Summary
Dietary restriction (DR), one of the most robust life-extending manipulations, is usually associated with reduced adiposity. This reduction is hypothesized to be important in the life-extending effect of DR, because excess adiposity is associated with metabolic and age-related disease. Previously, we described remarkable variation in the lifespan response of 41 recombinant inbred strains of mice to DR, ranging from life extension to life shortening. Here, we used this variation to determine the relationship of lifespan modulation under DR to fat loss. Across strains, DR life extension correlated inversely with fat reduction, measured at midlife (males, r = −0.41, P < 0.05, n = 38 strains; females, r = −0.63, P < 0.001, n = 33 strains) and later ages. Thus, strains with the least reduction in fat were more likely to show life extension, and those with the greatest reduction were more likely to have shortened lifespan. We identified two significant quantitative trait loci (QTLs) affecting fat mass under DR in males but none for lifespan—precluding the confirmation of these loci as coordinate modulators of adiposity and longevity. Our data also provide evidence for two QTLs previously shown to affect fuel efficiency under DR. In summary, the data do not support an important role for fat reduction in life extension by DR. They suggest instead that factors associated with maintaining adiposity are important for survival and life extension under DR.
Keywords: Dietary Restriction, Fat Mass, Lean Mass, Body Weight, Recombinant Inbred Mice, Quantitative Trait Loci, Lifespan
Introduction
The life-extending effect of dietary restriction (DR) has long been known (McCay et al., 1935; Weindruch and Walford, 1988), but the underlying molecular and physiological mechanisms are still obscure (Masoro, 2005). Reduction of fat mass has been argued to be a key factor (Barzilai, 1999; Barzilai and Gabriely, 2001; Barzilai and Gupta, 1999; Das et al., 2004; Masoro, 1999). The case favoring a role for reduced fat is based on the role of excess visceral adiposity in insulin resistance, type II diabetes, and metabolic syndrome (Despres and Lemieux, 2006). Furthermore, removal of visceral fat not only improves insulin sensitivity (Barzilai et al., 1998; Das et al., 2004) but also extends the lifespan of one strain of rat (Muzumdar et al., 2008). As acknowledged (Muzumdar et al., 2008), this view does not account for DR-induced lifespan extension in strains that do not exhibit metabolic disease or obesity (Masoro et al., 1992; McCarter et al., 2007) nor the beneficial effects of DR on pathological conditions and age-related dysfunctions (e.g. lymphoma, cataracts, etc.) not primarily linked to metabolic disease or obesity (Weindruch and Walford, 1988).
The argument against a role for reduced fat mass in the life-extending effect of DR is based on several observations. In a study using inbred rats, the DR rats with the greatest peak fat reserves had the greatest extension of life (Bertrand et al., 1980). Also, positive correlations have been observed in DR mice between lifespan and body weight (BW) (Goodrick et al., 1990; Harper et al., 2006; Rikke et al., 2010; Weindruch et al., 1986). In addition, the lifespan of genetically obese mice (ob/ob) under DR was found to be the same as the lifespan of their wild-type littermates under DR—even though the obese mice on DR had much higher levels of fat than their wild-type AL controls (Harrison et al., 1984).
Although absolute fat mass and life extension were positively correlated in one strain of rat under DR (Bertrand et al., 1980), no study has asked whether fat reduction per se is associated with life extension by DR. We therefore conducted a systematic, unbiased screen to determine the relationship between DR lifespan and adiposity across 41 recombinant inbred (RI) mouse strains. These strains exhibit extensive genetic variation in the lifespan response to DR (Liao et al., 2010a), ranging from lifespan lengthening to lifespan shortening. We also examined the relationship between lifespan and lean body mass.
Quantitative trait loci (QTL) mapping screens the genome for statistical associations between phenotypic and genotypic information, which facilitates finding genes that underlie quantitative traits (Abiola et al., 2003; Flint and Mott, 2001; Flint et al., 2005; Glazier et al., 2002; Korstanje and Paigen, 2002). Although a large number of QTLs and potential genes have been identified for BW, body composition, and lifespan under AL feeding (de Haan et al., 1998; de Haan and Williams, 2005; Gelman et al., 1988; Henckaerts et al., 2004; Jackson et al., 2002; Miller et al., 1998; Wuschke S. et al., 2006), no study has searched for genetic loci modulating adiposity and lean mass under DR. Earlier studies using ILSXISS RI mouse strains have reported marked genetic variation in the response of BW, growth, fertility, and body temperature to DR, and have begun to identify the genetic basis for this variation using QTL mapping (Rikke et al., 2006; Rikke and Johnson, 2007; Rikke et al., 2010). Here, we searched for QTLs in the ILSXISS RI panel specifying the modulation by DR of adiposity, lean mass, and lifespan as a first step to identify genes and pathways mediating the responses of these traits to DR.
Results
To test the hypothesis that fat reduction under DR is important for life extension, we measured the response of fat and lean mass in the ILSXISS recombinant inbred (RI) mouse strains (Williams et al., 2004)—38 strains for males, 33 for females. Mice were fed AL and DR (60% of AL) diets beginning at 2–5 months of age and body composition was measured using quantitative magnetic resonance (QMR) at 15–17 months of age. Correlation analysis was used to evaluate the association between body composition and lifespan (the lifespan data are the same as in Liao, et al., 2010a). Quantitative trait locus (QTL) mapping was used to identify the genetic regions that contribute to strain variation in the response of body composition and lifespan to DR.
Genetic variation in the response of body composition
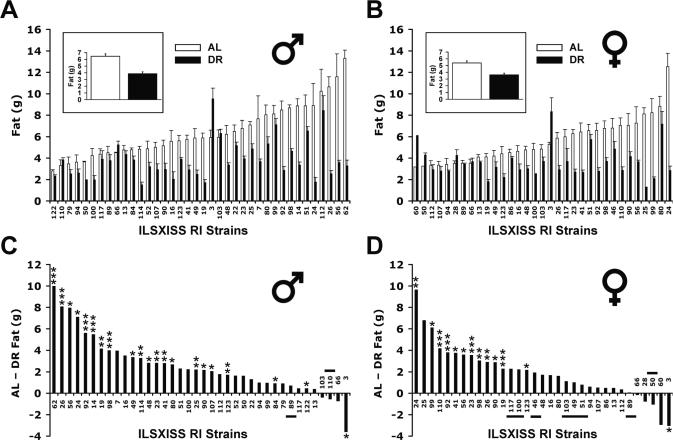
The RI strains exhibited marked genetic variation in absolute fat mass under both AL and DR conditions (Figs. 1A, B). Mean AL fat mass varied 4.8-fold in males and 3.9-fold in females. The strain variation under DR was even greater, varying 6.2-fold in males and 6.3-fold in females. The effect of strain on fat mass was highly significant for both sexes under both feeding conditions (all P < 0.0001, one-way ANOVA). Heritability under AL feeding was 52% for males (95% CI of 39%–63%) and 56% for females (95% CI of 42%–67%). Under DR, heritability was 75% for males (95% CI of 63%–82%) and 64% for females (95% CI of 48%–74%). Surprisingly, fat mass under DR did not correlate with fat mass under AL (Supplementary Table S1), indicating that the genetic modulation of adiposity differs under the two feeding conditions. Adiposity was positively correlated between males and females (Supplementary Table S2).
