Abstract
Background
Chromosomal translocations resulting in alternative fusions of the human TEL (ETV6) and JAK2 genes have been observed in cases of acute lymphoblastic leukemia and chronic myelogenous leukemia, but a full understanding of their role in disease etiology has remained elusive. In this study potential differences between these alternative TEL-JAK2 fusions, including their lineage specificity, were investigated.
Design and Methods
TEL-JAK2 fusion types derived from both T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia were generated using the corresponding zebrafish tel and jak2a genes and placed under the control of either the white blood cell-specific spi1 promoter or the ubiquitously-expressed cytomegalovirus promoter. These constructs were injected into zebrafish embryos and their effects on hematopoiesis examined using a range of molecular approaches. In addition, the functional properties of the alternative fusions were investigated in vitro.
Results
Injection of the T-cell acute lymphoblastic leukemia-derived tel-jak2a significantly perturbed lymphopoiesis with a lesser effect on myelopoiesis in zebrafish embryos. In contrast, injection of the atypical chronic myelogenous leukemia-derived tel-jak2a resulted in significant perturbation of the myeloid compartment. These phenotypes were observed regardless of whether expressed in a white blood cell-specific or ubiquitous manner, with no overt cellular proliferation outside of the hematopoietic cells. Functional studies revealed subtle differences between the alternative forms, with the acute lymphoblastic leukemia variant showing higher activity, but reduced downstream signal transducer and activator of transcription activation and decreased sensitivity to JAK2 inhibition. JAK2 activity was required to mediate the effects of both variants on zebrafish hematopoiesis.
Conclusions
This study indicates that the molecular structure of alternative TEL-JAK2 fusions likely contributes to the etiology of disease. The data further suggest that this class of oncogene exerts its effects in a cell lineage-specific manner, which may be due to differences in downstream signaling.
Keywords: oncogenesis, TEL-JAK2, leukemia, zebrafish
Introduction
The Janus kinase - signal transducer and activator of transcription (JAK-STAT) pathway lies downstream of cytokine receptors where it plays an important role in regulating proliferation, differentiation, and function of several cell types, particularly those of the immune and hematopoietic systems.1 Not surprisingly, therefore, deregulation of this pathway has been associated with various hematologic malignancies.2 For example, activating JAK1 mutations have been identified in T-cell acute lymphoblastic leukemia (ALL),3 where they are associated with poor prognosis,4 and acute myeloid leukemia,5 while activating mutations of JAK3 have been observed in acute megakaryoblastic leukemia.6,7 Activating JAK2 mutations have been observed in a high percentage of patients with classical myeloproliferative disorders,8-10 and at a lower frequency in those with acute myeloid leukemia and chronic neutrophilic leukemia.11,12 JAK and STAT proteins are also constitutively activated in hematopoietic cell lines transformed with different oncogenic kinases and in cells from patients with acute myeloid leukemia, ALL, chronic myelogenous leukemia (CML), and chronic lymphoblastic leukemia.13-15 In addition, JAK2 has been identified as a fusion partner in several leukemic oncogenes.16,17
TEL-JAK2 fusions are generated as a result of translocation of the JAK2 locus on 9p24 to the TEL/ETV6 locus on 12p13. In cases of ALL this is mediated by either of two t(9;12)(p24;p13) translocations: that seen in the pre-B-cell form of the disease creates a transcriptional fusion between exon 4 of TEL and exon 17 of JAK2, whereas that seen in the T-cell form fuses exon 5 of TEL and exon 19 of JAK2. In addition, a case of atypical CML has been reported which is the result of a unique complex t(9;15;12)(p24;q15;p13) translocation that creates a fusion between exon 5 of TEL and exon 12 of JAK2, with no involvement of BCR-ABL or other known oncogenes.16,18 Each of these TEL-JAK2 variants identified has in common the pointed (PNT) dimerization domain of TEL fused to the JH1 tyrosine kinase domain of JAK2, resulting in constitutive activation of the kinase as well as downstream signaling pathways.18-21 However, the CML version also contains the complete JH2 pseudokinase domain, which is known to be important in regulating kinase activity22,23 and recently identified as possessing dual-specificity kinase activity.24 The transformation properties of isolated TEL-JAK2 fusions have been demonstrated in vitro by their ability to enable cytokine-independent growth of murine hematopoietic Ba/F3 cells.18,19,21,25 Mice reconstituted with bone marrow cells transduced to express the T-cell ALL-associated TEL-JAK2 oncogene variant developed a fatal mixed myelo- and T-cell-lymphoproliferative disorder.19 Moreover, transgenic mice expressing TEL-JAK2 in the lymphoid lineage developed a rapid onset and fatal T-cell leukemia20 and B-cell lymphoma/leukemia.26 STAT5 is strongly activated by each of the TEL-JAK2 fusions and its activation is at least partially responsible for their transforming properties.20,27 Conversely, SOCS1, a negative regulator of the JAK-STAT pathway, is induced by TEL-JAK2 and can inhibit TEL-JAK2-mediated transformation.28-31 However, possible differences between the various TEL-JAK2 forms have yet to be established.
Zebrafish is now established as a useful model for the study of leukemic oncogenes.32-34 In previous studies, we used relevant zebrafish orthologs to recapitulate both a myeloproliferative disease-derived JAK2 mutant35 and the atypical CML-derived TEL-JAK2 fusion,34 and demonstrated their ability to perturb hematopoiesis in zebrafish embryos. Here we use this model to further investigate the alternative TEL-JAK2 fusions seen in ALL and CML, comparing their effects when expressed either in a white blood cell-specific or ubiquitous manner. This has delineated clear functional differences between the alternative TEL-JAK2 forms, as well as demonstrating lineage specificity for this class of oncogene.
