Abstract
Background
Interleukin-8 (IL8) receptors IL8RA and IL8RB on neutrophil membranes bind to IL8 and direct neutrophil recruitment to sites of inflammation, including acutely injured arteries. This study tested whether administration of IL8RA- and/or IL8RB-transduced rat aortic endothelial cells (ECs) accelerates adhesion of ECs to the injured surface, thus suppressing inflammation and neointima formation in balloon injured rat carotid arteries. We tested the hypothesis that targeted delivery of ECs by overexpressing IL8RA and RB receptors prevents inflammatory responses and promotes structural recovery of arteries following endoluminal injury.
Methods and Results
Young adult male rats received balloon injury of the right carotid artery and were transfused i.v. with ECs (total 1.5×106 cells at 1, 3, and 5 hrs post injury) transduced with adenoviral vectors carry IL8RA, IL8RB, IL8RA/RB (dual transduction) genes, AdNull (empty vector), or vehicle (no EC transfusion). ECs overexpressing IL8Rs inhibited pro-inflammatory mediators expression significantly (by 60–85%) and reduced infiltration of neutrophils and monocytes/macrophages into injured arteries at 1 day post injury, as well as stimulating a 2-fold increase in re-endothelialization at 14 days post injury. IL8RA-EC, IL8RB-EC, and IL8RA/RB-EC treatment reduced neointima formation dramatically (by 80%, 74%, and 95%) at 28 days post injury.
Conclusions
ECs with overexpression of IL8RA and/or IL8RB mimic the behavior of neutrophils that target and adhere to injured tissues, preventing inflammation and neointima formation. Targeted delivery of ECs to arteries with endoluminal injury provides a novel strategy for the prevention and treatment of cardiovascular disease.
Keywords: cell therapy, IL8 receptors, endothelial cells, restenosis, inflammation, reendothelialization
Introduction
The endothelium is a dynamic component of the cardiovascular system that plays an important role in health and disease. It is not only a barrier between the circulating blood and vascular smooth muscle cells (VSMCs), but also the source of mediators that regulate vascular tone and growth, platelet function and coagulation.1 Endothelial damage/dysfunction in vivo leads to loss of its anti-aggregative, anti-thrombotic, anti-inflammatory and anti-VSMC activation/growth properties.2,3
Neutrophils form the early line of defense against tissue injury. Neutrophils migrate to injured tissue in response to the chemoattractant interleukin-8 (IL8) that is expressed and released in large amounts by injured or infected tissues. IL8 binds to selective IL8RA and IL8RB receptors on the neutrophil surface.4,5 Activated IL8RA and/or IL8RB induce expression of chemotactic mediators that trigger local inflammation. Neutrophils are the main leukocyte subset that interacts with the damaged endothelium, triggers the initial pro-inflammatory response, and facilitates the influx of other classes of inflammatory cells e.g. monocytes/macrophages and T cells in the setting of acute vascular injury.6,7
Using the rat carotid artery balloon injury model, we tested the hypothesis that targeted delivery of endothelial cells (ECs) by overexpressing IL8RA and RB receptors prevents inflammatory responses and promotes structural recovery of arteries following endoluminal injury. Rat aortic ECs were transduced with adenoviral vectors carrying neutrophil IL8RA and RB receptors and intravenously (i.v.) transfused into rats with balloon injury of the common carotid arteries. We demonstrated that administration of ECs equipped with this homing device mimicked the behavior of neutrophils at the site of vascular injury, thus attenuating the infiltration of neutrophils and suppressing inflammation. We further demonstrated that ECs overexpressing IL-8 receptors accelerated re-endothelialization and inhibited neointima formation in injured arteries.
Methods
Animals and Procedures
Twelve-week-old male Sprague-Dawley rats were obtained from Charles River Breeding Laboratories and maintained at constant humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (6 A.M. to 6 P.M.) and fed a standard rat pellet diet ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Rats were weighed before and 2 or 4 wks after carotid injury.
One day before carotid injury, rats were anesthetized with 80 mg/kg ketamine and 5 mg/kg xylazine and subjected to cannulation of a femoral vein.8 On the next day, rats were subjected to balloon injury of the right carotid artery.9 The contralateral uninjured left carotid artery served as a control.
Plasmid and Adenoviral Vector Generation
ECs that overexpress IL8RA and/or IL8RB were generated using adenoviral vectors that contain human IL8RA and/or IL8RB cDNAs and the green fluorescent protein (GFP) gene using the AdEasy™ Adenoviral Vector System (Stratagene)10 as summarized in Online Supplemental Material and Supplemental Figure 1.
In Vitro Characterization of Rat Aortic ECs Transduced with IL8RA-GFP, IL8RB-GFP or AdNull-GFP Adenoviral vectors
Rat aortic endothelial cells (RAECs) and the EC growth medium MCDB-131C were purchased from VEC Technologies (Cat# RAEC/T-75, Cat# MCDB-131C). ECs were cultured to 80% confluence and transduced with the adenoviruses containing IL8RA-GFP, L8RB-GFP genes or AdNull-GFP (empty adenoviral vector control). More than 80% ECs became green 48 hrs after transduction. To examine the expression and distribution of IL8RA, IL8RB, or GFP in transduced ECs, cells were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton X-100 in PBS and then stained with selective primary antibodies against IL8RA (Cat# ab60254, Abcam) or IL8RB (Cat# ab24963, Abcam) and a Texas Red-labeled goat anti-mouse IgG secondary antibody (Cat# 115-075-003, Jackson ImmunoResearch Lab). After additional nuclear staining with DAPI (4’,6-diamidino-2-phenylindole, Sigma), ECs were mounted for confocal fluorescent microscopic analysis with a computerized Zeiss-Axioskop system.
Separate groups of ECs transduced with both IL8RA and IL8RB (IL8RA/RB) were used to examine the effects of overexpression of IL8RA and IL8RB on cell proliferation, migration and apoptosis in vitro. Untransduced ECs (Vehicle-EC) and ECs transduced with AdNull vectors were used as controls. Proliferative activity was assessed using direct cell counts at 1, 4, 9 and 24 hrs after cell seeding (cells reached 100% confluence at > 30 hrs culture). Migratory activity was assessed using Boyden chamber technique as described previously11 and in the Online Supplemental Material. Apoptotic activity was assessed using the TUNEL assay (Cell Technology).
To confirm that IL8RA-, IL8RB- and IL8RA/RB-ECs can functionally mimic neutrophil binding to IL8, the ability of transduced, ECs to attach to activated endothelial monolayers and to compete with neutrophils for binding to the monolayers was assessed. In the first experiment, the attachment of transduced ECs to endothelial monolayers was assessed. Endothelial monolayers in 24-well plates were treated with TNF-α (10 ng/ml) for 16 hrs before IL8RA, IL8RB, IL8RA/RB or AdNull ECs (1.0 × 106 cells) were added. At 15, 30, 45, and 60 min, ECs were washed 3 times with PBS and GFP-labeled ECs adherent to the activated monolayers were counted. In the second experiment, activated neutrophils were prepared as described previously11 and in the Online Supplemental Material. Various concentrations (0.5, 1.0 and 1.5 × 106 cells) of IL8RA/RB-ECs (GFP labeled) were applied to TNF-α (10 ng/ml) pre-treated endothelial monolayers. Neutrophils (1.0 × 107 cells, pre-labeled with CellTracker CM-Dil in red color) were added 30 min after the application of IL8RA/RB-ECs and incubated for an additional 1 hr before cell (red neutrophils and green ECs) counting.