Fig. 1.
Strain variation in fat mass of ILSXISS recombinant inbred (RI) mice under ad libitum (AL) and 40% dietary restriction (DR) diets. Fat mass was obtained using quantitative magnetic resonance (QMR) from ILSXISS recombinant inbred (RI) mice aged 15–17 months after they were under AL or DR diet since 2–5 months of age. Mean fat mass in the upper two panels is shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL means (A. males, 38 strains; B. females, 33 strains). Insets in panel A and B: the mean fat mass of all strains. Panels C (males) and D (females) illustrate the difference of fat mass between AL and DR groups, ranked from the strain with the greatest reduction in fat mass under DR to the strain with the least reduction (* p < 0.05, ** p < 0.01, *** p < 0.001 by t-test; no experiment-wise Bonferroni correction). The strains that showed significantly increased lifespan under DR (Liao et al., 2010a) are underlined or overlined. Error bars represent SEM.
Overall, 40% DR reduced fat mass by 38% in males and 33% in females (strains equally weighted, Figs. 1A, B). However, the extent of reduction varied markedly among strains (Figs. 1C, D): ranging from an 80% reduction to an unexpected ~60% increase (observed in ILSXISS 3 in both males and females, Figs. 1C, D). Remarkably, many strains showed no appreciable reduction in adiposity under DR. Fat reduction measured again at 20–22 months of age was comparable and significantly correlated with fat reduction assessed at 15–17 months of age (males: r = 0.74; females: r = 0.79; all P < 0.0001; Supplementary Fig. S1).
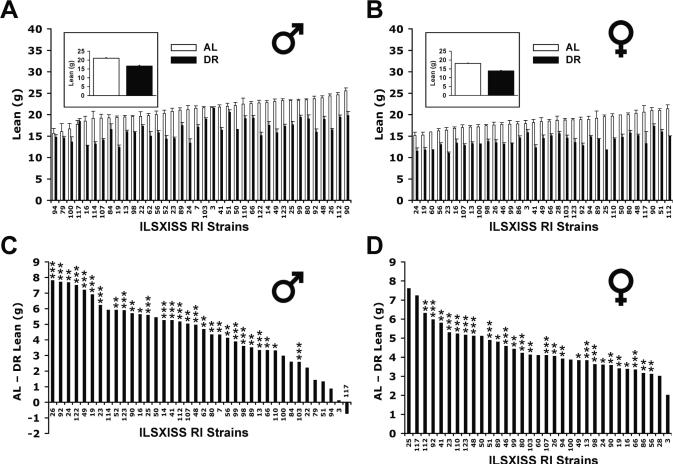
The strain variation in lean body mass under AL and DR diets was also significant, although less than that observed for adiposity (p < 0.0001, one-way ANOVA; Figs. 2A, B). The AL mean varied 1.8-fold in males and 1.7-fold in females. The DR mean varied 1.8-fold in males and 1.6-fold in females. Heritability under AL was 64% in males (95% CI of 52%–73%) and 46% in females (95% CI of 32%–58%), and under DR it was 72% in males (95% CI of 58%–80%) and 61% in females (95% CI of 44%–72%). Lean mass under DR, in contrast to fat mass, correlated with lean mass under AL (Supplementary Table S1), indicating partial genetic co-regulation under the two feeding conditions. Lean mass was positively correlated between males and females (Supplementary Table S2).
Fig. 2.
Strain variation in lean mass of ILSXISS recombinant inbred (RI) mice under ad libitum (AL) and 40% dietary restriction (DR) diets. Lean mass was obtained using quantitative magnetic resonance (QMR) from ILSXISS recombinant inbred (RI) mice aged 15–17 months after they were under AL or DR diet since 2–5 months of age. The mean lean mass in the upper two panels are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL means (A. males, 38 strains; B. females, 33 strains). Insets in panel A and B: the mean lean mass of all strains. Panels C (males) and D (females) illustrate the difference of lean mass between AL and DR, ranked from the strain with the greatest reduction in lean mass under DR to the strain with the least reduction (* p < 0.05, ** p < 0.01, *** p < 0.001 by t-test; no experiment-wise Bonferroni correction). Error bars represent SEM.
Overall, 40% DR reduced lean mass by 17% in males and 21% in females (strains equally weighted, Figs. 2A, B), with most strains exhibiting a reduction that was statistically significant (74% and 79% of the strains for males and females, respectively, using single strain P values < 0.05). The reduction also varied markedly among strains (Figs. 2C, D). This genetic variation was also present at 20–22 months of age (Supplementary Fig. S2) and correlated significantly with the variation at 15–17 months of age (males, r = 0.81; females, r = 0.70; all P < 0.0001).
BW also varied considerably among strains under both AL and DR feeding (Supplementary Fig. S3, p < 0.0001, one-way ANOVA) as reported previously at earlier ages (Rikke et al., 2006).
Relationships among adiposity, lean mass, BW, and lifespan
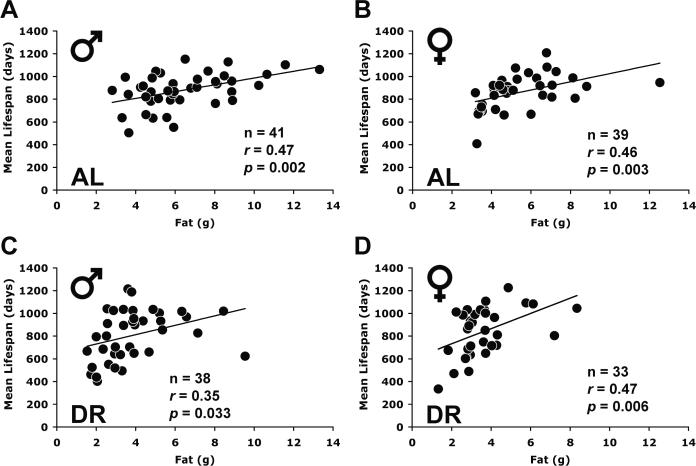
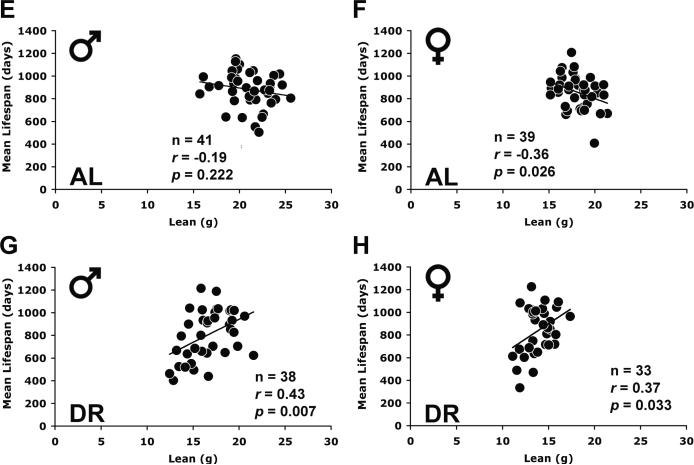
We calculated the correlations between absolute fat mass and lifespan across strains under AL and DR diets to determine whether absolute fat mass was a predictor of lifespan across inbred mouse strains. In DR mice, fat mass correlated positively with mean lifespan in both sexes (Table 1; Figs. 3C, D). BW under DR also correlated positively with lifespan, reflecting the fact that both lean mass and fat mass were positively correlated with lifespan (Table 1; Fig. 3); lean mass and fat mass also covaried positively with each other (Supplementary Table S3). Interestingly, fat mass and lifespan were also positively correlated in mice under AL feeding (Table 1; Figs. 3A, B). However, BW was not correlated with lifespan in AL mice because of a negative relationship between lean mass and lifespan (Table 1; Fig. 3). All of these correlations persisted at 20–22 months of age (Supplementary Table S4).
