabrication. After placing a drop of the GelMA/CaCO3 on the stage, the distance between the glass coverslip and the stage was adjusted initially to 100 μm for fabrication of the first layer. The UV image was then projected onto the glass coverslip for 8 sec at a power of 50 mW/cm2. After polymerization of the first layer, the stage was moved down to release the scaffold from the glass coverslip. The scaffold and stage were washed with PBS, a new drop of GelMA/CaCO3 was added to the top of the scaffold, and the coated glass coverslip was replaced. After changing the DMD image, the stage was moved to the new position (100 um), and the next layer was exposed with UV light. These steps were repeated for all 5 layers of the scaffold to construct the final layered 3D structure of approximately 500 μm or 0.5 mm high. Each longitudinal log is 20 μm wide and ~1800 μm long. These dimensions were chosen to represent the bundles of collagen fibrils that run circumferentially in the main central portion of the meniscus. The spacing between the logs (100 μm) was the lower end of pore diameter required for cellular infiltration. The supporting transverse logs are 50 μm wide and ~1730 μm long. These were constructed to provide stability and link the longitudinal layers. The distance between the glass coverslip and the stage was increased for each subsequent layer by increments of 100 μm, and the images were projected using the same intensity and duration as the initial layer. Figure 1A illustrates the specific image sequences used to generate the layered scaffold resembling a “log pile” to replicate the dominant structure of the circumferentially oriented collagen fibers in native menisci.
After fabrication, the complete scaffold was removed from the stage using a scalpel, placed in a bath of 10 mM HCl for 30 min. to dissolve the incorporated CaCO3, and thoroughly rinsed with PBS. In total, fabrication time for each 5-layer scaffold is approximately 35–40 min, including the scaffold projection, washing and removal of CaCO3.
2.6. Cell seeding and 3D culture
The GelMA scaffolds were placed into cell culture inserts (8 μm; BD Biosciences) inserted into 24 well plates and maintained in PBS until ready for cell seeding. To seed the cells, the PBS was removed and the cells were seeded at a density of 1×106 cell per ml in 50 μl of medium (50,000 cells per scaffold). The 50 μl cell suspension remained on the scaffold for 20 minutes to permit cell attachment before medium was added to the outer well. The medium moved up through the membrane at the base of the cell culture insert to bathe the cell seeded scaffold. However, higher volumes of media were avoided since many scaffolds floated, which may reduce cell migration into the scaffold from the surrounding membrane.
The medium used in 3D culture consisted of Dulbecco’s Modified Eagle’s Medium (DMEM) (Cellgro, Manassas, VA), 1x ITS+1 (Sigma; i.e. 10 mg/ml insulin, 5.5 mg/mL transferrin, 5 ng/ml selenium, 0.5 mg/ml bovine serum albumin, 4.7 mg/ml linoleic acid), 1.25 mg/ml human serum albumin (Bayer, Leverkusen, Germany), 100 nM dexamethasone (Sigma), 0.1mM Ascorbic acid 2-phosphate (Sigma), and Penicillin/Streptomycin/Gentamycin (Gibco, Carlsbad, CA) [39]. This mix was filter sterilized through a 0.22 micron filter. To stimulate meniscus neo-tissue formation, TGFβ1 (10ng/ml) was added to the medium immediately prior to use for all conditions.
2.7. Cell viability
The viability of cells cultured on GelMA scaffolds was observed using the live/dead kit consisting of Calcein-AM and Ethidium Homodimer-1 (Invitrogen) and a laser confocal microscope (LSM-510, Zeiss, Jena, Germany) as previously demonstrated [40].
2.8. Histology and immunohistochemistry
Two week old GelMA scaffolds seeded with meniscus fibrochondrocytes were cryofixed in tissue Tech (OCT compound, Sakura, Torrence, CA) on dry ice and cryo-sectioned ~7 um thick. The sections were stained with H&E and Safranin O-Fast Green. For detection of collagen type I by immunohistochemistry, cut sections were fixed in 4% formaldehyde for 10 minutes at room temperature and then treated with hyaluronidase for 2 hours [41] and incubated with primary antibodies against collagen type I (clone: I-8H5; MP Biomedicals, Santa Ana, CA) at 10 ug/ml for secondary antibody staining procedures and detection procedures were followed as previously described [42]. Isotype controls were used to control for non-specific staining.