In Vivo Transfusion Regimen
RAECs transduced with AdNull-GFP, IL8RA-GFP, IL8RB-GFP, or co-transduced with IL8RA-GFP and IL8RB-GFP were cultured in 100-mm culture dishes until > 80% cells expressed GFP (1.5 × 106 cells per dish). Cells were washed with 0.9% saline, collected with a cell scraper, and dispersed by gentle pipetting, then concentrated with centrifugation at 100× g and resuspended in 1.5 ml normal saline. Each rat was transfused three times with ECs (0.5 × 106 cells/500 µl) through a femoral venous catheter at 1, 3, and 5 hrs after carotid artery injury. Control rats received a corresponding volume of normal saline (Vehicle-control). Following carotid artery injury, rats (n=6/group) were randomly divided into five groups which received 1) saline (Vehicle-control), 2) ECs transduced with AdNull-GFP (AdNull-EC), 3) ECs transduced with IL8RA-GFP (IL8RA-EC), 4) ECs transduced with IL8RB-GFP (IL8RB-EC), or 5) ECs co-transduced with IL8RA-GFP and IL8RB-GFP (IL8RA/RB-EC).
Effects of IL8RA-, IL8RB-, IL8RA/RB-, and AdNull-ECs on Inflammation and Remodeling in Injured Arteries
Rats were sacrificed for assessment of attachment of transfused ECs to the injured endoluminal surface by confocal microscopic analysis of GFP cells (30 min post EC transfusion. Pro-inflammatory mediator mRNA expression was measured by real-time quantitative RT-PCR (24 hrs post EC transfusion). Infiltration of neutrophils (MPO staining) and monocyte/macrophages (ED-1 staining),12 as well as transfused ECs (GFP staining) was assessed by immunohistochemistry (24 hrs post EC transfusion), and re-endothelialization was measured by Evans blue staining (14 days post EC transfusion). Neointima formation was determined by quantitative morphometric analysis of elastin stained arteries (28 days post EC transfusion). The contralateral uninjured left common carotid arteries were used as controls.
Real-time Quantitative RT-PCR Analysis of Inflammatory Mediators
RNA was extracted from arteries, reverse transcribed to cDNA and amplified by PCR with specific primers (Online Supplemental Table 1) for tumor necrosis factor-alpha (TNF-α), monocyte chemotactic protein-1 (MCP-1), P-selectin; cytokine induced neutrophil chemoattractant-2-beta (CINC-2β, equivalent to human IL-8), vascular cell adhesion molecule 1 (VCAM-1), interleukin-1 beta (IL-1β), IL-6, and IL-10, and quantified using the iCycler (Applied Biosystems).13 Levels of specific mRNAs were normalized using ribosomal protein S9 mRNA, since expression of this housekeeping gene has been shown to remain stable in the rat carotid artery injury model.13
Immunohistochemical Analysis of Neutrophil, Monocyte/Macrophage, and Transfused EC Infiltration into Injured Carotid Arteries
One day after EC transfusion, formalin perfusion-fixed carotid arteries were harvested and embedded in paraffin. The avidin-biotin-peroxidase immunohistochemical technique was use to detect GFP-labeled IL8RA, IL8RB, IL8RA/RB, and AdNull transduced ECs, neutrophils and monocytes/macrophages in paraffin-embedded sections of carotid arteries using a Vector Laboratories kit (Biotechnology).11,14 Transfused ECs, neutrophils and monocytes/macrophages were recognized by specific primary antibodies against GFP, myeloperoxidase (MPO) (Santa Cruz Biotechnology) and ED1 (Serotec), respectively.
Evans Blue Staining for Re-Endothelialization
Balloon-injured carotid arteries harvested at 14 days post-injury were examined for re-endothelialization.15 Thirty minutes prior to sacrifice, rats received an intravenous injection of Evans blue dye (0.5 ml of 0.5% in saline, Fisher, Cat# 23860) and then perfused with 500 ml of 1X PBS. Carotid arteries were harvested and photographed, and the area of denuded endothelium was identified by blue staining. The ratio of area stained blue (no endothelium) to total common carotid artery length (from aortic arch to bifurcation of internal and external carotid arteries) was calculated.
Morphometric Analysis of Neointima Formation
Neointima to media ratios were calculated from 5 µm cross sections of the injured carotid arteries at 28 days post injury. Morphometric analysis of representative cross-sectional photomicrographs of injured carotid arteries was performed with a computer-based Bioquant II Morphometric system. At least 3 elastin-stained sections from the middle third of each vessel were examined, and the measurements were averaged for statistical analysis.16 The cross-sectional surface areas of the vessel within the external elastic lamina (EEL area), within the internal elastic lamina (IEL area), and within the lumen (lumen area) were measured. Neointima formation in the injured artery was expressed as the neointima (IEL area - Lumen area)-to-media (EEL area – IEL area) ratio.
Statistical Analysis
In each in vivo experiment, rats were age matched to minimize individual differences. Results were expressed as means±SEM. Statistical analysis was carried out using the SigmaStat statistical package (Version 3.5). The primary statistical test was two way and one way analysis of variance (ANOVA). When the overall F test result of ANOVA was significant, a multiple-comparison Tukey test was applied. Student’s t-test was used in two-mean comparisons. Differences were reported as significant when p values were <0.05.
Results
Adenoviral Transduction Increases IL8RA and IL8RB Expression in ECs, but Does Not Attenuate EC Proliferation, Migration or Apoptosis
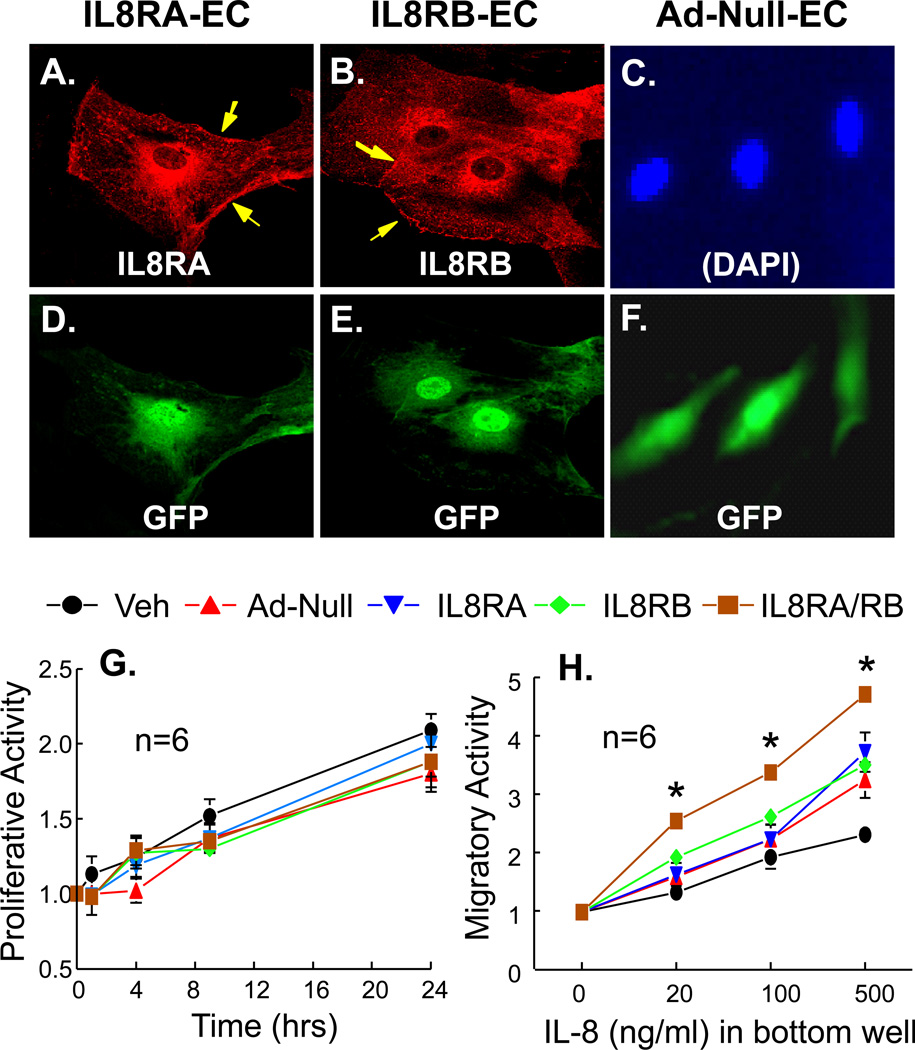
Confocal fluorescence microscopic analysis showed that IL8RA and IL8RB were over-expressed in the endoplasmic reticulum and on the cell membrane, but not in the nucleus of the IL8RA and IL8RB transduced ECs (Figures 1A-1C). GFP was expressed in the nuclei and perinuclear endoplasmic reticulum in IL8RA, IL8RB and AdNull transduced ECs (Figures 1D-1F). IL8RA and IL8RB were undetectable when Null-ECs were probed with anti-IL8RA or anti-IL8RB antibodies (data not shown), indicating that endogenous IL8RA and IL8RB levels were very low in these cells.