Design and Methods
Cloning of Flag-tagged tel-jak2a
Standard polymerase chain reaction (PCR) cloning techniques were used to splice together sequences encoding a double Flag tag, followed by residues 1-330 of zebrafish tel (equivalent to 1-337 of human TEL), followed by residues 776-1095 of zebrafish jak2a36 (equivalent to 812-1132 of human JAK2) to recapitulate the T-cell ALL-derived TEL-JAK2 fusion.18 After sequence verification, the fragment encoding Flag-tel-jak2a ALL was subcloned under the control of the zebrafish white blood cell-specific spi1 promoter in pA308, as described for the equivalent CML-derived fusion.34 Both were also cloned into pCS2+, to allow expression from the ubiquitous cytomegalovirus (CMV) promoter.37
Transient expression and analysis in human 293T cells
Human 293T cells were grown to 50-80% confluency before transfection with an empty vector (pCS2+) or constructs expressing either ALL- or CML-derived Flag-tel-jak2a using Fugene 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA). After incubation at 37°C in 10% (v/v) CO2 for 2 days, a total cell lysate was prepared and subjected to western blot analysis with murine anti-Flag (Upstate Biotechnology Inc., Lake Placid, NY, USA), or immunoprecipitated with anti-Flag followed by western blotting with anti-phosphotyrosine 4G10 (Upstate), as previously described.34 Alternatively, cells were fixed with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 0.5% (v/v) Triton X-100 in PBS, and washed three times with PBS, before blocking with 1% (w/v) bovine serum albumin in PBS. Cells were then incubated with 20 mg/mL anti-Flag antibody (Sigma) at 4°C overnight followed by incubation with a 1/2000 dilution of Alexa Fluor 568 goat anti-mouse IgG (Invitrogen) for 1 h. Excess antibodies were washed away by PBS. Finally, cells were subjected to nuclear staining with 1 μg/mL of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) stain (Sigma) and were visualized with an Olympus FluoView FV10i self-contained confocal laser scanning microscope system. In other experiments STAT3(m67)-luciferase, STAT5(β-cas)-luciferase, CMV-Renilla, CMV-stat1 and CMV-stat3 constructs were co-transfected and luciferase activity determined. JAK2 inhibitors (AG490 - 30 μM; WP1066 - 4 μM ) were added as required.
Zebrafish husbandry and manipulation
Wild-type stocks of zebrafish were obtained from St Kilda aquarium (St Kilda, Victoria, Australia) and MAS imports (Coburg, Victoria, Australia), and maintained according to standard husbandry practices, as described elsewhere.34 Microinjection was performed using Narishige micromanipulators mounted on a Leica stereomicroscope. Embryos at the one-cell stage were injected using a finely drawn capillary with approximately 1 nL of DNA solution diluted to 100 ng/μL in 0.25 M KCl. As controls, empty vector DNA and diluent only were injected in parallel. Embryos were subsequently allowed to develop in egg water [2.5% (w/v) Na2HPO4; pH 6.0-6.3] on a warming tray set at 28°C. In some experiments, embryos were incubated in the presence of a pharmacological JAK2 inhibitor, or a diluent control (dimethylsulfoxide; DMSO), as performed previously.38 Institutional and national guidelines for the care and use of laboratory animals were followed in all studies.
Molecular analyses
Techniques for whole mount in situ hybridization, immunohistochemistry with anti-Flag and anti-BrdU antibodies, diaminofluorene (DAF) and O-dianisidine staining of hemoglobin, May-Grünwald staining of blood smears and reverse transcriptase PCR analysis have been described previously.34,38,39 For quantitation of tel-jak2a expression, the primers 5′-TTCATGGACTACAAAGAC and 5′-GAATGTGCTGCAGGAGCT were used, with RNA treated extensively with DNaseI prior to reverse transcriptase PCR to destroy contaminating plasmid DNA.
Results
Construction and expression of tel-jak2a fusions
For this study, a Flag-tagged fusion gene corresponding to the TEL-JAK2 form seen in T-cell ALL18 was constructed using zebrafish tel and jak2a genes, guided by the high level of conservation at the fusion juncture (Online Supplementary Figure S1A). This was cloned downstream of the spi1 promoter, for white blood cell-specific expression,40 as previously described for the atypical CML-derived tel-jak2a.34 Both fusions were also cloned downstream of the CMV promoter for ubiquitous expression37 (Online Supplementary Figure S1A). The latter constructs were introduced into human 293T cells to verify the expression of the expected fusion proteins. Western blot analysis of total cell lysates using anti-Flag identified single bands with the expected molecular weight for each fusion which were absent in cells transfected with empty vector with GAPDH used as a loading control (Online Supplementary Figure S1B).
For the in vivo expression experiments, zebrafish embryos were microinjected with each of the four expression constructs at the one-cell stage. Quantitative reverse transcriptase PCR analysis on pooled embryos at 24 h post-fertilization (hpf) revealed higher expression from the CMV promoter compared to the spi1 promoter but that the ALL and CML versions were expressed at similar levels in each case (Online Supplementary Figure S1C). Staining of embryos with an anti-Flag antibody showed that those injected with spi1.tel-jak2a ALL or spi1.tel-jak2a CML showed discrete Flag-positive cells in the posterior intermediate cell mass and across the yolk (Online Supplementary Figure S1E,F), a pattern consistent with endogenous spi1-expression.40-42 In contrast, embryos injected with CMV.tel-jak2a ALL or CMV.tel-jak2a CML showed ubiquitous staining throughout the majority of the embryo (Online Supplementary Figure S1G,H). Only slight background staining was observed in uninjected embryos (Online Supplementary Figure S1D). This confirmed that all fusion constructs were expressed, and in appropriate cell populations.
Characterization of injected embryos
Injected embryos were examined for phenotypic perturbation from 24 hpf to 5 days post-fertilization (dpf) and compared to uninjected embryos or embryos injected with the relevant empty vector controls for the spi1 and CMV promoters (pA308 and pCS2+, respectively), with particular attention to any phenotypes related to perturbation of the hematopoietic compartment. Each construct was injected on at least five independent occasions to ensure reproducibility of the results, with more than 300 embryos injected with each oncogenic construct for an accurate representation of the effects observed. Injection of all tel-jak2a constructs produced phenotypes consistent with severe perturbation of the hematopoietic compartment during embryonic development. Ubiquitous expression of both tel-jak2a constructs also caused a number of other phenotypes, including head defects as well as occasional disruption of circulation, pigmentation and curvature of the tail (Online Supplementary Figures S2 and S3), although no cellular hyperproliferation was observed outside the hematopoietic compartment.