2.9. RNA isolation and RT-PCR
Total RNA was isolated from GelMA constructs using the RNAeasy mini kit (Qiagen, Hilden, Germany) and first strand cDNA was produced according to the manufacturer’s protocols (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed using TaqMan® gene expression reagents. COL1A1, Aggrecan, Sox9 and GAPDH were detected using Assays-on-Demand™ primer/probe sets (Applied Biosystems). GAPDH was used to normalize gene expression levels using the recommended ΔCt method, and fold-change was calculated using the 2-ΔΔCT formula [43].
2.10. Mechanical testing
The tensile strength of the GelMA construct along the axis of the “log-piles” was assessed using a custom built device consisting of 2 miniature brushless servo actuators (SMAC, Carlsbad, CA) and one 50 gram load cell (FUTEK, Irvine, CA). The scaffold was secured between 2 coverslips (100 μm-thick) using a cyanoacrylate glue. The base coverslip was glued down to a stainless steel block as an anchor, while the upper coverslip was attached to a steel plunger having a flat surface acted as the actuator for pulling the scaffolds (Supplementary data figure 1). LabVIEW (National Instruments, Austin, TX) software was employed for movement control and data acquisition on a laptop. The gel height was measured using electronic calipers. The force to pull the gel to breaking was monitored and recorded. Young’s modulus was calculated as previously reported [44].
2.11. Scanning electron microscopy (SEM)
Avascular meniscus cells cultured on GelMA for 14 days were washed with PBS and fixed with 2.5% w/v glutaraldehyde (Sigma, USA) for 1 h. After fixation they were washed three times with PBS for 10 min each wash. The scaffold was dehydrated in a graded series of ethanol (50%, 70%, and 90%) for 30 min each and left in 100% ethanol for 24 h at −20°C. The scaffold was dried in a critical point dryer (EMS 850, Electron Microscopy Science Co., Chelmsford, MA) and then surface metalized by sputter coating with iridium for SEM examination (XL30, FEI Co., Hillsboro, OR).
2.12. Organ culture repair model
Sections of human meniscus from one donor (18 year old male) were cultured in medium in 6-well plates for 2–3 days following processing. One full-thickness defect per tissue section was created with a scalpel parallel to the circumferential direction to simulate a longitudinal tear. Two week old cultured GelMA scaffolds with avascular meniscus fibrochondrocytes (22 year old male) was placed into the defect in an orientation where the “log-piles” were oriented parallel to the circumferential collagen bands. The tissues were maintained in differentiation medium (8 ml per well) for 3 weeks (with medium changes every 2–3 days) and processed for histology to examine scaffold/cell integration with the native tissue and for neo-tissue development.
3. Results
3.1. Human meniscus cells are organized by GelMA scaffold architecture
Following 2 weeks of culture, human avascular meniscus cells seeded onto GelMA scaffolds remained viable and were aligned along the “log-pile” strands of GelMA (Figure 2B and 2C). Scanning electron micrographs showed the alignment and intimate interaction between the meniscus cells GelMA scaffold (Figure 2D–G).
Fig. 2.

Cell viability and interaction with GelMA scaffold. A. Phase contrast of meniscus seeded GelMA scaffold (mag. 4x); B. Confocal image showing live cells (green) attached to GelMA “log-piles” (composite of a 3×3 scan at 10x mag.). C. Center region confocal image scan (mag. 10x). D–E Scanning electron micrographs of cell and GelMA interaction (Mag. D=1250x; E=2500x).