Table 1.
Correlation coefficients among strain means between mean lifespan and absolute fat mass, lean mass, and body weight (BW) at 15–17 months of age in both sexes and diets.
| Fat | Lean | BW | ||
|---|---|---|---|---|
| AL lifespan | ♂ (n=41) | 0.47** | −0.19 | 0.18 |
| AL lifespan | ♀ (n=39) | 0.46** | −0.36* | 0.11 |
| DR lifespan | ♂ (n=38) | 0.35* | 0.43** | 0.42** |
| DR lifespan | ♀ (n=33) | 0.47** | 0.37* | 0.51** |
P < 0.05;
P < 0.01: Pearson correlation coefficient (r), 2-tailed tests.
The lifespan data are from Liao et al., 2010a.
n: number of RI strains.
Fig. 3.
Scattergram showing the correlation between absolute fat mass, lean mass and lifespan in ad libitum (AL) and 40% dietary restriction (DR) diets for mice aged 15–17 months. Mean values of each ILSXISS recombinant inbred (RI) strain (A, C, E, G: males; B, D, F, H: females) are shown. Mean lifespan was derived from Liao et al. (2010a). The lines represent linear regression. The P values of the Pearson correlation coefficients (r) are all from 2-tailed tests. n: number of strains.
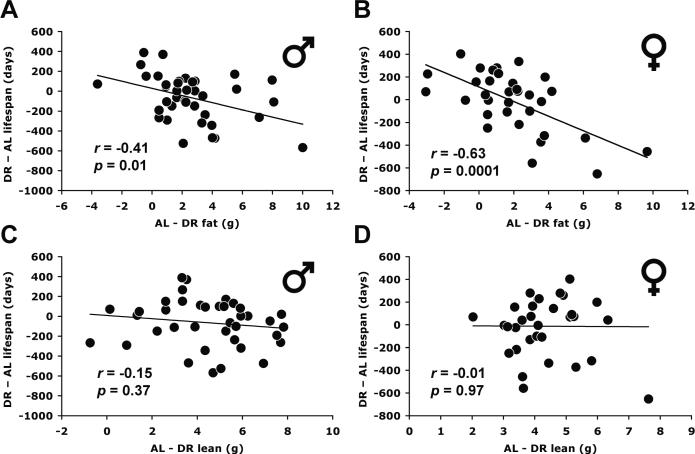
To address directly the relationship between fat reduction by DR and lifespan extension, we defined fat reduction as the difference score between AL fat mass and DR fat mass and compared this construct to the difference score defining lifespan extension (DR lifespan minus AL lifespan) across all strains (Figs. 4A, B). Fat reduction correlated inversely with lifespan extension in both males (r = −0.41, P = 0.01) and females (r = −0.63, P = 0.0001). Thus, the strains with the least reduction of fat were more likely to have lifespan extension and those with the greatest reduction of fat were more likely to have lifespan shortening (Figs. 4A, B). The same negative correlation was also found at 20–22 months of age (males, r = −0.60, P < 0.0001; females, r = −0.60, P = 0.0003). In fact, of the 10 strains with significantly increased lifespan under DR (Liao et al., 2010a), none had a significant reduction in fat (Figs. 1C, D). We also compared the reduction in lean mass, calculated as lean mass under AL minus lean mass under DR, to the extension of longevity. The reduction in lean mass did not correlate with lifespan extension in either males or females (Figs. 4C, D). The same result was obtained at 20–22 months of age (males, r = −0.26; females, r = −0.13). The result differs from the positive correlation between absolute lean mass and lifespan under DR because the difference scores incorporate AL lean mass and AL lifespan and their negative correlation.
Fig. 4.
Correlation between fat reduction and lifespan modulation under 40% dietary restriction (DR). X-axis: fat (or lean) reduction was measured by subtracting fat (or lean) mass under DR from fat (or lean) mass under AL feeding for each strain. Y-axis: lifespan modulation was measured by subtracting mean lifespan under AL from mean lifespan under DR (Liao et al., 2010a). Fat and lean mass were derived from 15–17 months of age. Reduction in lean body mass was not correlated with differential lifespan (C: males; D: females). The lines represent linear regression. The P values of the Pearson correlation coefficients (r) are all from 2-tailed tests.
QTL analysis
We next sought to identify chromosomal regions that modulate these genetic traits. To identify these loci, we conducted QTL mapping. The DR strain means for fat mass, lean mass, and lifespan were regressed on their respective AL means (Rikke et al., 2010). The regression corrects for the strain differences in the absence of DR without making any assumptions about whether a difference score or percent change is more appropriate (Kaiser, 1989).
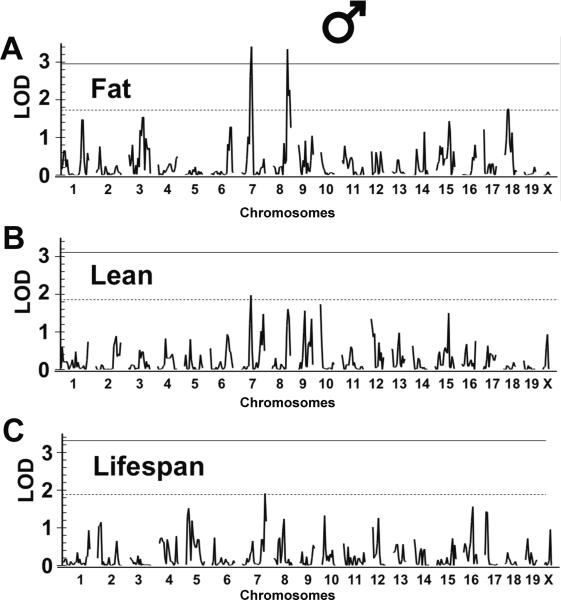
In males, we identified two significant QTLs on chromosomes 7 and 8 and one suggestive QTL on chromosome 18 affecting DR fat mass (Fig. 5A; Table 2). We designated the significant QTLs as “fat response to DR, QTL1 (Fdr1) and Fdr2. The ISS allele of the chromosome 7 QTL (peak LOD at marker D7Mit91) was associated with greater fat mass; the ISS allele of the chromosome 8 QTL (peak D8Mit200) was associated with lower fat mass (Table 2). These alleles also appeared to affect lean body mass in the same direction, and Fdr1 coincided with a suggestive QTL for lean mass (Fig. 5B, Table 2).