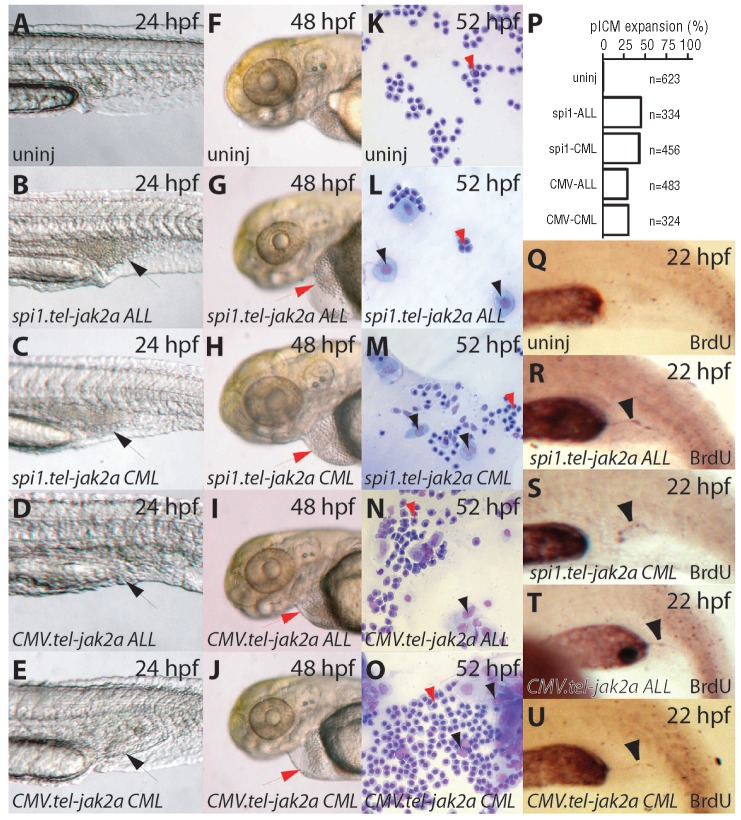
At 24 hpf, all constructs produced an expansion of the posterior intermediate cell mass (Figure 1B-E,P), a site of zebrafish hematopoiesis at this time,43 which was not observed in uninjected embryos (Figure 1A) or those injected with empty vectors (pA308 or pCS2+) (data not shown). This was unrelated to the infrequent circulation defects (data not shown). By 48 hpf, increased numbers of large, non-hemoglobinized cells were observed in the circulation in injected embryos (Figure 1G-J), compared to those not injected (Figure 1F). To examine the nature of the large non-hemoglobinized cells in more detail, blood smears were prepared and examined histologically. Samples taken from embryos injected with any of the tel-jak2a oncogenes contained significant numbers of white blood cells (Figure 1L-O), whereas blood from uninjected embryos contained almost exclusively red blood cells (Figure 1K). BrdU incorporation experiments confirmed that the posterior intermediate cell mass expansion observed in tel-jak2a-expressing embryos was due to increased cell proliferation (Figure 1Q-U).
Figure 1.
Hematopoietic defects caused by tel-jak2a fusions. (A-J) Light microscopy. Images of uninjected embryos (uninj: A, F) or embryos injected with spi1.tel-jak2a ALL (B, G), spi1.tel-jak2a CML (C, H), CMV.tel-jak2a ALL (D, I), or CML.tel-jak2a CML (E, J) at the times indicated. Expanded posterior intermediate cell mass regions in panels B-E are indicated by black arrowheads and enlarged pericardia in panels G-J indicated by red arrowheads. (K-O) Blood analysis. May-Grünwald stained blood smears from 52 hpf embryos, either uninjected (uninj: K), or injected with spi1.tel-jak2a ALL (L), spi1.tel-jak2a CML (M), CMV.tel-jak2a ALL (N), or CMV.tel-jak2a CML (O). Red arrowheads indicate red blood cells and black arrowheads indicate white blood cells. (P) Quantification of posterior intermediate cell mass expansion. (Q-U). Cell proliferation. BrdU incorporation in uninjected embryos (Q) or those injected with spi1.tel-jak2a ALL (R), spi1.tel-jak2a CML (S), CMV.tel-jak2a ALL (T) or CMV.tel-jak2a CML (U).
Molecular analysis of hematologic compartments
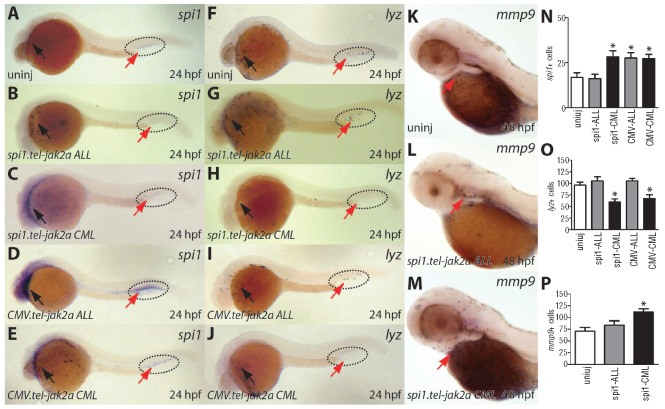
To further investigate the hematologic perturbation apparent in these embryos, in situ hybridization was performed using specific molecular markers and stains. At 24 hpf, there was an increase in cells expressing spi1, an early white blood cell marker,41,42 in embryos injected with spi1.tel-jak2a CML (Figure 2C,N) and CMV.tel-jak2a CML (Figure 2E,N), compared to uninjected embryos (Figure 2A,N). However, this was restricted to the rostral (anterior) population, with the caudal (posterior) population significantly depleted with spi1.tel-jak2a CML. The spi1.tel-jak2a CML and CMV.tel-jak2a CML embryos also showed a decrease in cells expressing lysozyme (lyz), a marker for mature myeloid cells derived from the rostral compartment44 (Figure 2H,J,O), although cells expressing mmp945 were increased with spi1.tel-jak2a CML (Figure 2M,P), compared to uninjected embryos (Figure 2F and Figure 2K, respectively). In contrast, expression of the ALL-derived fusion did not alter the number of lyz+ (Figure 2G,I) or mmp9+ (Figure 2L,P) cells, and only CMV.tel-jak2a ALL led to an expansion of spi1+ cell numbers in the posterior intermediate cell mass (Figure 2D,N). This suggests that the effects on myelopoiesis were largely specific for the CML-derived tel-jak2a fusion gene. In addition, ubiquitous expression of ALL or CML-derived tel-jak2a fusions resulted in ectopic expression of spi1 in a range of anterior tissues (Figure 2D,E) at 24 hpf.
Figure 2.
Characterization of myelopoiesis in embryos expressing tel-jak2a fusions. Uninjected embryos (uninj: A, F, K) or embryos injected with spi1.tel-jak2a ALL (B, G, L), spi1.tel-jak2a CML (C, H, M), CMV.tel-jak2a ALL (D, I) or CMV.tel-jak2a CML (E, J) were analyzed at 24 hpf for expression of the specific markers, spi1 (A-E), lysozyme (lyz) (F-J) and matrix metalloproteinase 9 (mmp9) (K-M), by in situ hybridization. The red arrows indicate the position of the yolk sac and the dotted line the area of the posterior intermediate cell mass. The numbers of spi1 (N), lyz (O) and mmp9 (P) cells were determined and are expressed as the mean ± SEM.