3.2. Meniscus-like neotissues are formed on GelMA scaffolds
The Young’s modulus of the scaffold was 14.3 ± 4.0 kPa (Figure 3). No change in mechanical properties was detected over time in culture medium alone or when seeded with cells (Figure 3). The extent of methacrylation was calculated to be greater than 90%, with a mass swelling ratio of 5.9, indicating that the mechanical properties of the GelMA were close to maximum [45]. Histological analysis indicated the formation of multilayered and aligned neotissue (Figure 4A–E). These tissues lacked glycosaminoglycans (GAG), as seen in the Safranin-O staining (Figure 4D), yet contained collagen type I (Figure 4E), which complements the gene expression profile of high COL1A1 mRNA levels (Figure 4G). Increased Sox9 also indicates re-differentiation to the meniscus phenotype (data shown as gene expression relative to the monolayer cultured cells).
Fig. 3.
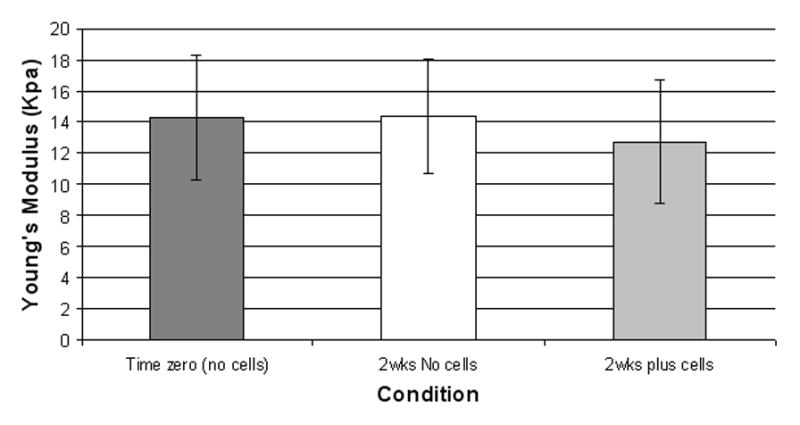
Measurement of Young’s modulus. Mean ± SD of calculated Young’s modulus (kPa) for scaffolds at time zero, and after 2 week in culture medium, with and without cells.
Fig. 4.

Histology, immunohistochemistry (IHC) and RT-PCR. A. H&E stain; B. Inset showing production of cell layers on the GelMA; C. H&E stain; D. Safranin O-Fast Green stain; E. Type I collagen IHC (red color).; G. Gene expression profile of meniscus cells cultured on GelMA for 2 weeks relative to monolayer expanded cell gene expression values denoted by red dotted line. (Panels A,C–E are magnification 40x).
3.3. Cell seeded GelMA scaffolds integrate with native meniscus tissue
Human meniscus cells seeded on GelMA scaffolds for 2 weeks were placed into surgically created defects in meniscus tissue (Figure 5A and 5B). After 3 weeks of free swelling culture in 6-well plates, histological analysis revealed the generation of neotissues between the implanted scaffolds and the native tissue (Figure 5C–N). The neotissues present are multilayered (Figure 5D) and have virtually no GAG staining (Figure 5G).
Fig. 5.

Human meniscus ex vivo repair model. A. Defects were surgically produced in human meniscus tissue and 2 week old meniscus cell seeded GelMA scaffolds were inserted and culture for a further 4 weeks. B. Inset showing a closer view of defect. C–N. Histology of two different defects showing areas of neotissue formation that integrate with surrounding meniscus tissue (H&E stain: C–E and I–K; Safranin O-fast green stains: F–H and L–N). Magnifications shown on each panel.
4. Discussion
Projection stereolithography to produce specified micropatterns is a useful means to produce scaffolds that emulate the structure of native tissue architecture. We show that the GelMA micro patterned scaffolds produced in this study maintain human meniscus cell viability without cytotoxic effects, produce organized cellular alignment in response to the scaffold microstructure, and promote the formation of meniscus-like tissues. Prefabrication of organized GelMA scaffolds with an architecture mimicking meniscus collagen bundle organization shows promise for the repair of meniscal tears as demonstrated by formation of integrated neotissue in the ex vivo meniscus defect model. Scaling-up of this approach may be useful to repair larger defects.