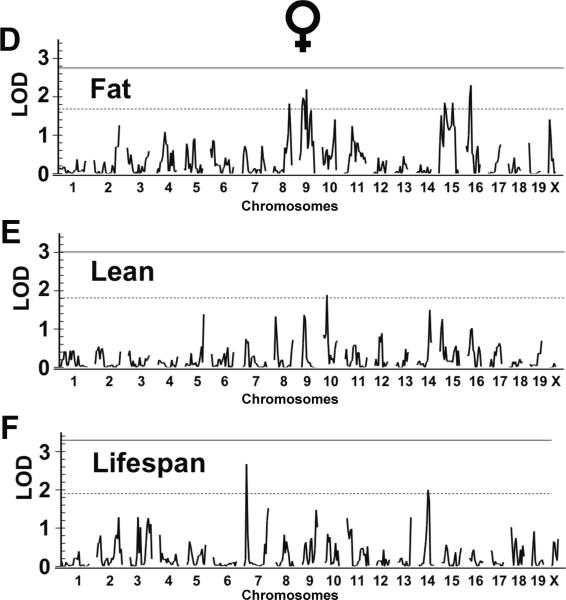
Fig. 5.
Quantitative trait loci (QTL) mapping for specific dietary restriction (DR) effects. QTL mapping for fat (A & D), lean (B & E) and lifespan (C & F) response to DR were screened across the genome for the ILSXISS recombinant inbred (RI) strains aged 15–17 months after they were under AL or DR diet since 2–5 months of age. Chromosome location is on the x–axis, and logarithm of odds (LOD) score is on the y–axis. Solid lines and dash lines indicate the genome-wide significant (P < 0.05) and suggestive (P < 0.63) threshold of LOD, respectively, as determined by permutation tests using 10,000 permutations.
Table 2.
QTLs for response of fat mass, lean mass, and lifespan to DR for the ILSXISS recombinant inbred (RI) strains. Significant QTLs (Fdr1 and Fdr2 on chr 7 and 8, respectively) are indicated in Bold.
| Trait | Sex | Chr | Locus | LOD | P value | Marker position (cM) | Effect Size (%) and Direction (+/−) |
|---|---|---|---|---|---|---|---|
| Fat | Male | 7 | D7Mit85 | 2.6 | 0.0005 | 26.5 | +27 |
| 7 | D7Mit91 | 3.4 | 0.00007 | 28.1 | +34 | ||
| (0.015) # | (16.5~39.7) * | (+20) b | |||||
| 8 | D8Mit200 | 3.3 | 0.00009 | 58 a | −22 | ||
| (0.027) * | |||||||
| 8 | D8Mit120 | 2.1 | 0.00184 | 61 | −15 | ||
| 8 | D8Mit148 | 2.3 | 0.00127 | 67 | −16 | ||
| 18 | D18Mit200 | 1.7 | 0.00465 | −8 | |||
| 18 | D18Mit94 | 1.8 | 0.00443 | −8 | |||
| Female | 8 | D8Mit120 | 1.8 | 0.00371 | 61 | −23 | |
| 9 | D9Mit256 | 1.7 | 0.00475 | 27 | −21 | ||
| 9 | D9Mit4 | 2.0 | 0.00261 | 29 | −24 | ||
| 9 | D9Mit300 | 1.9 | 0.0031 | 31 | −23 | ||
| 9 | D9Mit289 | 2.2 | 0.0015 | 38 | −26 | ||
| 15 | D15Mit84 | 1.8 | 0.00352 | 21.1 | −23 | ||
| 15 | D15Mit86 | 1.7 | 0.00539 | 22.2 | −21 | ||
| 15 | D15Mit93 | 1.8 | 0.00354 | 43.7 | −23 | ||
| 16 | D16Mit103 | 2.0 | 0.00255 | 22.2 | +24 | ||
| 16 | D16Mit58 | 2.3 | 0.00113 | 23.1 | +27 | ||
| Lean | Male | 7 | D7Mit91 | 2.0 | 0.00257 | 28.1 | +21 |
| Female | 10 | D10Mit106 | 1.9 | 0.0031 | 17 | +23 | |
| Lifespan | Male | 7 | D7Mit292 | 1.9 | 0.00309 | 69 | +19 |
| Female | 7 | D7Mit154 | 2.7 | 0.00046 | 4 | +27 | |
| 14 | D14Mit71 | 2.0 | 0.00244 | 44 | −21 |
All other QTLs are suggestive.
Chr: chromosome.
LOD: logarithm of odds.
P value: single marker.
Genome-wide P value.
Effect size (%): Percentage of phenotypic variance accounted for by QTL, which is overestimated because of low statistical power; Direction (+/−) for the effect size refers to the direction of the ISS allele
95 % confident interval (CI) of Fdr1 QTL.
95 % CI is not available due to the estimated chromosome region is larger than chr 8.
corrected effect size.
For the lifespan response to DR in males, a suggestive QTL was found near the distal end of chromosomes 7 (Fig. 5C, Table 2). This QTL did not overlap with Fdr1. At Fdr1 there was a small QTL peak (single-marker P = 0.08) in which the ISS allele was associated with greater lifespan consistent with the positive genetic correlation that we observed between DR fat and lifespan.
In females, we identified four suggestive QTLs affecting the body fat response (on chrs 8, 9, 15, 16; Fig. 5D, Table 2). The locus on chromosome 8 overlaps with Fdr2, and the ISS allele is associated with reduced fat as it is in males. The suggestive QTLs on chromosomes 9 and 15 overlap with and provide further validation of the significant QTLs that we identified previously as affecting fuel efficiency in response to DR (Rikke et al., 2010), the ILS alleles of both QTLs are associated with greater BW, growth, and fat. A suggestive QTL affecting lean body mass was found on chromosome 10 (Fig. 5F, Table 2). Two suggestive QTLs affecting the female lifespan response to DR were found on chromosomes 7 and 14 (Fig. 5G, Table 2).
For AL mice, we identified a significant QTL on chromosome 1 (LOD peak at D1Mit135; 59.7 cM) affecting female lifespan, which we have designated LS3 (Supporting Fig. S4; genome-wide P = 0.038; 95% CI of 56.8–62.7 cM; effect size ~18 %). No significant QTLs were identified by mapping on the difference scores for fat mass, lean mass, or lifespan. This indicates that using difference scores reduced the power to detect QTLs.