Figure 1.
(A-F) Confocal fluorescence micrographs showing over-expression of IL8RA or IL8RB in rat aortic endothelial cells (ECs). ECs were transduced with adenoviral vectors carrying (A, D) IL8RA, (B, E) IL8RB genes, or (C, F) AdNull (EC transduced with the empty adenoviral vector) with a green fluorescent protein (GFP) marker. Arrow heads indicate expression of IL8RA or IL8RB on the EC membrane. The expression of IL8RA, IL8RB, and GFP was controlled by separate CMV promoters. (D, E, F) Immunoflurorescence stain showing expression of GFP in the same ECs. (C) DAPI staining of the nuclei of cells in (F). (G) Proliferative activity (assessed by actual cell counts) of adenoviral transduced ECs over-express IL8RA, IL8RB, or both RA and RB (IL8RA/RB). Vehicle (Veh) control is ECs without adenoviral transduction. Ad-Null control is ECs transduced with empty adenovirus. (H) Migratory activity (assessed by Boyden chamber technique for 12 hrs) of ECs over-express IL8RA, IL8RB or both IL8RA and IL8RB. The bottom wells contain various concentration of IL-8. Results are means±SEM, (n)=wells or chambers. Two way ANOVA was used to analyze the data in 1G and 1H. * p<0.05, IL8-RA/RB vs. other groups in panel H.
Adenoviral transduction did not alter baseline or IL8-stimulated EC proliferation, apoptosis or migration (comparison between AdNull-EC and Vehicle-EC [without adenoviral transduction]). There were no statistically significant differences in mean values of proliferative activity between experimental groups (Figure 1G). IL8 dose dependently (20–500 ng/ml) enhanced migration of vehicle-control, AdNull, IL8RA, IL8RB and IL8RA/RB ECs. IL8-stimulated cell migration was significantly greater in IL8RA/RB-EC (2 way ANOVA, p<0.05) compared to the other 4 experimental groups (Figure 1H). Baseline apoptotic activity was undetectable in all three cell types, and IL8 treatment did not alter apoptosis in any cell type (data not shown). Thus, overexpression of IL8RA and/or IL8RB did not attenuate basal or stimulated proliferation, migration or apoptosis of transduced ECs.
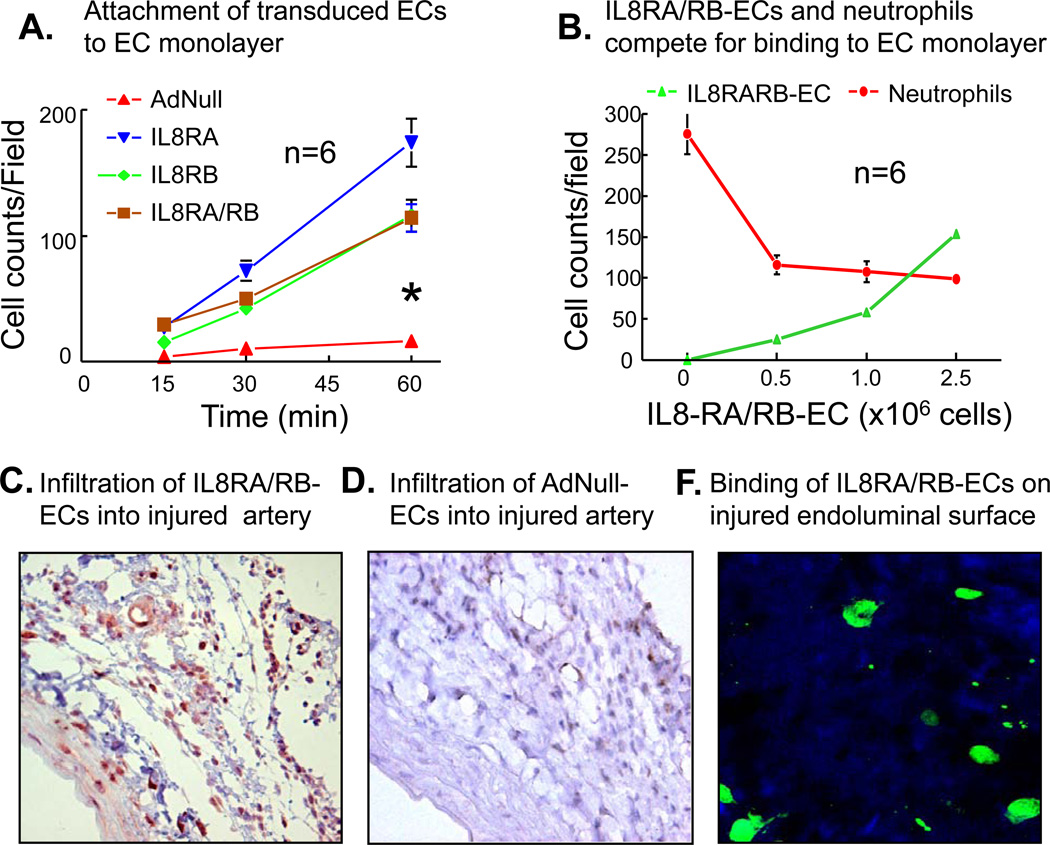
Attachment of IL8RA-, IL8RB- and IL8RA/RB-ECs to endothelial monolayers was time dependently greater than that of AdNull-EC (2 way ANOVA, p<0.05) (Figure 2A). Further, IL8RA/RB-EC dose dependently inhibited the binding of neutrophils to endothelial monolayers (Figure 2B). In an additional experiment, we showed that neutrophils could also inhibit the binding of IL8RA/RB-EC to endothelial monolayers (Online Supplemental Figure 2).
Figure 2.