To our knowledge, scaffolds employing DMD microfabrication have not been used for meniscus tissue engineering nor for meniscal defect repair. Specific combinations of GelMA and microengineering have been tested with various cell types and for directing specific cell patterns including fibroblasts, myoblasts, endothelial cells and cardiac stem cells [37], human umbilical vein endothelial cell [46], NIH-3T3 cells and human mesenchymal stem cells [47]. Spatial organization of embryoid bodies have been made in GelMA [48] and Aubin et al. [37] demonstrated that GelMA hydrogels could be useful for creating complex, cell-responsive microtissues, such as endothelialized microvasculature. Other studies using projection printing technology have been used with numerous different cell types [1, 31, 34, 49, 50]. Combined application of the GelMA hydrogels and dielectrophoresis (DEP) is another method for creating highly complex microscale scaffold/tissue constructs using myoblast or endothelial cells [47].
The neotissues produced on GelMA scaffolds appeared fibrocartilage-like with high expression of COL1A1 (mRNA and protein) as reported elsewhere for native [51, 52, 53, 54] and engineered meniscus [15]. Similar to the vascular region, we did not observe increased aggrecan expression or detect COL2A1. The increase in Sox9 expression indicates that longer term cultures may be needed to induce the expression of COL2A1 and aggrecan [55]. Perhaps mechanical stimulation [27, 56, 57], hypoxic conditions [58, 59], or other growth factors such as TGFβ3 [60, 61], IGF-1 [62] and FGF2 [62, 63] might enhance tissue generation relative to the non-mechanical loading, normoxic conditions and TGFβ1 used in this current study. Sox9 expression may also indicate the beginning of a shift towards a more “chondrocyte-like” phenotype that is characteristic of the avascular region [64, 65, 66].
The in vitro model developed here demonstrated the potential for cell seeded GelMA to integrate with native tissues. The open structure of the GelMA scaffold mimicking native collagen fiber bundles may have also encouraged integration. Pabbruwe et al. [18] seeded MSC into collagen scaffolds and implanted into ovine meniscus explants. Better integration was seen with scaffolds when the open/spongy structure was adjacent to the tissue. As discussed, the neotissues formed on GelMA may be more reminiscent of repair in the vascular zone of meniscus. Transplanting vascular zone tissue into avascular zone defects was shown to better integrate with the surrounding avascular tissue in comparison to re-implanted avascular zone tissue [50]. Combining GelMA with other biocompatible materials, like fibrin glue, may further promote defect repair. Synovial tissue grafts were implanted in to human meniscal lesions and were found to integrate better compared to fibrin glue in terms of cell infiltration and improved tissue formation in the defect [67]. Therefore synoviocytes might be useful as they are more readily available than meniscal cells.
The use of the DMD projection stereolithography system allowed for the formation of 3D GelMA scaffolds that featured aligned gelatin fibers mimicking the collagen bundles of native meniscal tissue. In contrast to other 3D patterning approaches, DMD projection printing offers advantages in speed, flexibility, and scalability. Generally, the use of photopolymer-based approaches allows for complex patterns that may be difficult to construct using cast-molding and electrospinning. While 3D plotting can offer fabrication of sophisticated architectures, the approach is often limited by its slow processing speed and the low mechanical strength of the final scaffolds [21]. The micromirror array allows for the use of rapidly interchangeable photomasks during the layer-by-layer fabrication process, providing more rapid construction of the patterned substrates. This ability to generate a complete layer in one simultaneous exposure confers improved scalability to the projection stereolithography platform. Additionally, as each layer is created in a discrete step with replenishment of the prepolymer, a multi-layered composite 3D structure can be created easily by exchanging the prepolymer composition at various points. The projection printing method is limited mainly by its lower resolution (about 5 microns) when compared to 2-photon polymerization methods as well as by the post-curing steps that may be necessary to provide additional mechanical integrity. Because polymerization of each additional layer further irradiates the preceding layers, cumulative exposure and thereby crosslinking density may vary throughout the layers if the prepolymer solution is not properly rinsed and replenished with each step. Additionally, not all biopolymers may be amenable to the modifications necessary to make them photopolymerizable [15].