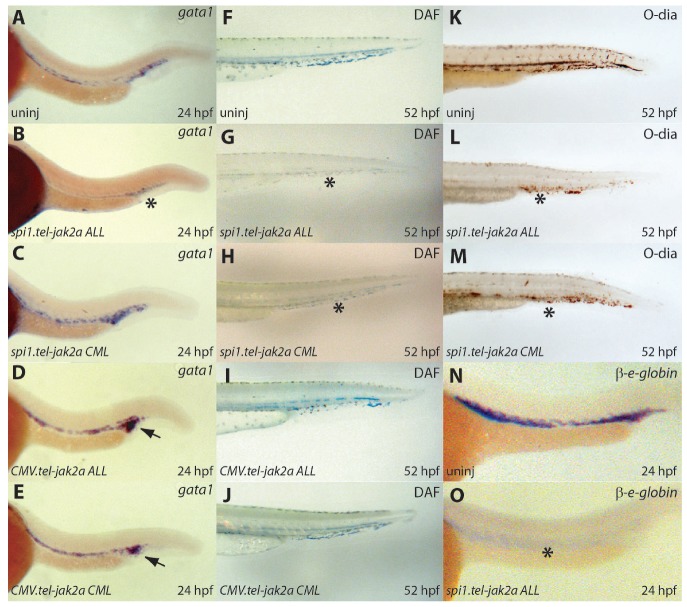
At 24 hpf, there was a slight increase in the expression of gata1, an early erythroid marker,46,47 in embryos injected with spi1.tel-jak2a CML (Figure 3C), CMV.tel-jak2a ALL (Figure 3D), and CMV.tel-jak2a CML (Figure 3E) compared to uninjected controls (Figure 3A). In contrast, there was a slight but consistent decrease in embryos injected with spi1.tel-jak2a ALL (Figure 3B). Consistent with a visible anemia observed by light microscopy from 48 hpf, a significant decrease in DAF staining was seen in embryos injected with spi1.tel-jak2a ALL (Figure 3G) or spi1.tel-jak2a CML (Figure 3H) compared to uninjected controls at 52 hpf (Figure 3F). This was confirmed by O-dianisidine staining of hemoglobin (Figure 3K-M), which correlated with levels of β-embryonic globin expression (Figure 3N-O, and data not shown). Ubiquitous expression of these two tel-jak2a variants, on the other hand, caused no major differences in the number of hemoglobinized cells, although there was some disruption of circulation through the tail (Figure 3I,J).
Figure 3.
Characterization of erythropoiesis in embryos expressing tel-jak2a fusions. Uninjected embryos (uninj: A, F, K, N) or embryos injected with spi1.tel-jak2a ALL (B, G, L, O), spi1.tel-jak2a CML (C, H, M), CMV.tel-jak2a ALL (D, I), or CMV.tel-jak2a CML (E, J) were analyzed by whole-mount in situ hybridization (WISH) for expression of the erythroid specific markers gata1 (A-E) and β-embryonic globin (β-e-globin) (N-O) at 24 hpf or stained with DAF (F-J) or O-dianisidine (K-M) at 52 hpf to detect hemoglobin (F-J). Reduced gata1 expression in panel (B), β-e-globin expression in panel (O), and hemoglobin in panels (G-H) and (L-M) are indicated with black asterisks, while increased gata1 expression is shown with a black arrow in panels (D) and (E).
The lymphoid compartment was also investigated by analysis of the expression of the lymphoid-specific genes, rag148 and ikaros.49 In embryos injected with the spi1.tel-jak2a ALL oncogene, expression of rag1 (Figure 4B) and ikaros (Figure 4G) was increased compared to that in uninjected embryos (Figure 4A,F). However, embryos injected with the three other tel-jak2a constructs exhibited normal rag1 and ikaros expression patterns (Figure 4C-E,H-J). Further analysis of embryos injected with the spi1.tel-jak2a ALL oncogene at 7 dpf demonstrated strong spi1 expression (Figure 4L) that was absent in controls (Figure 4K), indicating that the spi1 promoter remains strongly active in the thymus of these embryos.
Figure 4.
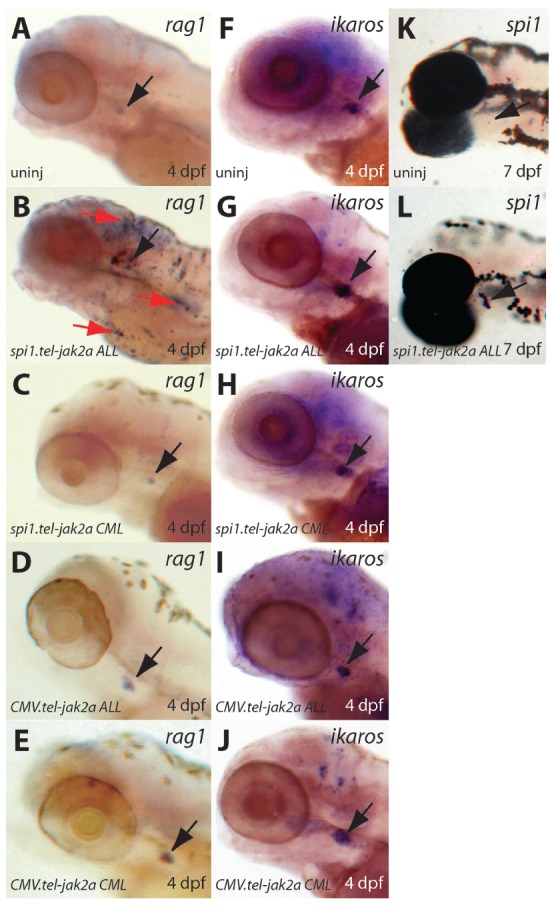
Characterization of lymphopoiesis in embryos expressing tel-jak2a fusions. Uninjected embryos (uninj: A, F, K) or embryos injected with spi1.tel-jak2a ALL (B, G, L), spi1.tel-jak2a CML (C, H), CMV.tel-jak2a ALL (D, I), or CMV.tel-jak2a CML (E, J) were analyzed by in situ hybridization for expression of the lymphoid specific markers rag1 (A-E) and ikaros (F-J) at 4 dpf and spi1 (K, L) at 7 dpf. Black arrows show the position of the thymus in each case, with red arrows indicating rag1+ cells through the embryo in panel B.