(A) The attachment rates of IL8RA, IL8RB, and IL8RA/RB ECs to an in vitro endothelial monolayer were faster than that of ECs transduced with empty adenviral vector (AdNull-EC). Endothelial monolayer was stimulated with TNF-α (10 ng/ml) for 16 hrs before AdNull, IL8RA, IL8RB, or IL8RA/RB ECs (with GFP green color) applied to the monolayer. The asterisk indicates that the slope of the lines of IL8RA, IL-RB and IL8RA/RB ECs were steeper (p<0.05) than that of AdNull-ECs. The attachment rates were not statistically different among the IL8RA, IL8RB and IL8RA/RB groups. Results are means±SEM, n=wells/per time point. Two way ANOVA was used to analyze the data in 2A. (B) IL8RA/RB-ECs inhibited binding of neutrophils to in vitro endothelial monolayer in a dose dependent manner. Endothelial monolayer was stimulated with TNF-α (10 ng/ml) for 16 hrs before various concentrations of IL8RA/RB-ECs (GFP color) applied to the monolayer. Neutrophils (labeled with CellTracker CM-Dil, red color) were added 30 min after the application of IL8RA/RB-EC and incubated for additional 1 hr before cell (red and green color) counting in the same well. Results are means±SEM, n=wells/per group. (C) Representative GFP immuno-flurorescence stained micrograph (100X) showing accumulation of GFP-labeled IL8RA/RB-ECs in the injured rat carotid artery (especially in the adventitia and vasa vasorum) 24 hrs after balloon injury of the carotid artery and transfusion of IL8RA/RB-ECs. Transfused ECs were immunostained with GFP primary antibodies. (D) Representative GFP immuno-flurorescence stained micrograph (100X) of GFP-labeled AdNull-ECs in the injured rat carotid artery 24 hrs after injury and cell transfusion. (F) Representative confocal fluorescence micrographs (400X) showing GFP-labeled IL8RA/RB-ECs attached to the endoluminal surface of balloon injured rat carotid artery 30 min after transfusion of IL8RA/RB-ECs. Injured artery was opened longitudinally to expose the endoluminal surface and washed 3 times with PBS before the micrograph was taken.
GFP positive ECs accumulated in injured arteries (especially in adventitia and vasa vasorum) by 24 hrs after injury and IL8RA/RB-EC transfusion (0.5 × 106 cells, i.v.), indicating that ECs equipped IL8RA and IL8RB infiltrated into the injured arteries (Figure 2C). In rats transfused with AdNull-ECs, only a few GFP-positive ECs could be found in adventitia (Figure 2D). Further, Transfused IL8RA/RB-ECs attached to the endoluminal surface of carotid arteries as early as 30 min after balloon injury and EC transfusion (Figure 2F).
ECs Transduced with IL8RA, IL8RB and IL8RA/RB Inhibit Inflammation in Injured Carotid Artery
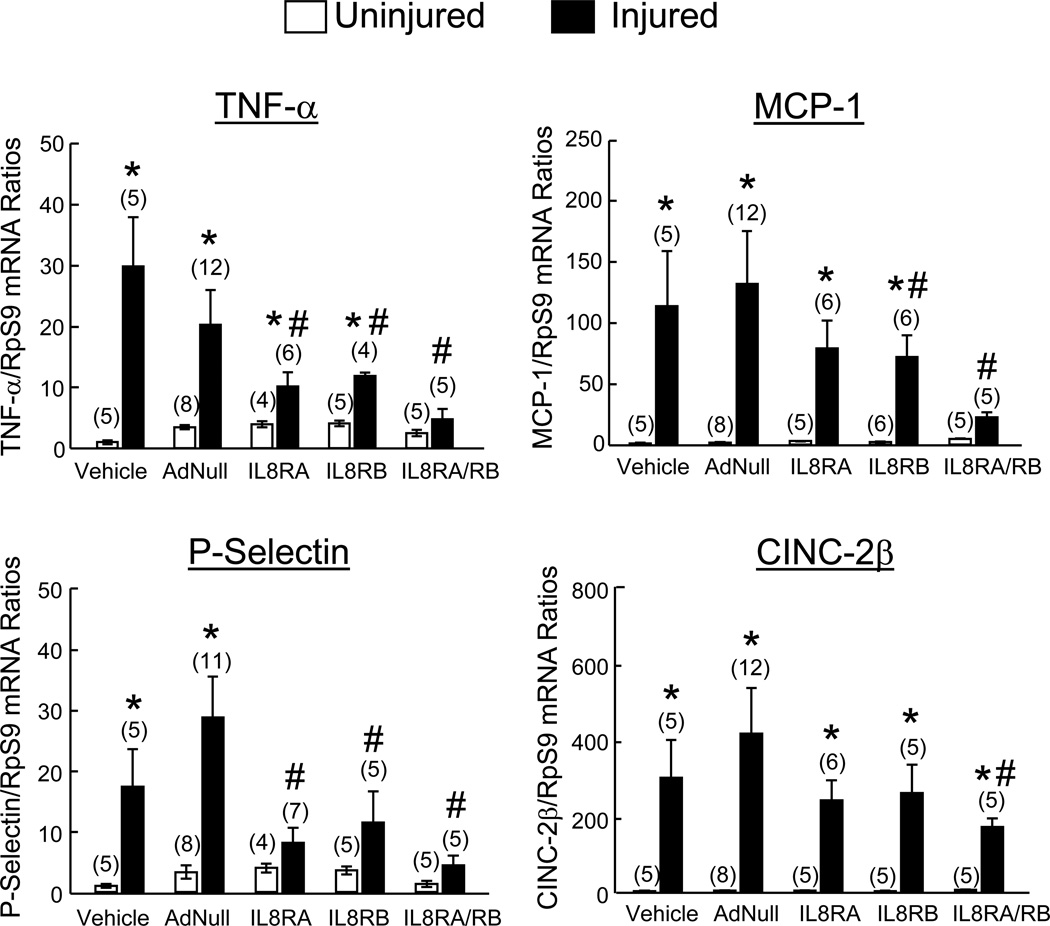
Real-time quantitative RT-PCR analysis of 24 hr injured and control uninjured carotid arteries from rats transfused with AdNull-ECs or vehicle (no EC transfusion) showed that all inflammatory mediators measured (TNF-α, MCP-1, P-Selectin, CINC-2β, VCAM-1, IL-1β, IL-6, and IL-10) were expressed at very low levels in uninjured vessels and increased markedly after injury (Figure 3). IL8RA-, IL8RB- and IL8RA/RB-EC transfusion resulted in significant reductions in mRNA levels of the inflammatory cytokine TNF-α, the adhesion molecule P-selectin, and the chemoattractants MCP-1 and CINC-2β compared to vehicle and AdNull-EC groups. The reductions of TNF-α, MCP-1, P-selectin and CINc-2β by IL8RA/RB-EC treatment were the greatest among the 3 groups. Expression of VCAM-1, IL-1β, IL-6, and IL-10 mRNA in injured carotid arteries was not altered by IL8RA/RB-EC treatment, and did not differ significantly from levels in injured arteries of and AdNull-EC and vehicle treated rats (data not shown).
Figure 3.
Effects of IL8RA, IL8RB, IL8RA/RB, or AdNull EC transfusion on mRNA expression of chemokines and adhesion molecules in injured and contralateral uninjured control arteries at 24 hrs post injury. Each rat received femoral venous transfusions of EC (0.5×106 cells/injection in 500 µl saline) at 1, 3, and 5 hrs post carotid artery injury. mRNA levels measured by real-time qRT-PCR were first normalized using ribosomal protein S9 (RpS9) mRNA to correct for differences in total RNA loading and then standardized to the mean value of the respective vehicle+uninjured group, which was assigned a value of 1. Vehicle group received balloon injury of the right carotid artery but did not receive EC transfusion. Results are means±SEM, (n)=number of rats. One way ANOVA was used to analyze the data. * p<0.05 vs. respective uninjured groups. # p<0.05 vs. respective Vehicle and AdNull-EC groups. TNF-α-tumor necrosis factor-alpha; MCP-1-monocyte chemotactic protein-1; CINC-2β-cytokine induced neutrophil chemoattractant-2-beta (equivalent to human IL-8).