Despite the favorable interaction of human meniscus cells with the GelMA scaffolds (low toxicity and neo-tissue formation), we noted a few less-favorable outcomes. A promising integration between the neo-tissue and the native meniscus tissue was observed in the ex vivo defect model, yet the relatively weak tensile mechanical properties of the existing GelMA scaffolds may not be suitable to withstand the mechanical forces in the in vivo knee environment. The stiffness of the DMD fabricated scaffold was lower than reported for solid GelMA, reflecting the effect of the spaces between the “logs” [45]. The stiffness to withstand clinically relevant forces is much higher (~150kPA) and not likely to be achievable with GelMA [68]. To overcome this variable, other hydrogels possessing tunable mechanical properties such as polyethylene glycol (PEG) [30, 49, 69] or alginate [27] could be used employing the DMD-PSL system. We also noted an issue with uniform cell seeding. A majority of seeded cells remained on the surface of the GelMA construct. While using projected printing technologies described here, it would be conceivable to accurately incorporate a chemo-attractant molecules, like PDGF [70] into the internal layers of the scaffold to encourage better scaffold cell infiltration.
Conclusions
In summary, we have demonstrated the utility of using DMD-PSL using GelMA for rapid production of organized scaffolds that emulate meniscus collagen bundles, which directed cell alignment and supported the development of viable neo-meniscus tissue in vitro. Moreover, we show the promise of using these scaffolds in repairing meniscus lesions in an ex vivo organ culture model. Scaling-up of this approach may be useful to repair larger defects.
Supplementary Material
Measurement of Young’s modulus. Overview of system used measure the tensile properties of the GelMA scaffolds. The GelMA constructs were glued between two coverslips and the upper coverslip was attached to a steel plunger having a flat surface acted as the actuator for pulling the scaffolds. LabVIEW (National Instruments) software was employed for movement control and data acquisition on a laptop. The gel height was measured using electronic calipers. The force to pull the gel to breaking was monitored and recorded. Young’s modulus was calculated as previously described [44].
Acknowledgments
Funding provided by Donald and Darlene Shiley, California Institute of Regenerative Medicine (TR1-01216), National Institutes of Health (P01 AG007996) and R01EB012597, and National Science Foundation (CMMI-1120795). Additional funding for Peter H Chung was provided by a National Science Foundation Graduate Research Fellowship (DGE-1144086). We greatly appreciate technical assistance by Jin Woo Lee, Keith Yu, and Samuel Huang (production of GelMA scaffolds), Jihye Baek (SEM imaging), Xian Chen and Sujata Sovani (TNBS assay, cell cultures, IHC and gene expression analyses), Jonathan Netter (assistance with mechanical testing), Margaret Chadwell (histology), and Judy Blake (manuscript formatting and copyediting).
Footnotes
Author Contributions
SPG, SC and DDD orchestrated overall experimental design, SPG and DDD wrote the manuscript in close collaboration with the other authors. SPG designed and directed cell culture studies and analyses. SC, PS and P Chung designed and directed construction of the GelMA scaffolds. P Chen designed and conducted the mechanical testing. All authors discussed the results and approved the final version of the manuscript.
Competing Financial Interests statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shawn P Grogan, Email: Grogan.Shawn@scrippshealth.org, Shiley Center for Orthopaedic Research and Education at Scripps Clinic, 11025 North Torrey Pines Road, Suite 200, La Jolla, CA 92037, Phone: +1 (858) 332 0609, Fax: +1 (858) 332 0669.
Peter H Chung, Email: peterchung@ucsd.edu, Department of NanoEngineering, University of California, San Diego, Atkinson Hall, Room 2314, 9500 Gilman Drive #0448, San Diego, CA, 92093, Phone: +1 (858) 822 7856, Fax: +1 (858) 534 9553.
Pranav Soman, Email: psoman@ucsd.edu, Department of NanoEngineering, University of California, San Diego, Atkinson Hall, Room 2314, 9500 Gilman Drive #0448, San Diego, CA, 92093, Phone: +1 (858) 822, 7856 Fax: +1 (858) 534 9553.
Peter Chen, Email: Chen.Peter@scrippshealth.org, Bioengineering Department, University of California, San Diego, 9500 Gilman Drive, San Diego, CA, 92093, Phone: +1 (858) 534 4218, Fax: +1 (858) 534 5722.