Discussion
Our results indicate that reduction of total fat stores correlates inversely with life extension by DR: strains with the least reduction in fat were significantly more likely to show life extension. Strikingly, none of the strains showing lifespan extension exhibited a significant reduction in adiposity. Absolute fat mass under DR, which was uncorrelated with absolute fat mass under AL feeding, was also positively correlated with DR lifespan. Both ways of examining the data suggest that the maintenance, not reduction, of adiposity under DR, or factors associated therewith, are important to DR's life-extending effect.
A limitation of this study is that our measures of body composition were conducted (for logistical reasons) relatively late in life: from 15–17 months onward. Therefore, the genetic relationship between fat mass and lifespan under DR could be different at younger ages. The following argument makes this possibility unlikely. Although we have no data for adiposity at ages earlier than 15 months, BW reduction at younger ages, which correlates with fat reduction at 15–17 months of age (r = 0.87 for females and r = 0.88 males; Ps < 0.0001), was significantly correlated with DR life extension. In males, BW reduction was inversely correlated with life extension at 8 and 12 months of age (r = −0.31, P = 0.047; r = −0.45, P = 0.004, respectively) and at 12 months of age in females (r = −0.39, P = 0.02). Therefore, assuming that body fat and BW reduction were correlated at these younger ages as they were at 15–17 months and older, it seems unlikely that the genetic relationship between lifespan and body fat measured at older ages was appreciably different at younger ages. Another issue concerns the relative importance of body compositional changes to the longevity effect of DR. The correlations between fat reduction, absolute fat mass, and lean body mass with lifespan range between 0.35 and −0.63, thus only accounting for a fraction of the variance in lifespan. The large amount of variation unexplained by these factors implicates other factors in the lifespan modulation by DR—a result consistent with the view that DR acts through multiple physiological processes and biochemical pathways (Masoro, 2005).
Why is maintenance of adiposity associated with lifespan extension under DR, and conversely, loss of adiposity with lifespan shortening under DR? Insight may be gained by considering the major compensatory adaptations employed to reduce energy expenditure and preserve fat mass during nutrient deprivation, namely: reductions in body temperature, metabolic rate, and motor activity (Waterlow, 1986)— especially since the first two responses have been implicated as potential anti-aging mechanisms in DR (Conti et al., 2006; Ferguson et al., 2008; Rikke and Johnson, 2004, 2007) (reviewed by Masoro, 2005).
We examined the relationship between the responses of fat mass and body temperature to DR by comparing our data for fat to the temperature data obtained by Rikke et al (2007) in different cohorts of the same strains. In contrast to the expectation that lower body temperature would be associated with energy conservation and thus the preservation of fat, we found that fat mass and body temperature were positively correlated (r = 0.56, p = 0.003, n = 27 strains): strains with lower body temperature under DR had less fat (and BW). The strength of this relationship is remarkable given that the body temperature and body composition responses to DR were measured in separate cohorts and colonies, and surprising given that the body temperature and BW responses to DR were not correlated when measured in the same cohort (Rikke and Johnson, 2007). These results do not support a physiological trade-off between temperature reduction and fat maintenance.
Interestingly, reduction of body temperature was also a negative predictor of life extension in this study (r = −0.47, P = 0.008, n = 31 strains; females; males were not studied in the earlier report): strains with the greatest reduction in body temperature were at greatest risk for life shortening and those maintaining higher body temperature were more likely to have extended lifespan under DR. This relationship was dependent on the correlation between the body temperature and body fat responses, suggesting a coordinated genetic modulation of all three responses. Therefore, the results suggest that, if anything, the genetic profile associated with extended lifespan under DR in these strains minimizes losses in body temperature as well as body fat. These findings also contradict a simple model in which lower temperature per se (independent of lower metabolic rate) extends lifespan (Conti, 2008; Conti et al., 2006; Koizumi et al., 1992; Rikke and Johnson, 2004; Turturro and Hart, 1991).
Another energy-conserving response to reduced food availability is a reduction in lean body mass, which reduces whole animal metabolic rate (Hambly and Speakman, 2005; Li et al., 2010). In contrast to the expectation that reduced lean mass as an energy preserving response would be greater in strains that maintained adiposity, we found that it was positively correlated with the reduction in fat mass (males: r = 0.54, P < 0.001; females: r = 0.33, P = 0.06). Thus, the data do not support a significant role for reduced whole animal metabolic rate, to the extent that it is determined by reducing lean body mass, in either the maintenance of fat mass or life extension under DR. These results, however, do not exclude the possibility that reductions in resting metabolic rate per unit lean mass could be an adaptive response to preserve adiposity. DR rats have reduced plasma thyroid hormone (Herlihy et al., 1990), a hormonal response that is consistent with reduced resting metabolic rate. However, several studies have reported that resting metabolic rate normalized to lean body mass is not decreased by DR after the initial period of body mass adjustment to reduced caloric intake (Hambly and Speakman, 2005; McCarter and Palmer, 1992).
Reduced motor activity is another adaptive response to conserve energy and preserve fat (Hambly and Speakman, 2005). The LSXSS RI strains (derived from the same parental stock as the ILSXISS strains used in this study) exhibit dramatically reduced runwheel activity—by about 50% when fed 60% AL, with only 1 strain out of 14 showing increased activity (Rikke et al., 2003). However, the genetic variation in runwheel and home-cage activity was not correlated with the BW response in that study (Rikke et al., 2006). Therefore, given the high correlation between BW and body fat in this study, it appears unlikely that the motor response to DR is having an appreciable effect on the fat response in these strains.
Despite the lack of support for body fat being genetically maintained as a consequence of reduced energy utilization by classic physiological responses, the observation that maintenance of adiposity is a predictor of lifespan extension by DR supports a fuel efficiency model (Rikke et al., 2010). Fuel efficiency is a surrogate measure of metabolic efficiency defined in terms of maintaining higher BW and growth rate (Rikke et al., 2010). We have previously shown that this construct predicts DR's coordinated genetic effect on lifespan and female fertility. This study further supports this model by showing that the maintenance of body fat correlates positively with BW and lifespan under DR. Furthermore, the two QTLs that we previously identified as affecting fuel efficiency, on chromosome 9 and 15, proved to be suggestive QTLs in this study, affecting the body fat response in the direction expected (ILS alleles associated with maintaining higher body fat and BW).