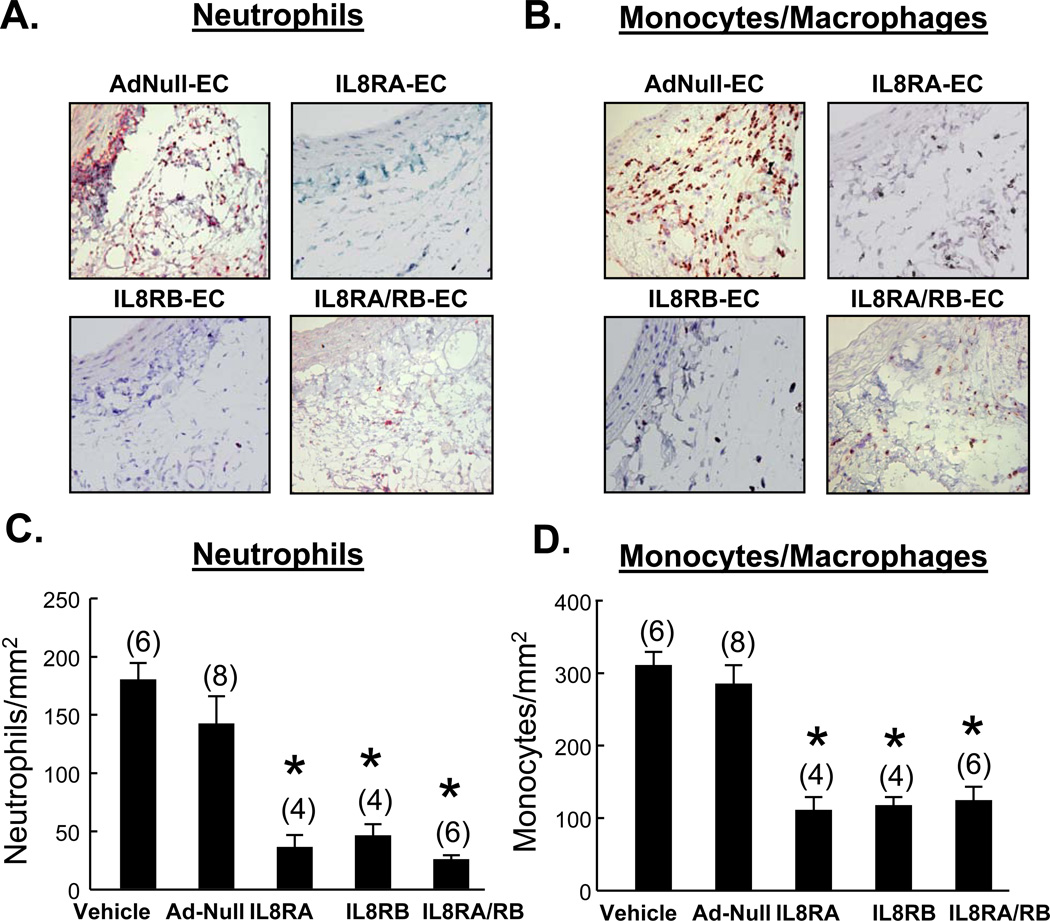
Immunohistochemical staining demonstrated large numbers of MPO+ neutrophils and ED1+ monocytes/macrophages in the adventitial domains of 24 hr injured arteries from vehicle treated and AdNull-EC treated rats; neutrophil and monocyte/macrophage numbers were greatly reduced by IL8RA-, IL8RB-, and IL8RA/RB-EC treatment (Figure 4). The number of neutrophils in adventitia of injured carotid artery was negatively correlated with the number of GFP-labeled ECs and positively correlated with the number of monocytes/macrophages (Online Supplemental Figure 3). Infiltrating neutrophils and monocytes/macrophages could not be detected in the media of injured arteries in any treatment group.
Figure 4.
Effects of IL8RA, IL8RB, IL8RA/RB, or AdNull EC transfusion on neutrophil and monocyte/macrophage infiltration into adventitia of injured carotid artery 24 hr after transfusion of ECs overexpressing IL8RA, IL8RB, or both IL8RA/RB. Neutrophils and monocytes/macrophages were immunostained with myeloperoxidase (MPO) and ED1 primary antibodies, respectively. (A and B) Representative micrographs showing the MPO+ and CD1+ cells in adventitia, respectively, and (C and D) Bar graphs showing neutrophil and monocyte/macrophage densities in adventitia of injured carotid arteries of rats transfused with AdNull, IL8RA, IL8RB, or Ad-IL8RA/RB ECs. Vehicle group received balloon injury but did not receive EC transfusion. Results are means±SEM, (n)=number of rats. One way ANOVA was used to analyze the data. * p<0.05 vs. respective Vehicle and AdNull-EC groups.
ECs Transduced with IL8RA/RB Accelerate Re-Endothelialization of Injured Carotid Artery
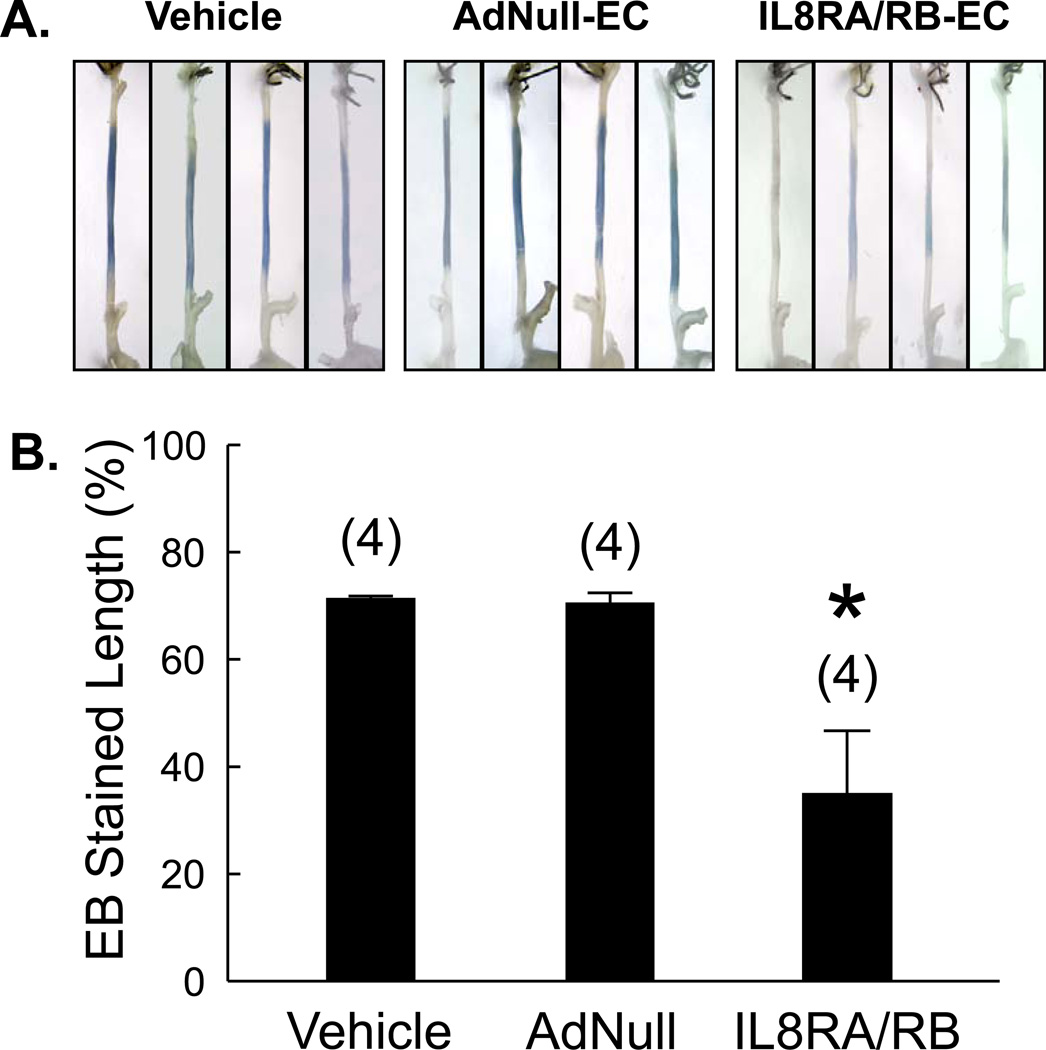
To elucidate the effects of IL8RA/RB-EC transfusion on re-endothelialization, Evans blue staining of 14 day injured and uninjured carotid arteries of rats transfused with IL8RA/RB-ECs, AdNull-ECs or vehicle (no EC transfusion) was performed. Arteries from rats treated with IL8RA/RB-ECs had smaller areas of blue staining relative to arteries from rats that received AdNull-ECs or vehicle (Figure 5A). Uninjured contralateral carotid arteries were virtually negative for Evans blue staining (data not shown). Quantitative assessment showed that transfusion with IL8-RA/RB-ECs resulted in a greater than 2-fold reduction in the denuded (blue) area at 14 days after the injury, indicating a ~2-fold increase in re-endothelialization at this time point (Figure 5B).