Biochemical analysis of TEL-JAK2 variants
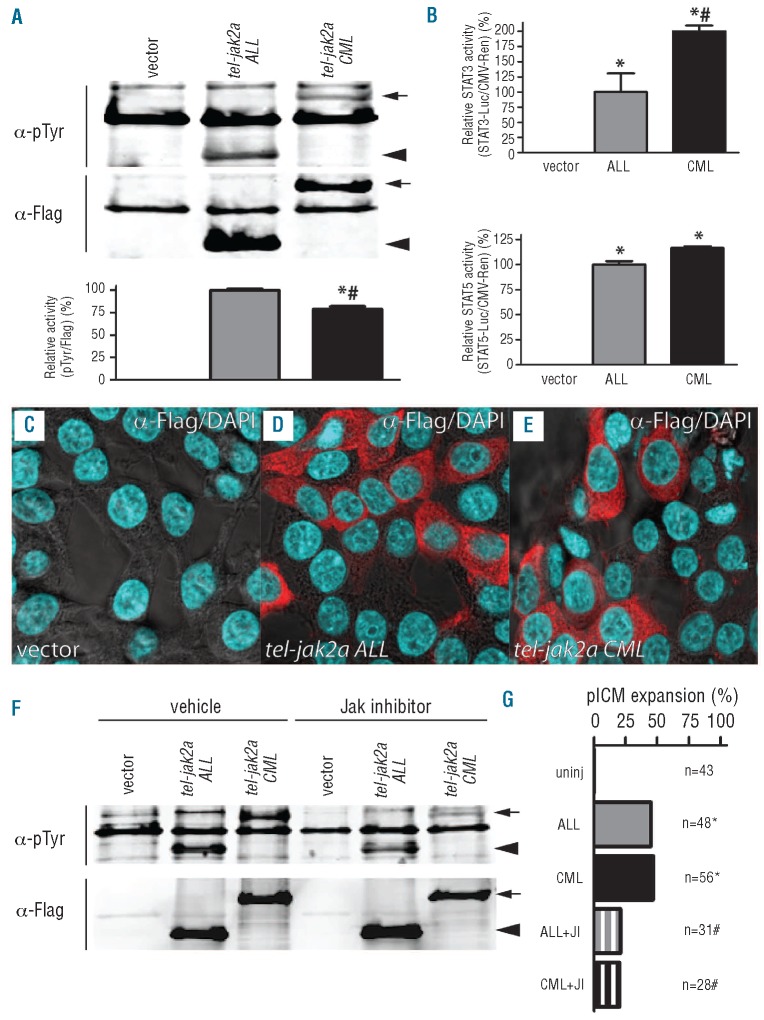
We next sought to investigate potential functional differences between the different TEL-JAK2 variants to explain their lineage specificity. To compare their relative activities, both forms were immunoprecipitated from transfected HEK293T cell lysates with anti-Flag antibody with the resultant immune complexes analyzed by western blot with anti-phosphotyrosine antibody as a surrogate marker of activity (Figure 5A). This revealed that both fusion proteins were phosphorylated, probably through auto-phosphorylation as occurs in mammalian TEL-JAK fusion proteins.21 Re-probing the blot with anti-Flag antibody allowed normalization of the phosphotyrosine band intensity, which revealed a greater activity of the ALL variant compared to the CML variant. To assess potential biochemical differences further, activation of the downstream STAT3 and STAT5 was analyzed using relevant luciferase reporters normalized relative to a CMV-Renilla control. This indicated that the levels of activation of both STAT3 and STAT5 were slightly greater in the CML variant, with the largest difference seen with STAT3 (Figure 5B). To investigate possible involvement of STAT1, zebrafish stat1 or stat3 expression constructs were co-transfected with the STAT3-luciferase reporter, which can also respond to STAT1. However, only transfection of stat3 led to increased luciferase activity (data not shown), implying that stat1 is not involved. The relative localization of the two TEL-JAK2 variants was also analyzed by immunohistochemistry with anti-Flag antibody. This identified equivalent cytoplasmic expression patterns for both the ALL and CML variants (Figure 5C-E). Finally, the sensitivity of each variant to JAK2 inhibitors was investigated. By analysis of phosphotyrosine staining in transfected HEK 293T cells, both variants showed reduced activity with the inhibitor AG490, although the CML variant was more sensitive than the ALL variant (inhibition of 72% compared to 35%) (Figure 5F). A similar effect was also seen with the alternative inhibitor WP1066 (data not shown). Treatment of tel-jak2a-injected embryos with the JAK2 inhibitor AG490 largely blunted the expanded posterior intermediate cell mass mediated by both variants (Figure 5G).
Figure 5.
Functional properties of tel-jak2a fusions. (A) Activity of tel-jak2a fusions. Lysates from HEK293T cells transfected with the indicated constructs were immunoprecipitated with α-Flag and analyzed by western blot with α-phosphotyrosine (α-pTyr) and α-Flag (upper panels), with the relative levels of tyrosine phosphorylation (pTyr/Flag) determined by image analysis, with tel-jak2a ALL set at 100% (lower panel). The tel-jak2a CML protein is indicated with an arrow and the tel-jak2a ALL protein with an arrowhead. (B) Downstream STAT activation by tel-jak2a fusions. HEK293T cells were co-transfected with empty vector, CMV.tel-jak2a ALL and CMV.tel-jak2a CML as indicated, along with STAT-luciferase and CMV.Renilla constructs, with the relative activation of STAT3 (upper panel) or STAT5 (lower panel) reporter determined relative to CMV.tel-jak2a ALL at 100%. (C-E) Localization of tel-jak2a fusions. HEK293T cells were transfected with the indicated constructs and co-stained with α-Flag/Alexa fluor 568nm (red) and DAPI (blue). (F-G) Sensitivity of tel-jak2a fusions to JAK2 inhibitors. HEK293T cells were transfected and analyzed as in panel (A) in the absence or presence of 30 μM AG490 (F). Alternatively, zebrafish were injected with the indicated constructs with or without 30 μM AG490 and scored for posterior intermediate cell mass expansion (G).