A related question raised by this study concerns the observation that absolute adiposity was positively correlated with lifespan not only in DR mice but also in mice under AL feeding. Although this seemingly runs counter to the evidence that obesity is a risk factor for early mortality in humans as well as rodents, strains in this study may not be obese under AL feeding. Obesity, defined operationally, would be a level of adiposity increasing risk for morbidity and mortality. Obese (ob/ob) mice, which develop diabetes and have short lifespan, have 67% fat (Harrison et al., 1984). By contrast, most strains in our study have less than 30% of fat at 15–17 months of age (Supporting Fig. S5). Epidemiologic studies show that both too much or too little fat are deleterious for lifespan (Flegal et al., 2007; Orpana et al., 2010) --- a result that produces an inverted U-shaped curve for the relationship between adiposity and longevity. Although obesity is strongly associated with hyperglycemia and insulin resistance, lipoatrophy, a condition of depleted fat reserves, is also a risk factor—not only for insulin resistance (Fontana and Klein, 2007; Reitman et al., 1999), but also elevated inflammatory tone (Herrero et al., 2010). Thus, the RI strains in this study may not be in the range of excess adiposity that puts them at risk for premature death under AL feeding. Moreover, strains with the shortest lifespans and the lowest level of adiposity may be in the range associated with potentially life-shortening lipoatrophic sequelae (Fig. 1A,B). Such lipoatrophy may also play a role in the lifespan-shortening effect of DR observed in many of the RI strains, the surprising finding reported earlier (Liao et al., 2010a, b; Rikke et al., 2010).
Our data indicate that reduction of total fat mass below an as yet undefined threshold is a risk factor for lifespan shortening under DR; however the data do not rule out the possibility that selective reduction in visceral adiposity may still play a role in lifespan extension in some or all strains (Muzumdar et al., 2008). The beneficial effects of subcutaneous fat over visceral fat have been reported (Tran et al., 2008). Interestingly, DR in C57BL/6J females, redistributed fat stores in favor of subcutaneous depots over visceral (Varady et al., 2010), and a limitation of our study is that fat depots were not distinguishable by our measurement technique. Given the different effects of subcutaneous, visceral, and brown fat on metabolic function (Rosen and Spiegelman, 2006; Tran et al., 2008), it will be informative to determine 1) whether the inverse relationship between fat and lifespan modulation holds for all or only selected fat depots, and 2) whether strains in which DR extended lifespan show a redistribution of fat stores favoring subcutaneous depots, despite minimal reduction in total fat stores. In addition, DR not only may redistribute fat mass, but it also may modify the secretary profiles of adipokines from fat, which have effects on systemic metabolism as well as inflammation (Rosen and Spiegelman, 2006). For example, DR increases the plasma level of adiponectin, which is secreted exclusively from adipose tissue and is involved in glucose homeostasis and insulin sensitivity (Berg et al., 2001; Zhu et al., 2004). DR also decreases tumor necrosis factor-α (TNF-α) from adipose tissue, which impairs insulin signaling and thereby increases insulin resistance (Barzilai and Gupta, 1999; Bordone and Guarente, 2005). It will be informative to measure the secretory profiles of adipose tissue from these mouse strains under DR. Strain variation of those traits may be also critical for animal survival under DR.
The inverse correlation between lifespan extension and fat reduction in response to DR demonstrates that these two traits share genetic pathways. However, the absence of significant QTLs affecting lifespan indicates that the QTL effect sizes for longevity are below our limit of reliable detection; in this study a QTL would have to explain 43% of the genetic variation to be detected with 80% power (Belknap et al., 1996). In our studies, power for detecting QTLs the size of Fdr1 and Fdr2 was less than 50%, which greatly limited our ability to establish whether or not these QTLs also affected lifespan. Nonetheless, we did find evidence that the ISS allele on Fdr1 in males was associated with positive effect on the fat mass and lifespan response to DR, and the ISS allele at Fdr2 was associated with a negative effect on both traits. Additional studies using more powerful statistical analyses and genetic resources will be needed to validate these results.
In summary, this study demonstrates remarkable strain variation in fat reduction by DR in RI mouse strains and shows that factors associated with maintaining, rather than reducing fat, are important to the mechanism of survival and life extension under DR. These results also provide further support for the metabolic efficiency model underlying the prolongevity effect of DR.
Experimental procedures
Mouse strains and husbandry
This study used 41 ILSXISS recombinant inbred (RI) mouse strains (Williams et al., 2004) (formerly called LXS) originally developed to analyze genetic variation in alcohol sensitivity (Bennett et al., 2006). Briefly, ILSXISS RI strains were derived from an F1 cross between two progenitor inbred strains, Inbred Long Sleep (ILS) and Inbred Short Sleep (ISS) strains. The F1s were then crossed to produce heterozygous F2s. The F2s were inbred using 20 or more consecutive generations of brother/sister matting to generate up to 75 strains of ILSXISS lines (Williams et al., 2004). Since each RI strain is inbred, any given genetic locus has either an ISS or ILS allele. These strains have distinct strain distribution patterns of their ISS and ILS alleles that provide a powerful means of associating genetic variation with causal genetic loci (Rikke and Johnson, 2007; Williams et al., 2004).
The animal husbandry was described in a previous report (Liao et al., 2010a). Briefly, mice were typically maintained 5/cage in a specific-pathogen-free vivarium dedicated to murine aging research. Males (38 strains) and females (33 strains) were separately housed in the same room with a 10:14 hour light/dark cycle (lights out at 5:00 PM). DR was implemented at 2–5 months of age immediately at 60% of AL intake calculated for each strain on the basis of AL food intake measured weekly and adjusted for wastage. The rations were given daily just before lights out. At 12 months of age, the DR rations were fixed to avoid tracking the reduction of food intake that can occur with aging. The diet was Harian-Teklad 7912, which is an irradiated, non-purified mouse chow (> 19% crude protein, > 5% crude fat, < 5% crude fiber).
Measurements of body composition and body weight
The whole-body-composition analysis was conducted using quantitative magnetic resonance (QMR) machine (Echo Medical System, Houston, TX, USA) (Tinsley et al., 2004); the AL and DR mice being analyzed over the same time period. This machine utilizes nuclear magnetic resonance (NMR) to reliably and accurately analyze the physical state of the tissue (Taicher et al., 2003; Tinsley et al., 2004), which provides an estimate of total body fat, lean mass, and free water. The procedure involved immobilizing the mice in plastic restrainer tubes (no sedation) placed in the QMR machine. Scanning takes less than 2 minutes per mouse. BW was also recorded at the same time and every 3 weeks from weaning to death.
Statistical analysis
The effect of strain on adiposity and lean mass were calculated by oneway analysis of variance (ANOVA). AL and DR groups in each strain were compared using unpaired t-tests. Correlations were assessed using Pearson product-moment analysis (two-tailed). Statistical tests were conducted using Statistical Package for Social Sciences 16.0 for Mac (SPSS® Inc., Chicago, IL); p values less than 0.05 were considered significant.
To determine whether fat and lean mass were heritable and thus suitable for linkage analysis, the heritability (h2RI, narrow sense) was calculated as previously described (Rikke et al., 2004). Basically, heritability is estimated from the components of variance between and within strains calculated from ANOVA.
Regression
The fat response to DR is defined as the strain means for DR fat regressed on the AL means, which removes any correlation with the AL variation. Although DR fat was not correlated with AL fat, we regressed DR fat on AL fat for genetic mapping in both sexes to clarify the specific effects of DR on fat reduction. Such an adjustment removes the confound between variation under AL and variation imposed by DR, and enhanced statistical power for identifying QTL for DR response with this approach has been shown (Rikke et al., 2006; Rikke et al., 2010). For QTL mapping, the lean mass response and lifespan response to DR were defined similarly by regressing on their respective AL strain means.