Figure 5.
Re-endothelialization of injured carotid arteries is promoted by IL8RA/RB-EC transfusion. (A) Isolated common carotid arteries from rats 2 wks post balloon injury. Rats were perfused with Evans blue (EB) dye prior to dissection. Common carotid arteries were cut from the aortic arch to the bifurcation of the internal and external carotid arteries. Intact endothelium excludes the EB dye. (B) Percentage of de-endothelialized carotid artery assessed by EB dye technique (length of blue region/length between aortic arch and bifurcation). Results are means±SEM, (n)=number of rats. One way ANOVA was used to analyze the data. * p<0.05 vs. both Vehicle and AdNull-EC groups.
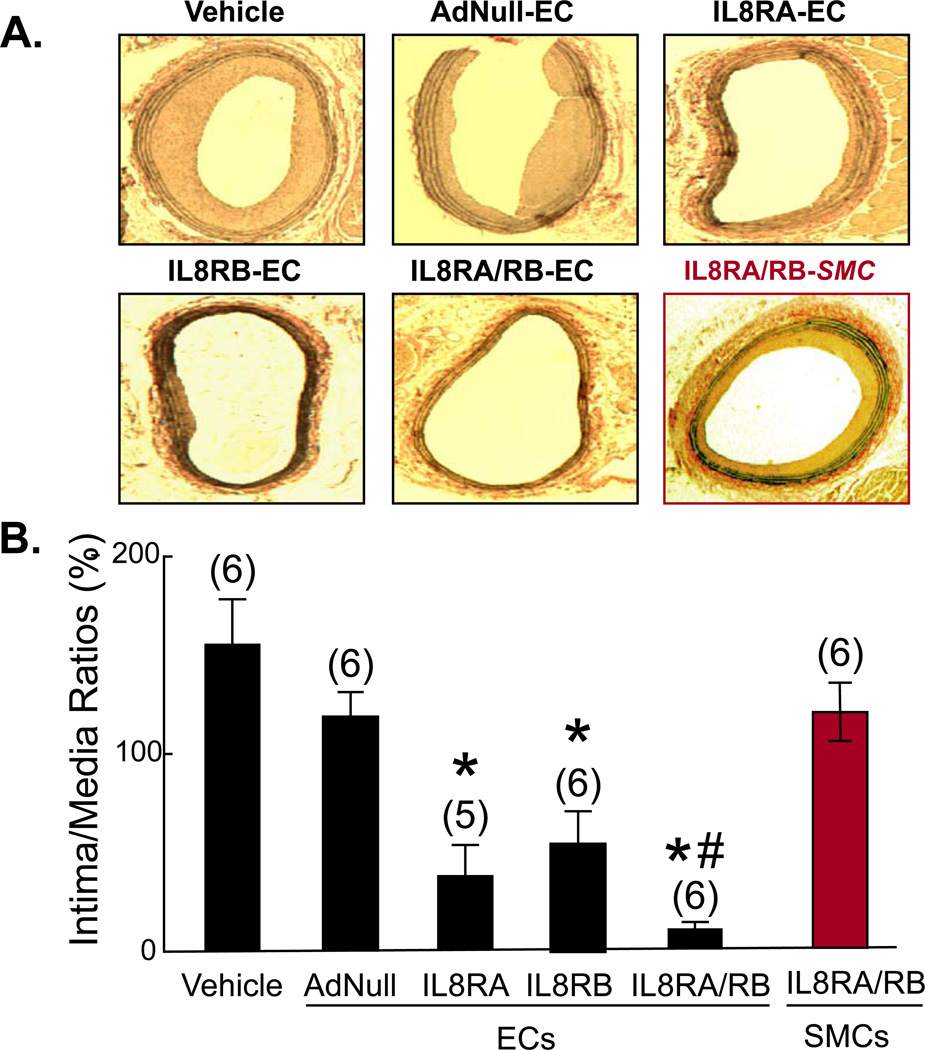
ECs Transduced with IL8Rs Decrease Neointima Formation in Injured Carotid Artery
Morphometric analysis showed that at 4 wk post-balloon injury, the neointima-to-media area ratios (I/M) of injured carotid arteries were significantly less in rats transfused with ECs that overexpress IL8RA and/or IL8RA (Figure 6A). Treatment with IL8RA-EC, IL8RB-EC, and IL8RA/RB-EC reduced I/M ratios by 80%, 74%, and 95% (compared to AdNull-EC group), respectively (Figure 6B). The mean values of medial areas were not statistically significantly different among the treatment groups (Online Supplemental Figure 4). The vasoprotective effects of transfusion with ECs transduced with both IL8RA and IL8RB were greater than those of transfusion with ECs transduced with either IL8RA or IL8RB alone. As an additional control, we found that transfusion with rat aortic smooth muscle cells transduced with IL8RA and IL8RB genes (1.5 × 106 cells) did not inhibit neointima formation (Figure 6).
Figure 6.
(A) Representative elastin-stained cross sections of right carotid arteries 4-wk post balloon injury. IL8RA-EC, IL8RB-EC, and ILRA/RB-EC rats received transfusions of ECs that overexpress IL8RA and/or IL8RB, respectively. Transfusion with the same number of rat vascular smooth muscle cells transduced with IL8RA and IL8RB adenovial vectors (IL8RA/RB-SMC) was used as additional control. (B) Quantification of neointima formation at 4-wk post balloon injury of the carotid artery. Results are means±SEM of neointima-to-media ratios, (n)=number of rats. One way ANOVA was used to analyze the data. * p<0.05 vs. Vehicle, AdNull-EC and IL8RA/RB-SMC groups; # p<0.05 vs. IL8RB group.
Discussion
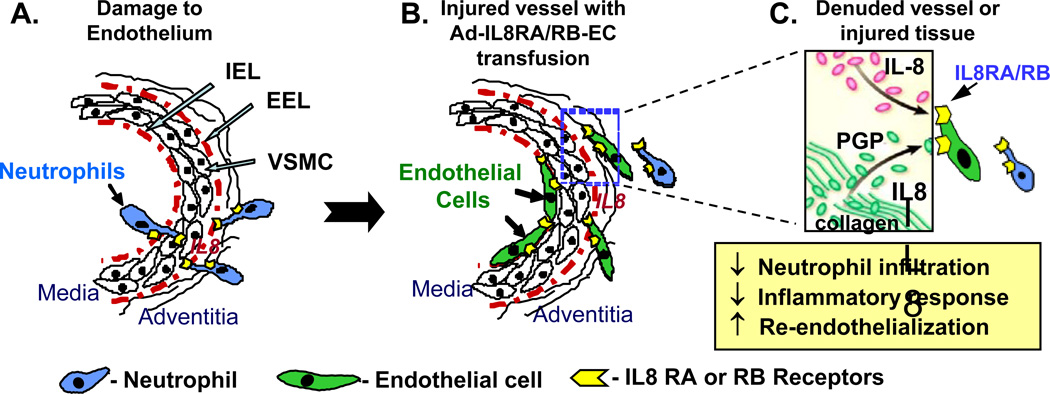
This study has shown for the first time that intravenous transfusion of ECs transduced with IL8RA- and/or IL8RB results in a significantly reduced inflammatory response, enhanced re-endothelialization and diminished neointima formation in balloon injured rat carotid arteries. These results support our hypothesis that ECs that overexpress IL8RA and/or IL8RB mimic the behavior of neutrophils that target and adhere to injured tissues, thus minimizing the inflammatory response to injury, accelerating re-endothelialization and inhibiting subsequent neointima formation (Figure 7). Targeting delivery of ECs equipped with the homing device (IL8RA and RB receptors) to the site of arterial injury, as demonstrated in this study, provides a novel strategy for the prevention and treatment of cardiovascular diseases.