Discussion
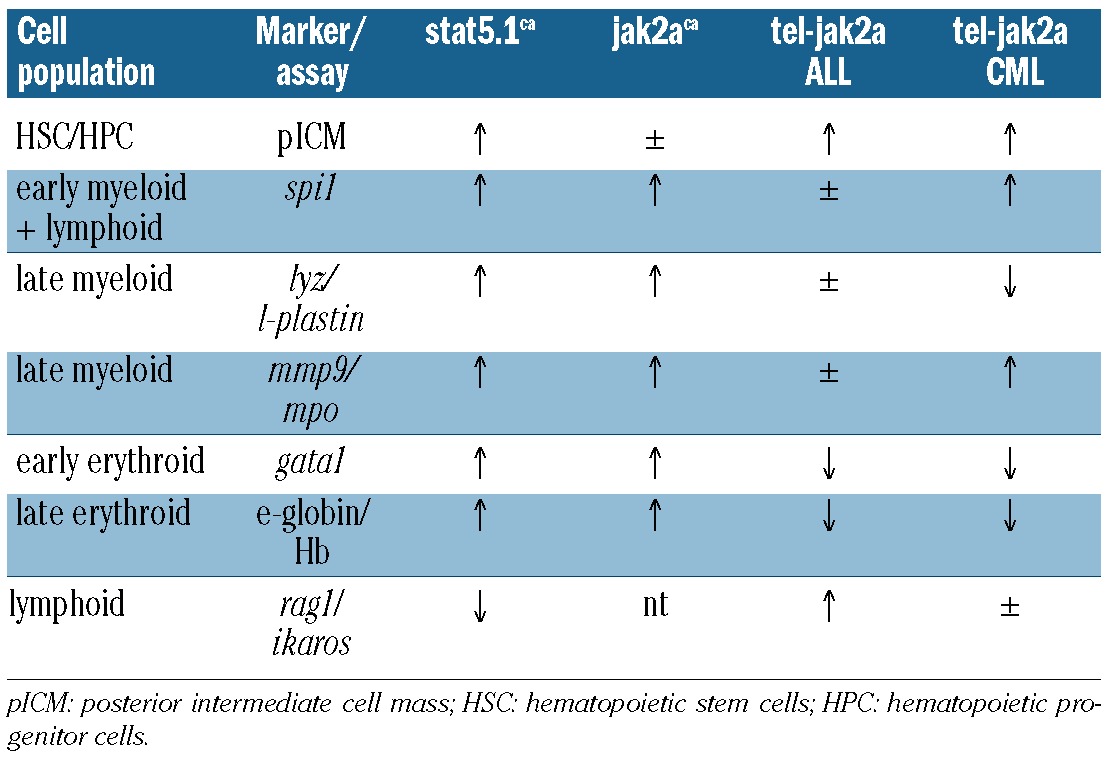
We and others have previously shown that zebrafish are susceptible to the pathological actions of a number of genes implicated in hematologic malignancies and diseases, including Myc, RUNX1-CBF, JAK2 and STAT5,32,33,35,38,50 as well as a zebrafish tel-jak2a fusion gene corresponding to the TEL-JAK2 oncogene found in atypical CML34 (Table 1). Collectively this work has demonstrated that zebrafish represents a suitable model organism for the study of leukemic oncogenes. Here we have taken advantage of this model to investigate two fundamental unanswered questions regarding TEL-JAK2 fusions. Firstly, are there any intrinsic differences between the alternative forms of TEL-JAK2 seen in lymphoid and myeloid leukemia that contribute to the type of malignancy induced? This was examined by directly comparing versions of tel-jak2a derived from T-cell ALL and atypical CML. Secondly, is TEL-JAK2 a leukemia-specific oncogene, or is this association simply the result of being expressed in cells of this lineage via the TEL promoter? We investigated this question by comparing white blood cell-specific expression with ubiquitous expression of the alternative tel-jak2a fusions. Our results shed considerable light on both questions.
Table 1.
Summary of consequences of JAK2 perturbation in zebrafish.
Expression of the T-cell ALL-derived zebrafish tel-jak2a gene under the control of the white blood cell-specific spi1 promoter resulted in an expansion of the posterior intermediate cell mass, increased numbers of white blood cells in the circulation and anemia. Further analysis using in situ hybridization with lineage-specific markers revealed a later expansion of lymphoid cells. The late latency of the lymphoid phenotypes is consistent with the ontogeny of lymphoid development in zebrafish, which commences at around 3.5 dpf,51 and is a consequence of the conserved ability of the spi1 promoter to mediate expression in early lymphoid lineages.52 The CML-derived tel-jak2a oncogene also resulted in elevated numbers of white blood cells and anemia. However, in this case there was a decrease in mature myeloid cells suggesting a block in myelopoiesis, as well as an increase in the number of immature erythroid cells, but no effects on cells of the lymphoid lineage. Collectively, this suggests that while both CML and ALL versions of tel-jak2a can exert a potent proliferative effect on early hematopoietic cells, different cell lineages are susceptible in each case. In contrast, the effects on the erythroid lineage were similar to those of the CML-derived fusion, with an increase in immature erythroid cells but with manifest anemia, suggesting a differentiation block in an erythroid progenitor population. This is in contrast to the effects of activating JAK2 mutants that lead to enhanced erythropoiesis.53 There are two potential explanations for the difference. Firstly, the tel-jak2a fusions are expressed from the spi1 promoter and so will not be expressed in erythroid cells, apart from the earliest precursors, in contrast to the JAK2 mutants that are expressed throughout erythropoiesis. Secondly, the activating JAK2 mutants act via receptor-associated pathways,54 whereas the TEL-JAK2 forms lack the domains required for receptor association and are present throughout the cytoplasm, as we have demonstrated in this study.
Ubiquitous expression of either tel-jak2a variant caused some non-hematopoietic defects, including vasculature defects and general head and tail defects, which were also observed with activating forms of stat5 (data not shown), suggesting this is the likely downstream pathway. Importantly, however, while ubiquitous expression of either tel-jak2a variant resulted in expansion of white blood cells, no other cellular hyperproliferation was observed, indicating that the effects of this class of oncogenes are limited to cells of the hematopoietic lineage. Activating JAK2 mutations are known to promote cytokine receptor hypersensitivity10,55 and altered regulation of downstream pathways.56 Since the majority of cytokine receptors are expressed on immune and hematopoietic cells, this might provide some explanation for this observation.
Other studies have attempted to investigate the downstream pathways important for the effects of TEL-JAK2. These have identified STAT5,27 TYK2,57 SOCS1,29 NF-κB,58 PI-3K,59 and various MAPK60 as potentially playing a role. Several studies have investigated the efficacy of inhibitors that can suppress the effects of TEL-JAK2.61,62 We showed that both forms of TEL-JAK2 were sensitive to JAK2 inhibitors, although the ALL form appeared slightly less sensitive. This information is clinically significant, and highlights the usefulness of zebrafish as a pre-clinical model to dissect these pathways.
The presence of a complete JH2 domain is the only difference between the two forms, and so this region of the fusion oncoprotein must be responsible. The mechanism by which this so-called “pseudo-kinase domain” facilitates the effect has remained unknown. This domain is known to act as a regulator of JAK kinase activity,23,63 with mutation of this domain leading to myeloproliferative disorders in humans, mice, fish and flies.22,35,38,64,65 Consistent with this, the ALL version that lacks the JH2 domain showed greater activity, which is in accordance with other studies on human TEL-JAK2 fusions.66 Somewhat surprisingly, however, the CML version showed greater STAT activation, particularly of STAT3, which represents a novel downstream target for TEL-JAK2. Analysis of the conserved tyrosine residues within the JH2 domain which are unique to the CML-derived TEL-JAK2 provide a potential mechanism to explain this difference (Online Supplementary Figure S4). One of these, Y570, has the sequence DYG(E/Q)(V/L) which is in good agreement with consensus STAT5 docking sites.67,68 Another, at Y637 has the sequence YLK(R/K), which fits the alternative STAT3 site of Y-hydrophobic-basic-large hydrophilic.69 Recently, the JH2 domain was also found to possess both tyrosine and serine kinase activity,24 which might also contribute to the differential levels of STAT activation – and potentially the phenotypic differences – observed. It is worth noting that activating JH2 domain mutants of JAK2 have also been identified in human lymphoid disorders.70
Supplementary Material
Acknowledgments
Funding: the authors acknowledge support from the Sylvia and Charles Viertel Foundation (ACW), NHMRC (ACW), Cancer Council of Victoria (ACW, SMNO), and Deakin University International Research Scholarship Scheme (SMNO, PR, JK). This project utilized the resources of FishWorks: Collaborative Infrastructure for Zebrafish Research, an Australian Research Council LIEF initiative.