Quantitative Trait Loci (QTL) mapping
The phenotypic data (the mean of each strain and group) were subjected to a QTL mapping using Map Manager QTXb20 (http://www.mapmanager.org/mmQTX.html) (Manly et al., 2001). The program uses marker regression and an additive regression model to detect genetic loci influencing complex traits. The strain distribution pattern of 330 simple sequence length polymorphism (SSLP) markers was used in the QTL analysis. The positions of these microsatellite markers were updated using the Mouse Genome Database (http://www.informatics.jax.org/) as of January 2010. Briefly, the strength of the association between the genotypes and phenotypes is calculated as a logarithm of odds (LOD). The significant QTLs exceed the threshold of genome-wide P < 0.05 and suggestive QTLs exceed P < 0.63 (cutoff that permits one false positive) determined empirically by permutation testing (10,000 trials) (Churchill and Doerge, 1994).
Supplementary Material
Scattergrams showing the correlations of fat in AL and DR mice measured at 15–17 and 20–22 months of age. Absolute fat measured at 15–17 months was correlated with that at 20–22 months in both males (A) and females (B) under both AL (◯) and DR (●) feedings. DR-induced fat reduction (i.e., AL minus DR) at 15–17 months was also correlated with that at 20–22 months of age (C. males; D. females). The lines (C&D) represent linear regression. All Ps < 0.0001: Pearson correlation coefficient (r), 2-tailed tests.
Scattergram showing the correlations of lean in AL and DR mice measured at 15–17 and 20–22 months of age. Absolute lean measured at 15–17 months was correlated with that at 20–22 months in both males (A) and females (B) under both AL (◯) and DR (●) feedings. DR-induced lean reduction (i.e., AL minus DR) at 15–17 months was also correlated with that at 20–22 months of age (C. males; D. females). The lines (C&D) represent linear regression. All Ps < 0.0001: Pearson correlation coefficient (r), 2-tailed tests.
Strain variation in body weight (BW) of ILSXISS recombinant inbred (RI) mice aged 15–17 months under ad libitum (AL) and 40% dietary restriction (DR) diets. The mean BW in the upper two panels are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL means (A. males, 38 strains; B. females, 33 strains). Insets in panel A and B: the mean BW of all strains. Panels C (males) and D (females) illustrate the difference of BW between AL and DR, ranked from the strain with the greatest reduction in BW under DR to the strain with the least reduction (* p < 0.05, ** p < 0.01, *** p < 0.001 by t-test; no experiment-wise Bonferroni correction). Error bars represent SEM.
Quantitative trait loci (QTL) mapping for lifespan in females under ad libitum (AL). QTL mapping for lifespan was screened across the genome for the ILSXISS recombinant inbred (RI) strains in females under AL. Chromosome location is on the x–axis, and logarithm of odds (LOD) score is on the y–axis. Solid lines and dash lines indicate the genome-wide significant (P < 0.05) and suggestive (P < 0.63) threshold of LOD, respectively, as determined by permutation tests using 10,000 permutations.
Strain variation in % fat mass of ILSXISS recombinant inbred (RI) mice aged 15–17 months under ad libitum (AL) and 40% dietary restriction (DR) diets. The % fat mass (A. males, 38 strains; B. females, 33 strains) are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL % fat.
Acknowledgements
We thank Vivian Diaz's crew at the Aging and Longevity Assessment Core for outstanding animal husbandry and care. This project was supported by grants from the National Institute on Aging (1 RO1 AG024354), the Glenn Foundation, and the Ellison Medical Foundation.
References
- Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, Blankenhorn EP, Blizard DA, Bolivar V, Brockmann GA, et al. The nature and identification of quantitative trait loci: a community's view. Nat Rev Genet. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N. Author's response to commentary on “Revisiting the role of fat mass in the life extension induced by caloric restriction”. J Gerontol A Biol Sci Med Sci. 1999;54:B98. doi: 10.1093/gerona/54.3.b89. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. Journal of Clinical Investigation. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Gabriely I. The role of fat depletion in the biological benefits of caloric restriction. J Nutr. 2001;131:903S–906S. doi: 10.1093/jn/131.3.903S. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci. 1999;54:B89–96. doi: 10.1093/gerona/54.3.b89. discussion B97–88. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Mitchell SR, O'Toole LA, Helms ML, Crabbe JC. Type I and type II error rates for quantitative trait loci (QTL) mapping studies using recombinant inbred mouse strains. Behav Genet. 1996;26:149–160. doi: 10.1007/BF02359892. [DOI] [PubMed] [Google Scholar]
- Bennett B, Carosone-Link P, Zahniser NR, Johnson TE. Confirmation and fine mapping of ethanol sensitivity quantitative trait loci, and candidate gene testing in the LXS recombinant inbred mice. J Pharmacol Exp Ther. 2006;319:299–307. doi: 10.1124/jpet.106.103572. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bertrand HA, Lynd FT, Masoro EJ, Yu BP. Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J Gerontol. 1980;35:827–835. doi: 10.1093/geronj/35.6.827. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- de Haan G, Gelman R, Watson A, Yunis E, Van Zant G. A putative gene causes variability in lifespan among genotypically identical mice. Nat Genet. 1998;19:114–116. doi: 10.1038/465. see comment. [DOI] [PubMed] [Google Scholar]
- de Haan G, Williams RW. A genetic and genomic approach to identify longevity genes in mice. Mech Ageing Dev. 2005;126:133–138. doi: 10.1016/j.mad.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Despres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. see comment. [DOI] [PubMed] [Google Scholar]
- Flint J, Mott R. Finding the molecular basis of quantitative traits: successes and pitfalls. Nat Rev Genet. 2001;2:437–445. doi: 10.1038/35076585. [DOI] [PubMed] [Google Scholar]
- Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet. 2005;6:271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. Jama. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Gelman R, Watson A, Bronson R, Yunis E. Murine chromosomal regions correlated with longevity. Genetics. 1988;118:693–704. doi: 10.1093/genetics/118.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Hambly C, Speakman JR. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes Res. 2005;13:1548–1557. doi: 10.1038/oby.2005.190. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckaerts E, Langer JC, Snoeck H-W. Quantitative genetic variation in the hematopoietic stem cell and progenitor cell compartment and in lifespan are closely linked at multiple loci in BXD recombinant inbred mice. Blood. 2004;104:374–379. doi: 10.1182/blood-2003-12-4304. [DOI] [PubMed] [Google Scholar]
- Herlihy JT, Stacy C, Bertrand HA. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech Ageing Dev. 1990;53:9–16. doi: 10.1016/0047-6374(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AU, Galecki AT, Burke DT, Miller RA. Mouse loci associated with life span exhibit sex-specific and epistatic effects. J Gerontol A Biol Sci Med Sci. 2002;57:B9–B15. doi: 10.1093/gerona/57.1.b9. [DOI] [PubMed] [Google Scholar]
