Abstract
Two phosphorus-containing glycolipids have previously been observed in Clostridium acetobutylicum. We had shown that the concentration of one of them increases in response to increased unsaturation of the membrane lipid hydrocarbon chains, suggesting a potential role in the regulation of lipid polymorphism in this organism. Mass spectrometry shows that these glycolipids are ethanolamine phosphate (Etn-P)-containing derivatives of a mono- and diglycosyldiradylglycerol. The content of both diglycosyldiradylglycerol and the Etn-P-monoglycosyldiradylglycerol, which increase upon increased unsaturation of the membrane, also increase upon addition of octanol to the medium. Thus, it appears that the Etn-P-monoglycosyldiradylglycerol along with the diglycosyldiradylglycerol may serve to stabilize the membrane bilayer during membrane stress caused by the presence of the solvents produced during fermentation.
Keywords: glycolipid, plasmalogen, phosphoglycolipid, solvent
1. Introduction
Clostridium acetobutylicum has been used in the commercial production of acetone, butanol and ethanol by means of the so-called ABE fermentation for nearly a century. It can use a wide variety of sugars and polysaccharides including starch and xylan. Industrial utilization of the ABE fermentation declined after World War II. As the price of petroleum decreased and that of fermentable materials such as molasses increased, the chemical production of acetone and butanol supplanted the ABE process in many parts of the world. Recent increases in the price of petroleum have led to a re-emergence of interest in the ABE fermentation with the goal of decreasing the energy costs of separating the end-products from the fermentation broth and increasing the yields through genetic engineering [1,2].
One of the barriers to higher yields appears to be the toxicity of the fermentation products [3,4]. A major target affected by solvents is the cell membrane and numerous studies have shown that bacteria can respond to solvents such as acetone-butanol by changing the compositions of the hydrocarbon chains of the lipids and the lipid class compositions [3,5–9]. In an earlier study we showed that the relative amounts of several lipids in C. acetobutylicum changed in response to systematic changes in the degree of unsaturation of the membrane acyl and alk-1’-enyl chains. Increasing the degree of unsaturation of the hydrocarbon chains in lipids such as phosphatidylethanolamine (PE) and monoglycosyldiacylglycerol (MGDG) produces increased curvature strain in the membrane which if not compensated can eventually lead to the formation of non-lamellar phases such as the reversed hexagonal and cubic phases resulting in loss of the barrier function. Increase in the size of the polar head groups by methylation of PE to form phosphatidylcholine or by addition of a second sugar to a monoglycosyldiacylglycerol (MGDG) to form diglycosyldiacylglycerol (DGDG) serves to stabilize the bilayer arrangement [8,10,11]. C. acetobutylicum has both PE and MGDG; however, it is unable to methylate PE. Clostridium butyricum compensates for increased membrane lipid unsaturation by forming a glycerol acetal of plasmenylethanolamine [12] and C. acetobutylicum is also able to form this glycerol acetal. When the cells were fed mixtures of palmitic and oleic acids, the relative amount of the glycerol acetal of plasmenylethanolamine did not change until the membrane hydrocarbon chains were almost entirely unsaturated. As expected, as the degree of unsaturation increased there was an increase in DGDRG and a decrease in MGDRG. There was also an increase in the less polar of two phosphoglycolipids (PGL) [7]. We have now identified these PGL as ethanolamine-phosphate (Etn-P) derivatives of two glycolipids in C. acetobutylicum. In addition to responding to the degree of unsaturation of the membrane lipids, we now show that the Etn-P-MGDRG increases in response to addition of octanol to the culture medium. Thus, the response of Ca to both increased unsaturation and the addition of a solvent is to modify MGDG by adding either a second sugar or by addition of Etn-P to the sugar.
2. Methods
C. acetobutylicum ATCC 824 was the gift of Eleftherios Papoutsakis and Bryan Tracy, University of Delaware. For lipid isolation bacteria were grown overnight on Reinforced Clostridial Medium without agar, which contained per liter: yeast extract, 10.5 g; peptone, 12.5 g; glucose, 5 g; soluble starch, 1 g; NaCl, 5 g; sodium acetate, 3 g; and cysteine HCl•H2O, 0.6 g. The pH was adjusted to approx. 7.1 with NaOH. The glucose solution was autoclaved separately.
Bacteria were harvested by centrifugation at 2900 × g for 10 min and washed twice in 20 mM MOPS buffer, pH 7.2. The wet cell pellets were extracted with chloroform/methanol/water by the method of Bligh and Dyer [13], as modified [14]. The dried lipids were dissolved in chloroform, and stored at −20 °C. under argon.