Acknowledgments:
the authors thank Duncan Shirley and Melanie Condron for expert technical support, Simon Yoong and Graham Lieschke for helpful discussions at the commencement of this project, as well as Daniel McCulloch, Suzita Mohammed-Noor and Cristal Peck for assistance with preparation of the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059-63 [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan LA, Liongue C, Lewis RS, Stephenson SEM, Ward AC. Cytokine receptor signaling through the Jak/Stat/Socs pathway in disease. Mol Immunol. 2007;44(10):2497-506 [DOI] [PubMed] [Google Scholar]
- 3.Jeong EG, Kim MS, Nam HK, Min CK, Lee S, Chung YJ, et al. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res.2008;14(12):3716-21 [DOI] [PubMed] [Google Scholar]
- 4.Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med.2008;205(4):751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang Z, Zhao Y, Mitaksov V, Fremont DH, Kasai Y, Molitoris A, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111(9):4809-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10(1):65-75 [DOI] [PubMed] [Google Scholar]
- 7.Kiyoi H, Yamaji S, Kojima S, Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. Leukemia. 2007;21(3):574-6 [DOI] [PubMed] [Google Scholar]
- 8.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054-61 [DOI] [PubMed] [Google Scholar]
- 9.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med.2005;352(17):1779-90 [DOI] [PubMed] [Google Scholar]
- 10.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-8 [DOI] [PubMed] [Google Scholar]
- 11.Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106(4):1207-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Kim YG, Soung YH, Han KJ, Kim SY, Rhim HS, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25(9):1434-6 [DOI] [PubMed] [Google Scholar]
- 13.Gouilleux-Gruart B, Gouilleux F, Desaint C, Claisse JF, Capiod JC, Delobel J, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87(5):1692-7 [PubMed] [Google Scholar]
- 14.Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159(10):4720-8 [PubMed] [Google Scholar]
- 15.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95(1):19-29 [PubMed] [Google Scholar]
- 16.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90(7):2535-40 [PubMed] [Google Scholar]
- 17.Bousquet M, Quelen C, De Mas V, Duchayne E, Roquefeuil B, Delsol G, et al. The t(8;9)(p22;p24) translocation in atypical chronic myeloid leukaemia yields a new PCM1-JAK2 fusion gene. Oncogene. 2005;24(48):7248-52 [DOI] [PubMed] [Google Scholar]
- 18.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309-12 [DOI] [PubMed] [Google Scholar]
- 19.Schwaller J, Frantsve J, Aster J, Williams IR, Tomasson MH, Ross TS, et al. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J. 1998;17(18):5321-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carron C, Cormier F, Janin A, Lacronique V, Giovannini M, Daniel M-T, et al. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95(12):3891-9 [PubMed] [Google Scholar]
- 21.Lacronique V, Boureux A, Monni R, Dumon S, Mauchauffe M, Mayeux P, et al. Transforming properties of chimeric TEL-JAK proteins in Ba/F3 cells. Blood. 2000;95(6):2076-83 [PubMed] [Google Scholar]
- 22.Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, et al. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol.1997;17(3):1562-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feener EP, Rosario F, Dunn SL, Stancheva Z, Myers MGJ. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signalling. Mol Cell Biol.2004;24(11):4968-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol.2011;18(9):971-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho JM-Y, Beattie BK, Squire JA, Frank DA, Barber DL. Fusion of the ets transcription factor TEL to Jak2 results in constitutive Jak-Stat signaling. Blood. 1999;93(12):4354-64 [PubMed] [Google Scholar]
- 26.dos Santos NR, Ghysdael J. A transgenic mouse model for TEL-JAK2-induced B-cell lymphoma/leukemia. Leukemia. 2006;20(1):182-5 [DOI] [PubMed] [Google Scholar]
- 27.Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell.2000;6(3):693-704 [DOI] [PubMed] [Google Scholar]
- 28.Monni R, Santos SC, Mauchauffe M, Berger R, Ghysdael J, Gouilleux F, et al. The TEL-Jak2 oncoprotein induces Socs1 expression and altered cytokine response in Ba/F3 cells. Oncogene. 2001;20(7):849-58 [DOI] [PubMed] [Google Scholar]
- 29.Frantsve J, Schwaller J, Sternberg DW, Kutok J, Gilliland DG. Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteosomal-mediated degradation. Mol Cell Biol.2001;21(10):3547-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem.2001;276(16):12530-8 [DOI] [PubMed] [Google Scholar]
- 31.Rottapel R, Ilangumaran S, Neale C, La Rose J, Ho JMY, Nguyen MHH, et al. The tumor suppressor activity of SOCS-1. Oncogene. 2002;21 (28):4351-62 [DOI] [PubMed] [Google Scholar]
- 32.Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129(8):2015-30 [DOI] [PubMed] [Google Scholar]
- 33.Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299(5608):887-90 [DOI] [PubMed] [Google Scholar]
- 34.Onnebo SMN, Condron MM, McPhee DO, Lieschke GJ, Ward AC. Hematopoietic perturbation in zebrafish expressing a tel-jak2a fusion. Exp Hematol. 2005;33(2):182-8 [DOI] [PubMed] [Google Scholar]
- 35.Ma AC, Fan A, Ward AC, Liongue C, Lewis RS, Cheng SH, et al. A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp Hematol. 2009;37(12):1379-86 [DOI] [PubMed] [Google Scholar]
- 36.Oates AC, Brownlie A, Pratt SJ, Irvine DV, Liao EC, Paw BH, et al. Gene duplication of zebrafish JAK2 homologs is accompanied by divergent embryonic expression patterns: only jak2a is expressed during erythropoiesis. Blood. 1999;94(8):2622-36 [PubMed] [Google Scholar]
- 37.Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectoderm cells to a neural fate. Genes Dev.1994;8(12):1434-47 [DOI] [PubMed] [Google Scholar]
- 38.Ma AC, Ward AC, Liang R, Leung AY. The role of jak2a in zebrafish hematopoiesis. Blood. 2007;110(6):1824-30 [DOI] [PubMed] [Google Scholar]