- Kaiser L. Adjusting for baseline: change or percentage change? Stat Med. 1989;8:1183–1190. doi: 10.1002/sim.4780081002. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Tsukada M, Wada Y, Masuda H, Weindruch R. Mitotic activity in mice is suppressed by energy restriction-induced torpor. J Nutr. 1992;122:1446–1453. doi: 10.1093/jn/122.7.1446. [DOI] [PubMed] [Google Scholar]
- Korstanje R, Paigen B. From QTL to gene: the harvest begins. Nat Genet. 2002;31:235–236. doi: 10.1038/ng0702-235. comment. [DOI] [PubMed] [Google Scholar]
- Li X, Cope MB, Johnson MS, Smith DL, Jr., Nagy TR. Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity (Silver Spring) 2010;18:456–462. doi: 10.1038/oby.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010a;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. No evidence that competition for food underlies lifespan shortening by dietary restriction in multiply housed mice: response to commentary. Aging Cell. 2010b;9:450–452. [Google Scholar]
- Manly KF, Cudmore RH, Jr., Meer JM. Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome. 2001;12:930–932. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Commentary on “Revisiting the role of fat mass in the life extension induced by caloric restriction”. J Gerontol A Biol Sci Med Sci. 1999;54:B97. doi: 10.1093/gerona/54.3.b89. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, McCarter RJ, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. Journal of Gerontology. 1992;47:B202–208. doi: 10.1093/geronj/47.6.b202. erratum appears in J Gerontol 1993 Mar;48(2):B73. [DOI] [PubMed] [Google Scholar]
- McCarter R, Mejia W, Ikeno Y, Monnier V, Kewitt K, Gibbs M, McMahan A, Strong R. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62:1059–1070. doi: 10.1093/gerona/62.10.1059. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol. 1992;263:E448–452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Miller RA, Chrisp C, Jackson AU, Burke D. Marker loci associated with life span in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 1998;53:M257–263. doi: 10.1093/gerona/53a.4.m257. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpana HM, Berthelot J-M, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity (Silver Spring) 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- Reitman ML, Mason MM, Moitra J, Gavrilova O, Marcus-Samuels B, Eckhaus M, Vinson C. Transgenic mice lacking white fat: models for understanding human lipoatrophic diabetes. Annals of the New York Academy of Sciences. 1999;892:289–296. doi: 10.1111/j.1749-6632.1999.tb07802.x. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Battaglia ME, Allison DB, Johnson TE. Murine weight loss exhibits significant genetic variation during dietary restriction. Physiol Genomics. 2006;27:122–130. doi: 10.1152/physiolgenomics.00068.2006. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp Gerontol. 2004;39:927–930. doi: 10.1016/j.exger.2004.03.020. erratum appears in Exp Gerontol. 2004 Sep;39(9):1431. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. Physiological genetics of dietary restriction: uncoupling the body temperature and body weight responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1522–1527. doi: 10.1152/ajpregu.00215.2007. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Liao C-Y, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. 2010;45:691–701. doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Quantitative trait Loci specifying the response of body temperature to dietary restriction. J Gerontol A Biol Sci Med Sci. 2004;59:118–125. doi: 10.1093/gerona/59.2.b118. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Analytical & Bioanalytical Chemistry. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obesity Research. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Hart RW. Longevity-assurance mechanisms and caloric restriction. Ann N Y Acad Sci. 1991;621:363–372. doi: 10.1111/j.1749-6632.1991.tb16992.x. [DOI] [PubMed] [Google Scholar]
- Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J nutr biochem. 2010;21:188–195. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Waterlow JC. Metabolic adaptation to low intakes of energy and protein. Annu Rev Nutr. 1986;6:495–526. doi: 10.1146/annurev.nu.06.070186.002431. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, III: Charles C Thomas Publisher; 1988. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Williams RW, Bennett B, Lu L, Gu J, DeFries JC, Carosone-Link PJ, Rikke BA, Belknap JK, Johnson TE. Genetic structure of the LXS panel of recombinant inbred mouse strains: a powerful resource for complex trait analysis. Mamm Genome. 2004;15:637–647. doi: 10.1007/s00335-004-2380-6. [DOI] [PubMed] [Google Scholar]
- Wuschke S, Dahm S, Schmidt C, Joost H-G, H. A-H. A meta-analysis of quantitative trait loci associated with body weight and adiposity in mice. Int J Obes. 2006:1–13. doi: 10.1038/sj.ijo.0803473. [DOI] [PubMed] [Google Scholar]
- Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scattergrams showing the correlations of fat in AL and DR mice measured at 15–17 and 20–22 months of age. Absolute fat measured at 15–17 months was correlated with that at 20–22 months in both males (A) and females (B) under both AL (◯) and DR (●) feedings. DR-induced fat reduction (i.e., AL minus DR) at 15–17 months was also correlated with that at 20–22 months of age (C. males; D. females). The lines (C&D) represent linear regression. All Ps < 0.0001: Pearson correlation coefficient (r), 2-tailed tests.
Scattergram showing the correlations of lean in AL and DR mice measured at 15–17 and 20–22 months of age. Absolute lean measured at 15–17 months was correlated with that at 20–22 months in both males (A) and females (B) under both AL (◯) and DR (●) feedings. DR-induced lean reduction (i.e., AL minus DR) at 15–17 months was also correlated with that at 20–22 months of age (C. males; D. females). The lines (C&D) represent linear regression. All Ps < 0.0001: Pearson correlation coefficient (r), 2-tailed tests.
Strain variation in body weight (BW) of ILSXISS recombinant inbred (RI) mice aged 15–17 months under ad libitum (AL) and 40% dietary restriction (DR) diets. The mean BW in the upper two panels are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL means (A. males, 38 strains; B. females, 33 strains). Insets in panel A and B: the mean BW of all strains. Panels C (males) and D (females) illustrate the difference of BW between AL and DR, ranked from the strain with the greatest reduction in BW under DR to the strain with the least reduction (* p < 0.05, ** p < 0.01, *** p < 0.001 by t-test; no experiment-wise Bonferroni correction). Error bars represent SEM.
Quantitative trait loci (QTL) mapping for lifespan in females under ad libitum (AL). QTL mapping for lifespan was screened across the genome for the ILSXISS recombinant inbred (RI) strains in females under AL. Chromosome location is on the x–axis, and logarithm of odds (LOD) score is on the y–axis. Solid lines and dash lines indicate the genome-wide significant (P < 0.05) and suggestive (P < 0.63) threshold of LOD, respectively, as determined by permutation tests using 10,000 permutations.
Strain variation in % fat mass of ILSXISS recombinant inbred (RI) mice aged 15–17 months under ad libitum (AL) and 40% dietary restriction (DR) diets. The % fat mass (A. males, 38 strains; B. females, 33 strains) are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL % fat.