Martin K Lotz, Email: mlotz@scripps.edu, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10555 North Pines Road, MEM-161, La Jolla, CA 92037, Phone: +1 (858) 784 8960, Fax: +1 (858) 784 2744.
Shaochen Chen, Email: chen168@ucsd.edu, Department of NanoEngineering, University of California, San Diego, Atkinson Hall, Room 2314, 9500 Gilman Drive #0448, San Diego, CA, 92093, Phone: +1 (858) 822 7856, Fax: +1 (858) 534 9553.
Darryl D D’Lima, Email: Dlima.Darryl@scrippshealth.org, Shiley Center for Orthopaedic Research and Education at Scripps Clinic, 11025 North, Torrey Pines Road, Suite 200, La Jolla, CA 92037, Phone: +1 (858) 332 0166, Fax: +1 (858) 332 0669.
References
- 1.Garrett WE, Jr, Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88:660–7. doi: 10.2106/JBJS.E.01208. [DOI] [PubMed] [Google Scholar]
- 2.Fetzer GB, Spindler KP, Amendola A, Andrish JT, Bergfeld JA, Dunn WR, et al. Potential market for new meniscus repair strategies: evaluation of the MOON cohort. J Knee Surg. 2009;22:180–6. doi: 10.1055/s-0030-1247746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford GM, Hegmann KT, White GL, Jr, Holmes EB. Associations of body mass index with meniscal tears. Am J Prev Med. 2005;28:364–8. doi: 10.1016/j.amepre.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillquist J, Hamberg P, Lysholm J. Endoscopic partial and total meniscectomy. A comparative study with a short term follow up. Acta Orthop Scand. 1982;53:975–9. doi: 10.3109/17453678208992857. [DOI] [PubMed] [Google Scholar]
- 7.Englund M, Roos EM, Roos HP, Lohmander LS. Patient-relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology (Oxford) 2001;40:631–9. doi: 10.1093/rheumatology/40.6.631. [DOI] [PubMed] [Google Scholar]
- 8.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 9.Barrett GR, Field MH, Treacy SH, Ruff CG. Clinical results of meniscus repair in patients 40 years and older. Arthroscopy. 1998;14:824–9. doi: 10.1016/s0749-8063(98)70018-0. [DOI] [PubMed] [Google Scholar]
- 10.Reguzzoni M, Manelli A, Ronga M, Raspanti M, Grassi FA. Histology and ultrastructure of a tissue-engineered collagen meniscus before and after implantation. J Biomed Mater Res B Appl Biomater. 2005;74:808–16. doi: 10.1002/jbm.b.30314. [DOI] [PubMed] [Google Scholar]
- 11.Ronga M, Grassi FA, Manelli A, Bulgheroni P. Tissue engineering techniques for the treatment of a complex knee injury. Arthroscopy. 2006;22:576, e1–3. doi: 10.1016/j.arthro.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 12.Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79:1770–7. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Zaffagnini S, Giordano G, Vascellari A, Bruni D, Neri MP, Iacono F, et al. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg Sports Traumatol Arthrosc. 2007;15:175–83. doi: 10.1007/s00167-006-0144-4. [DOI] [PubMed] [Google Scholar]
- 14.Kamimura T, Kimura M. Repair of horizontal meniscal cleavage tears with exogenous fibrin clots. Knee Surg Sports Traumatol Arthrosc. 2011;19:1154–7. doi: 10.1007/s00167-011-1404-5. [DOI] [PubMed] [Google Scholar]
- 15.Freymann U, Endres M, Neumann K, Scholman HJ, Morawietz L, Kaps C. Expanded human meniscus-derived cells in 3-D polymer-hyaluronan scaffolds for meniscus repair. Acta Biomater. 2012;8:677–85. doi: 10.1016/j.actbio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–32. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- 17.Weinand C, Peretti GM, Adams SB, Jr, Randolph MA, Savvidis E, Gill TJ. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg. 2006;126:599–605. doi: 10.1007/s00402-005-0100-7. [DOI] [PubMed] [Google Scholar]
- 18.Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31:2583–91. doi: 10.1016/j.biomaterials.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med. 2004;32:146–58. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 20.Sandmann GH, Eichhorn S, Vogt S, Adamczyk C, Aryee S, Hoberg M, et al. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res A. 2009;91:567–74. doi: 10.1002/jbm.a.32269. [DOI] [PubMed] [Google Scholar]