- 39.O'Sullivan LA, Noor SM, Trengove MC, Lewis RS, Liongue C, Sprigg NS, et al. Suppressor of cytokine signaling 1 regulates embryonic myelopoiesis independently of its effects on T cell development. J Immunol. 2011;186(8):4751-61 [DOI] [PubMed] [Google Scholar]
- 40.Ward AC, McPhee DO, Condron MM, Varma S, Cody SH, Onnebo SMN, et al. The zebrafish spi1 promoter drives myeloid-specific expression in stable transgenic fish. Blood. 2003;102(9):3238-40 [DOI] [PubMed] [Google Scholar]
- 41.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98(3):643-51 [DOI] [PubMed] [Google Scholar]
- 42.Lieschke GJ, Oates AC, Paw BH, Thompson MA, Hall NE, Ward AC, et al. Zebrafish SPI-1(PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Dev Biol.2002;246(2):274-95 [DOI] [PubMed] [Google Scholar]
- 43.Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92(23):10713-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crosier PS, Bardsley A, Horsfield JA, Krassowska AK, Lavallie ER, Collins-Racie LA, et al. In situ hybridisation screen in zebrafish for the selection of gene encoding secreted proteins. Dev Dyn.2001;222(4):637-44 [DOI] [PubMed] [Google Scholar]
- 45.Yoong S, O'Connell B, Soanes A, Crowhurst MO, Lieschke GJ, Ward AC. Characterization of the zebrafish matrix metalloproteinase 9 gene and its developmental expression pattern. Gene Expr Patterns. 2007;7(1-2):39-46 [DOI] [PubMed] [Google Scholar]
- 46.Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124(20):4105-11 [DOI] [PubMed] [Google Scholar]
- 47.Meng A, Tang H, Ong BA, Farrell MJ, Lin S. Promoter analysis in living zebrafish embryos identifies a cis-acting motif required for neuronal expression of GATA-2. Proc Natl Acad Sci USA. 1997;94(12):6267-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trede NS, Zapata A, Zon LI. Fishing for lymphoid genes. Trends Immunol. 2001;22(6):302-7 [DOI] [PubMed] [Google Scholar]
- 49.Willett CE, Cortes A, Zuasti A, Zapata A. Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev Dyn.1999;214(4):323-36 [DOI] [PubMed] [Google Scholar]
- 50.Lewis RS, Stephenson SEM, Ward AC. Constitutive activation of zebrafish stat5 expands hematopoietic cell populations in vivo. Exp Hematol. 2006;34(2):179-87 [DOI] [PubMed] [Google Scholar]
- 51.Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20(4):367-79 [DOI] [PubMed] [Google Scholar]
- 52.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55-79 [DOI] [PubMed] [Google Scholar]
- 53.Campbell PJ, Griesshammer M, Dohner H, Kusec R, Hasselbalch HC, Larsen TS, et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood. 2006;107(5):2098-100 [DOI] [PubMed] [Google Scholar]
- 54.Staerk J, Kallin A, Royer Y, Diaconu CC, Dusa A, Demoulin JB, et al. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol Biol (Paris). 2007;55(2):88-91 [DOI] [PubMed] [Google Scholar]
- 55.Morgan KJ, Gilliland DG. A role for JAK2 mutations in myeloproliferative diseases. Annu Rev Med.2008;59:213-22 [DOI] [PubMed] [Google Scholar]
- 56.Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W, et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109(11):4924-9 [DOI] [PubMed] [Google Scholar]
- 57.Stoiber D, Kovacic B, Schuster C, Schellack C, Karaghiosoff M, Kreibich R, et al. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J Clin Invest.2004;114(11):1650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malinge S, Monni R, Bernard O, Penard-Lacronique V. Activation of the NF-kappaB pathway by the leukemogenic TEL-Jak2 and TEL-Abl fusion proteins leads to the accumulation of antiapoptotic IAP proteins and involves IKKalpha. Oncogene. 2006;25(25):3589-97 [DOI] [PubMed] [Google Scholar]
- 59.Nguyen MH, Ho JM, Beattie BK, Barber DL. TEL-JAK2 mediates constitutive activation of the phosphatidylinositol 3'-kinase/protein kinase B signaling pathway. J Biol Chem.2001;276(35):32704-13 [DOI] [PubMed] [Google Scholar]
- 60.Ho JM, Nguyen MH, Dierov JK, Badger KM, Beattie BK, Tartaro P, et al. TEL-JAK2 constitutively activates the extracellular signal-regulated kinase (ERK), stress-activated protein/Jun kinase (SAPK/JNK), and p38 signaling pathways. Blood. 2002;100(4):1438-48 [PubMed] [Google Scholar]
- 61.Funakoshi-Tago M, Tago K, Nishizawa C, Takahashi K, Mashino T, Iwata S, et al. Licochalcone A is a potent inhibitor of TEL-Jak2-mediated transformation through the specific inhibition of Stat3 activation. Biochem Pharmacol. 2008;76(12):1681-93 [DOI] [PubMed] [Google Scholar]
- 62.Dawson MA, Curry JE, Barber K, Beer PA, Graham B, Lyons JF, et al. AT9283, a potent inhibitor of the Aurora kinases and Jak2, has therapeutic potential in myeloproliferative disroders. Br J Haematol. 2010;150(1):46-57 [DOI] [PubMed] [Google Scholar]
- 63.Saharinen P, Silvennoinen O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J Biol Chem.2002;277(49):47954-63 [DOI] [PubMed] [Google Scholar]
- 64.De Keersmaecker K, Cools J. Chronic myeloproliferative disorders: a tyrosine kinase tale. Leukemia. 2006;20(2):200-5 [DOI] [PubMed] [Google Scholar]
- 65.Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22(1):87-95 [DOI] [PubMed] [Google Scholar]
- 66.Barahmand-Pour F, Meinke A, Groner B, Decker T. Jak2-Stat5 interactions analyzed in yeast. J Biol Chem.1998;273(20):12567-75 [DOI] [PubMed] [Google Scholar]
- 67.May P, Gerhartz C, Heesel B, Welte T, Doppler W, Graeve L, et al. Comparative study on the phosphotyrosine motifs of different cytokine receptors involved in STAT5 activation. FEBS Lett.1996;394(2):221-6 [DOI] [PubMed] [Google Scholar]
- 68.Pezet A, Ferrag F, Kelly PA, Edery M. Tyrosine docking sites of the rat prolactin receptor required for association and activation of Stat5. J Biol Chem.1997;272(40):25043-50 [DOI] [PubMed] [Google Scholar]
- 69.Ward AC, Hermans MHA, Smith L, van Aesch YM, Schelen AM, Antonissen C, et al. Tyrosine-dependent and independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood. 1999;93(1):113-24 [PubMed] [Google Scholar]
- 70.Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2009;106(23):9414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.