Figure 7.
Schematic illustration of the effects of targeted delivery of ECs over-expressing IL8RA and/or IL8RB on inflammatory mediator expression, neutrophil infiltration/activation and pro-inflammatory responses, and re-endothelialization and repair of blood vessels with endoluminal injury. (A) Native IL8RA and IL8RB on neutrophil membranes bind to IL8 with high affinity and play a critical role in neutrophil recruitment (e.g. adhesion and trans-endothelial migration) to sites of blood vessel injury. (B) ECs overexpressing IL8RA and/or IL8RB mimic the behavior of neutrophils that target and adhere to the sites of injury. (C) Proteolytic cleavage of collagen by matrix metalloproteinases (MMPs) generates an acetylated tripeptide, acPGP (Pro-Gly-Pro), which mimics key sequences of IL-8, stimulates IL8RA and IL8RB and prolongs influx of neutrophils.33,34 We postulate that IL8RA/RB-ECs compete with the binding of neutrophils to IL8 and/or the collagen fragment (Pro-Gly-Pro) expressed in injured tissue and thus inhibit neutrophil-induced inflammatory responses (e.g. infiltration of monocytes/macrophages and expression of pro-inflammatory cytokines) and accelerate healing of the injured tissue. IEL-internal elastic lamella; EEL-external elastic lamella; VSMC-vascular smooth muscle cell.
Cell-based therapies for vascular and cardiac diseases have proliferated over the past decade, but with limited success and only moderate benefit in clinical application.16–20 The major hurdles for successful cell therapies are 1) cell type selection (progenitor vs. differentiated cells), 2) time for cell delivery (acute vs. chronic treatment), 3) cell delivery mode (direct into tissue vs. peripheral administration), and 4) targeted delivery of cells to damaged tissue to maximize therapeutic effects. The current study demonstrates an innovative, relatively noninvasive strategy to overcome the above hurdles. We have shown that acute intravenous transfusion of healthy differentiated adult rat ECs overexpressing selective neutrophil IL8RA and IL8RB receptors within hours after endoluminal injury of rat carotid artery effectively inhibits inflammatory mediator expression, inflammatory leukocyte infiltration, re-endothelialization and neointima formation in injured vessels.
IL-8, a member of CXC chemokine subfamily, is a major chemoattractant/activator for neutrophils and has been implicated in a variety of inflammatory diseases.21 A large variety of cell types, including endothelial and epithelial cells, monocytes, T lymphocytes, neutrophils, and fibroblasts, express IL-8 mRNA and protein, and expression of IL8 is significantly increased in the setting of acute cell/tissue injury. In particular, IL8 plays a crucial role in recruiting neutrophils to sites of injury.22 Interactions between IL-8 and its G-protein receptors IL8RA and IL8RB (also called CXC receptor type-1 [CXCR1] and CXCR2) on neutrophil membranes accelerate adhesion and infiltration of neutrophils in sites of acute vascular injury and inflammation.23
In a more chronic setting, IL8 increases the adhesion of neutrophils to the endothelium and induces their transendothelial migration, initiating vascular dysfunction and vascular diseases, including atherosclerosis, aortic aneurysm formation, and hypertension.24 Activated neutrophils exert potent cytotoxic effects through the release of proteolytic enzymes and pro-inflammatory effects through the upregulation of cytokines, such as TNF-α, MCP-1, and P-Selectin that induce recruitment of monocytes to areas of injury. Monocyte-derived macrophages then produce additional cytokines and growth factors necessary for fully developed inflammation. These processes induce myofibroblast transformation and proliferation and neovasculariztion, leading to tissue repair, fibrosis and scar formation. Thus, blockade of initial neutrophil adhesion and infiltration has the potential for downregulating interactions between neutrophils and injured tissues, effectively attenuating inflammatory responses to tissue injury.
The acute inflammatory response to endoluminal vascular injury plays an important role in determining the extent of subsequent neointima formation and vascular remodeling.12,25,26 Our previous studies showed that medial smooth muscle cells (SMCs) are activated immediately following endoluminal arterial injury via a combination of EC denudation and direct mechanical trauma and release a variety of chemoattractants (e.g. CINC-2β [equivalent to human IL-8], and MCP-1) which reach the adventitial and peri-adventitial space by diffusion through the vessel wall and via the circulation (vasa vasorum).12,26 Adventitial cells are activated within hrs of endoluminal vascular injury and recruit inflammatory leukocytes (neutrophils initially and monocyte/macrophages later) to the adventitia in the early phase of the injury response.12 The influx of neutrophils triggers the inflammatory reaction by secreting cytokines (e.g. TNF-α, IL-1, IL-6) and contributes to tissue injury by releasing proteases (e.g. collagenase, elastase and myeloperoxidase [MPO]). Release of these mediators from neutrophils stimulates further neutrophil/monocyte, SMC and adventitial fibroblast activation and SMC and adventitial fibroblast proliferation and migration into neointima.12 We have shown that inhibition of these early pro-inflammatory responses with estrogen partially attenuates neointima formation in balloon-injured rat carotid arteries.12,26 In the current study, transfusion of IL8-RA/RB-ECs significantly attenuated expression of pro-inflammatory mediators and completely inhibited neointima formation in injured carotid artery, supporting our hypothesis that IL8RA/RB-ECs compete with neutrophils at the site of injury and protect injured tissues from inflammatory damage.
In the late phase of the vascular injury response, re-endothelialization of the damaged vessel limits the extent of vascular remodeling. We and others have demonstrated that SMC proliferation and neointima formation can be inhibited by facilitating re-endothelialization at sites of balloon injury,15,27 supporting the hypothesis that interventions that accelerate re-endothelializaton inhibit the vascular injury response. Regeneration and proliferation of ECs after vascular injury was long believed to be a local process regulated by adjacent ECs in the intact uninjured intima.28 This dogma was challenged by recent evidence that circulating endothelial progenitor cells or bone-marrow derived stem cells promote endothelial repair by seeding themselves on the damaged vascular surface and participating in re-endothelialization and repair after vascular injury.29–32 Our current data show enhanced re-endothelialization of the injured carotid artery in rats receiving transfusion of IL8RA/RB-ECs compared to Ad-Null and vehicle treated rats, suggesting that overexpression of IL8RA and IL8RB in ECs enhanced re-endothelializaton by increased the homing of ECs to injured arteries.