- 21.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–41. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–85. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 24.Kim HN, Kang DH, Kim MS, Jiao A, Kim DH, Suh KY. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng. 2012;40:1339–55. doi: 10.1007/s10439-012-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman JJ, Desai TA. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng. 2006;34:89–101. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- 26.Subia B, Kundu J, Kundu SC. Tissue Engineering. InTech; 2010. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. [Google Scholar]
- 27.Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011;17:1445–55. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–30. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 29.Zervantonakis IK, Kothapalli CR, Chung S, Sudo R, Kamm RD. Microfluidic devices for studying heterotypic cell-cell interactions and tissue specimen cultures under controlled microenvironments. Biomicrofluidics. 2011;5:13406. doi: 10.1063/1.3553237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fozdar DY, Soman P, Lee JW, Han LH, Chen S. Three-Dimensional Polymer Constructs Exhibiting a Tunable Negative Poisson’s Ratio. Adv Funct Mater. 2011;21:2712–20. doi: 10.1002/adfm.201002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han LH, Mapili G, Chen SC, Roy K. Projection Microfabrication of Three-Dimensional Scaffolds for Tissue Engineering. Journal of Manufacturing Science and Engineering. 2008;130:021005. [Google Scholar]
- 32.Mapili G, Lu Y, Chen S, Roy K. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75:414–24. doi: 10.1002/jbm.b.30325. [DOI] [PubMed] [Google Scholar]
- 33.Suri S, Han LH, Zhang W, Singh A, Chen S, Schmidt CE. Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomed Microdevices. 2011;13:983–93. doi: 10.1007/s10544-011-9568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang AP, Qu X, Soman P, Hribar KC, Lee JW, Chen S, et al. Rapid Fabrication of Complex 3D Extracellular Microenvironments by Dynamic Optical Projection Stereolithography. Adv Mater. 2012;24:4266–70. doi: 10.1002/adma.201202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–41. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
- 37.Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, et al. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941–51. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habeeb AF. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328–36. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 39.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–84. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Grogan SP, Aklin B, Frenz M, Brunner T, Schaffner T, Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198:5–13. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 41.Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16:398–404. doi: 10.1016/j.knee.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Hirvonen J, Helminen HJ, et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903–9. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 45.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauvin R, Chen YC, Lee JW, Soman P, Zorlutuna P, Nichol JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–34. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramon-Azcon J, Ahadian S, Obregon R, Camci-Unal G, Ostrovidov S, Hosseini V, et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip. 2012;12:2959–69. doi: 10.1039/c2lc40213k. [DOI] [PubMed] [Google Scholar]
- 48.Qi H, Du Y, Wang L, Kaji H, Bae H, Khademhosseini A. Patterned differentiation of individual embryoid bodies in spatially organized 3D hybrid microgels. Adv Mater. 2010;22:5276–81. doi: 10.1002/adma.201002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curley JL, Jennings SR, Moore MJ. Fabrication of micropatterned hydrogels for neural culture systems using dynamic mask projection photolithography. J Vis Exp. 2011 doi: 10.3791/2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials. 2004;25:2047–53. doi: 10.1016/j.biomaterials.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 51.Cheung HS. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect Tissue Res. 1987;16:343–56. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- 52.Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983;158:265–70. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- 53.Gao J. Immunolocalization of types I, II, and X collagen in the tibial insertion sites of the medial meniscus. Knee Surg Sports Traumatol Arthrosc. 2000;8:61–5. doi: 10.1007/s001670050013. [DOI] [PubMed] [Google Scholar]
- 54.Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res. 2005;23:142–9. doi: 10.1016/j.orthres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 55.de Mulder EL, Hannink G, Giele M, Verdonschot N, Buma P. Proliferation of meniscal fibrochondrocytes cultured on a new polyurethane scaffold is stimulated by TGF-ss. J Biomater Appl. 2011 doi: 10.1177/0885328211417317. [DOI] [PubMed] [Google Scholar]
- 56.Kanazawa T, Furumatsu T, Hachioji M, Oohashi T, Ninomiya Y, Ozaki T. Mechanical stretch enhances COL2A1 expression on chromatin by inducing SOX9 nuclear translocalization in inner meniscus cells. J Orthop Res. 2012;30:468–74. doi: 10.1002/jor.21528. [DOI] [PubMed] [Google Scholar]
- 57.Petri M, Ufer K, Toma I, Becher C, Liodakis E, Brand S, et al. Effects of perfusion and cyclic compression on in vitro tissue engineered meniscus implants. Knee Surg Sports Traumatol Arthrosc. 2012;20:223–31. doi: 10.1007/s00167-011-1600-3. [DOI] [PubMed] [Google Scholar]
- 58.Adesida AB, Grady LM, Khan WS, Millward-Sadler SJ, Salter DM, Hardingham TE. Human meniscus cells express hypoxia inducible factor-1alpha and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Res Ther. 2007;9:R69. doi: 10.1186/ar2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saliken DJ, Mulet-Sierra A, Jomha NM, Adesida AB. Decreased hypertrophic differentiation accompanies enhanced matrix formation in co-cultures of outer meniscus cells with bone marrow mesenchymal stromal cells. Arthritis Res Ther. 2012;14:R153. doi: 10.1186/ar3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ionescu LC, Lee GC, Huang KL, Mauck RL. Growth factor supplementation improves native and engineered meniscus repair in vitro. Acta Biomater. 2012;8:3687–94. doi: 10.1016/j.actbio.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal BB, Park SH, Gil ES, Kaplan DL. Stem cell-based meniscus tissue engineering. Tissue Eng Part A. 2011;17:2749–61. doi: 10.1089/ten.tea.2011.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox DB, Warnock JJ, Stoker AM, Luther JK, Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res Vet Sci. 2010;88:326–32. doi: 10.1016/j.rvsc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009;16:1363–72. doi: 10.1038/gt.2009.91. [DOI] [PubMed] [Google Scholar]
- 64.Furumatsu T, Kanazawa T, Yokoyama Y, Abe N, Ozaki T. Inner meniscus cells maintain higher chondrogenic phenotype compared with outer meniscus cells. Connect Tissue Res. 2011;52:459–65. doi: 10.3109/03008207.2011.562061. [DOI] [PubMed] [Google Scholar]
- 65.Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, et al. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525–35. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melrose J, Smith S, Cake M, Read R, Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225–35. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 67.Ochi M, Mochizuki Y, Deie M, Ikuta Y. Augmented meniscal healing with free synovial autografts: an organ culture model. Arch Orthop Trauma Surg. 1996;115:123–6. doi: 10.1007/BF00434537. [DOI] [PubMed] [Google Scholar]
- 68.Li S-T, Rodkey WG, Yuen D, Hansen P, Steadman JR. Type I collagen-based template for meniscus regeneration. In: Lewandrowski K-U, Wise DL, Trantolo DJ, Gresser JD, Yaszemski MJ, Altobelli DE, editors. Tissue Engineering and Biodegradable Equivalents Scientific and Clinical Applications. New York: Marcel Dekker, Inc; 2002. pp. 237–66. [Google Scholar]
- 69.Xia C, Fang NX. 3D microfabricated bioreactor with capillaries. Biomed Microdevices. 2009;11:1309–15. doi: 10.1007/s10544-009-9350-4. [DOI] [PubMed] [Google Scholar]
- 70.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–12. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurement of Young’s modulus. Overview of system used measure the tensile properties of the GelMA scaffolds. The GelMA constructs were glued between two coverslips and the upper coverslip was attached to a steel plunger having a flat surface acted as the actuator for pulling the scaffolds. LabVIEW (National Instruments) software was employed for movement control and data acquisition on a laptop. The gel height was measured using electronic calipers. The force to pull the gel to breaking was monitored and recorded. Young’s modulus was calculated as previously described [44].