Interestingly, IL8 shares neutrophil chemoattractant properties with a specific collagen-derived tripeptide fragment, N-acetylated proline-glycine–proline (acPGP), and expression of acPGP in injured tissue has been reported to induce neutrophil chemotaxis in murine models.22,33 acPGP is a proteolytic cleavage product of collagen by matrix metalloproteinases (e.g. MMP-8 and MMP-9) and prolyl endopeptidase, a key proteolytic enzyme involving in inflammatory process. acPGP has very similar 3-D structure as IL8 and acts as a potent neutrophil chemoattractant through binding to IL8RA and IL8RB on neutrophil membranes.22,33,34 Levels of MMPs and proteases, as well as generation of acPGP correlate closely with the level of inflammation in tissue, indicating that structural proteins (e.g. collagen or elastin) can regulate innate immunity by prolonging the influx of neutrophils into injured tissue.22 We postulate that acPGP also acts as a local ligand that attracts the adhesion of IL8RA/RB-ECs to the damaged endoluminal surface and adventitial tissue of the injured carotid artery (Figure 7).
In summary, our data demonstrate that transfusion of ECs overexpressing IL8RA and ILRB inhibit neointima formation in balloon injured carotid arteries. Since transfused IL8RA/RB-ECs were present both on the injured endoluminal surface and in the adventitia, these results support our hypothesis that inhibition of neointima formation is mediated by both decreasing the neutrophil-mediated pro-inflammatory response and accelerating re-endothelialization of arteries with endoluminal injury. We also demonstrated that inhibition of inflammation and neointima formation was greater following IL8RA/RB-EC treatment than following IL8RA-EC or IL8RB-EC treatment, indicating that IL8RA-IL8RB heterodimers have better functionality than either IL8RA or IL8RB homodimers in transduced ECs. The current study has significant clinical application. Equipped with the homing device, ECs will mimic the behavior of neutrophils that target and adhere to injured tissue and thus will inhibit inflammation and accelerate tissue repair. Targeted delivery of ECs to sites of cardiovascular injury provides a novel therapeutic strategy for cardiovascular disease.
Supplementary Material
Clinical Summary -CIRCULATIONAHA/2011/078436.
The endothelium is a functional component of the cardiovascular (CV) system that plays an important role in health and disease. Injury or dysfunction of endothelium can lead to many forms of CV disease (CVD), and the injured endothelium can serve as a therapeutic target in the prevention and treatment of CVD. The cell-based therapies have been shown to alleviate tissue injury and organ dysfunction in animal models of CVD. However, clinical trials with cell therapy for patients with CVD have yielded inconsistent results and, overall, modest benefit. A major unsolved problem for cell-based therapy is how to home transplanted cells to damaged organs in order to improve their survival and enhance tissue repair and organ function. We have developed an innovative strategy to overcome the above hurdle by intravenous transfusing rat endothelial cells (ECs) overexpressing neutrophil interleukin-8 (IL-8) receptors, IL8RA and IL8RB, into rats with experimental endoluminal injury of the carotid artery. We have shown that, equipped with the IL8R homing device, ECs mimic the behavior of neutrophils and compete with the binding of neutrophils to IL-8 over-expressed in injured vessels, thus inhibiting the neutrophil-induced inflammatory response and accelerating re-endothelialization and vascular repair. This targeted delivery of ECs to sites of CV injury provides a novel therapeutic strategy for CVD. The results of the current study open the door to new therapeutic applications of cell therapy in patients with CV injury.
Acknowledgments
Funding Sources: This work was supported, in part, by National Heart, Lung, and Blood Institute grants: HL080017, HL044195 (Chen YF), HL07457 (Oparil S) and by American Heart Association Grants 10POST3180007 (Gong K) and 09BGIA2250367 (Xing D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Butt M, Dwivedi G, Blann A, Khair O, Lip GY. Endothelial dysfunction: methods of assessment & implications for cardiovascular diseases. Curr Pharm Des. 2010;16:3442–3454. doi: 10.2174/138161210793563383. [DOI] [PubMed] [Google Scholar]
- 2.Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–130. [PubMed] [Google Scholar]
- 3.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 5.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 6.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, Vink A, de Kleijn DP. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30:1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 7.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oparil S, Wyss JM, Yang RH, Jin HK, Chen YF. Dietary Ca2+ prevents NaCl-sensitive hypertension in spontaneously hypertensive rats by a sympatholytic mechanism. Am J Hypertens. 1990;3:179S–188S. doi: 10.1093/ajh/3.8.179. [DOI] [PubMed] [Google Scholar]
- 9.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 10.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–25014. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing D, Feng W, Miller AD, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-α-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-β activation. Am J Physiol. 2007;292:H2607–H2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 12.Xing D, Miller A, Novak L, Chen YF, Oparil S. Ovarian hormone modulates leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- 13.Xing D, Hage FG, Chen YF, McCrory MA, Feng W, Skibinski GA, Majid-Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (FcγRI) dependent. Am J Pathol. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;10:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 15.Chen YF, Oparil S. Effects of sex steroid in vascular injury. In: Levin, Nadler, editors. Endocrinology of Cardiovascular Function. Kluwer Academic Publishers; 1998. pp. 45–59. [Google Scholar]
- 16.Perry TE, Song M, Despres DJ, Kim SM, San H, Yu ZX, Raghavachari N, Schnermann J, Cannon RO, 3rd, Orlic D. Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res. 2009;84:317–325. doi: 10.1093/cvr/cvp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deb A, Patterson C. Hard luck stories: the reality of endothelial progenitor cells continues to fall short of the promise. Circulation. 2010;121:850–852. doi: 10.1161/CIR.0b013e3181d4c360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121:898–905. doi: 10.1161/CIRCULATIONAHA.109.885459. [DOI] [PubMed] [Google Scholar]
- 19.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stellos K, Langer H, Gnerlich S, Panagiota V, Paul A, Schönberger T, Ninci E, Menzel D, Mueller I, Bigalke B, Geisler T, Bültmann A, Lindemann S, Gawaz M. Junctional adhesion molecule A expressed on human CD34+ cells promotes adhesion on vascular wall and differentiation into endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1127–1136. doi: 10.1161/ATVBAHA.110.204370. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkilde MM, Schwartz TW. The chemokine system - a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skelton NJ, Quan C, Reilly D, Lowman H. Structure of a CXC chemokine-receptor fragment in complex with interleukin-8. Structure. 1999;7:157–168. doi: 10.1016/S0969-2126(99)80022-7. [DOI] [PubMed] [Google Scholar]
- 24.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hay C, Micko C, Prescott MF, Liau G, Robinson K, De Leon H. Differential cell cycle progression patterns of infiltrating leukocytes and resident cells after balloon injury of the rat carotid artery. Arterioscler Thromb Vasc Biol. 2001;21:1948–1954. doi: 10.1161/hq1201.100256. [DOI] [PubMed] [Google Scholar]
- 26.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, Ferrara N, Symes JF, Isner JM. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91:2793–2801. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Moons L, Stassen JM, De Mol M, Bouché A, van den Oord JJ, Kockx M, Collen D. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150:761–776. [PMC free article] [PubMed] [Google Scholar]
- 29.Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 30.Hristov M, Zernecke A, Liehn EA, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost. 2007;98:274–277. [PubMed] [Google Scholar]
- 31.Chen L, Wu F, Xia WH, Zhang YY, Xu SY, Cheng F, Liu X, Zhang XY, Wang SM, Tao J. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res. 2010;88:462–470. doi: 10.1093/cvr/cvq207. [DOI] [PubMed] [Google Scholar]
- 32.Stellos K, Langer H, Gnerlich S, Panagiota V, Paul A, Schönberger T, Ninci E, Menzel D, Mueller I, Bigalke B, Geisler T, Bültmann A, Lindemann S, Gawaz M. Junctional adhesion molecule A expressed on human CD34+ cells promotes adhesion on vascular wall and differentiation into endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1127–1136. doi: 10.1161/ATVBAHA.110.204370. [DOI] [PubMed] [Google Scholar]
- 33.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 34.Henson PM, Vandivier RW. The matrix degrades, neutrophils invade. Nat Med. 2006;12:280–281. doi: 10.1038/nm0306-280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.