For quantification of the polar lipids the cells were labeled by overnight growth in 10 ml reinforced clostridial medium with 10 µCi (370 kBq) [1-14C] acetate (60 mCi/mmol−1, 2220 MBq/mmol−1) Perkin Elmer Life Sciences, Waltham, Massachusetts 02451, USA) at 37 °C. Cells were harvested by centrifugation, washed and the lipids extracted as described above. As needed, unlabeled carrier lipid was added. Radiolabeled lipids (4000 cpm) were separated by thin-layer chromatography on silica gel 60, 10 × 20 cm, thin-layer plates. The solvent consisted of chloroform/methanol/concentrated ammonia/water, 65:30:2.5:2.5 (by vol.) After separation they were visualized and quantified with a PhosphoImager (Typhoon 9410, Amersham Biosciences, Arlington Heights, IL), equipped with ImageQuant software.
Normal phase LC-ESI/MS of lipids was performed using an Agilent 1200 Quaternary LC system (Santa Clara, CA), coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA) as previously described [15]. Data acquisition and analysis were performed using the Analyst QS software (Applied Biosystems, Foster City, CA).
3. Results
3.1. Identification of the phosphoglycolipids of C. acetobutylicum
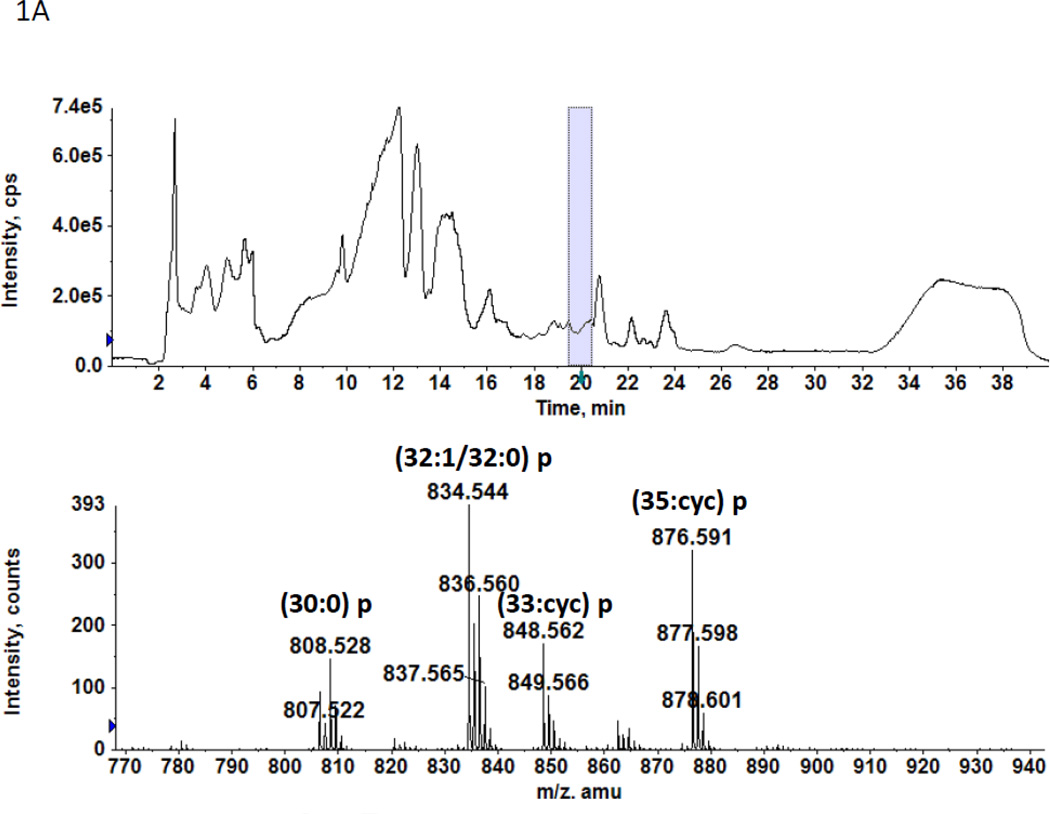
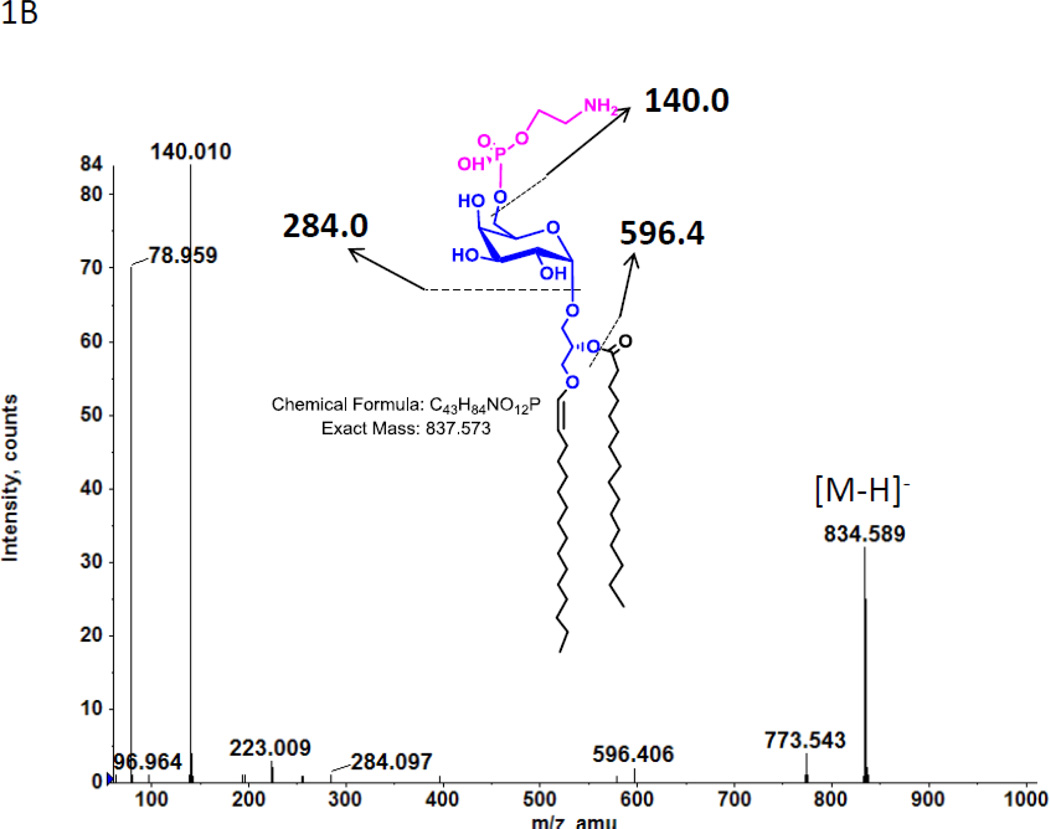
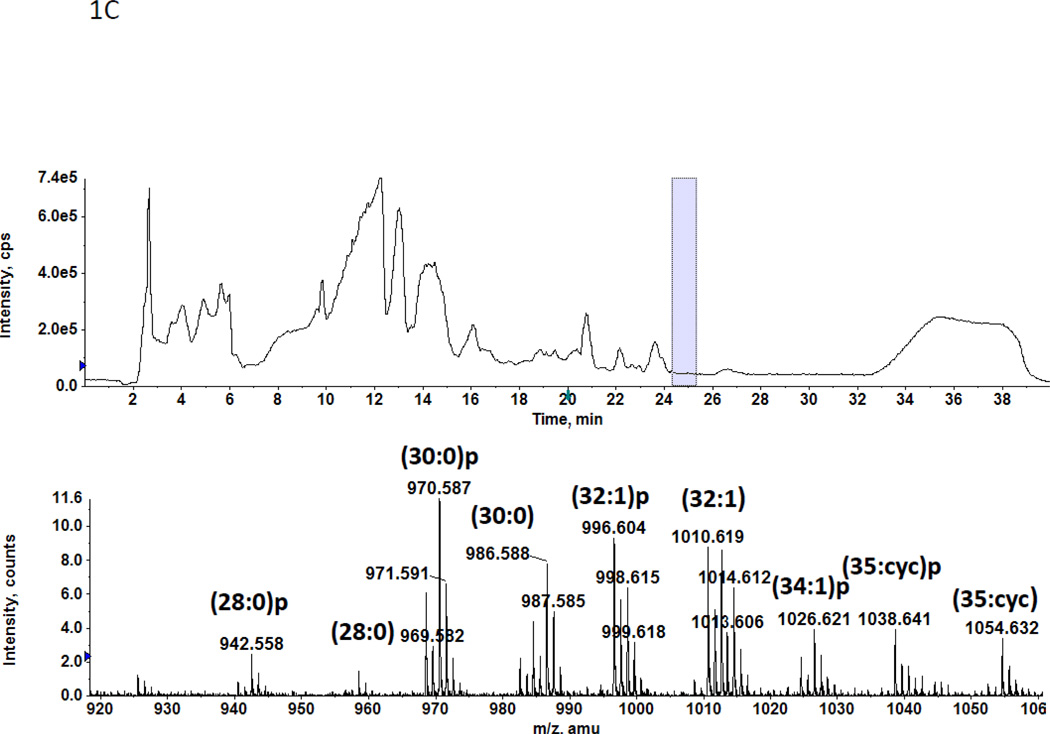
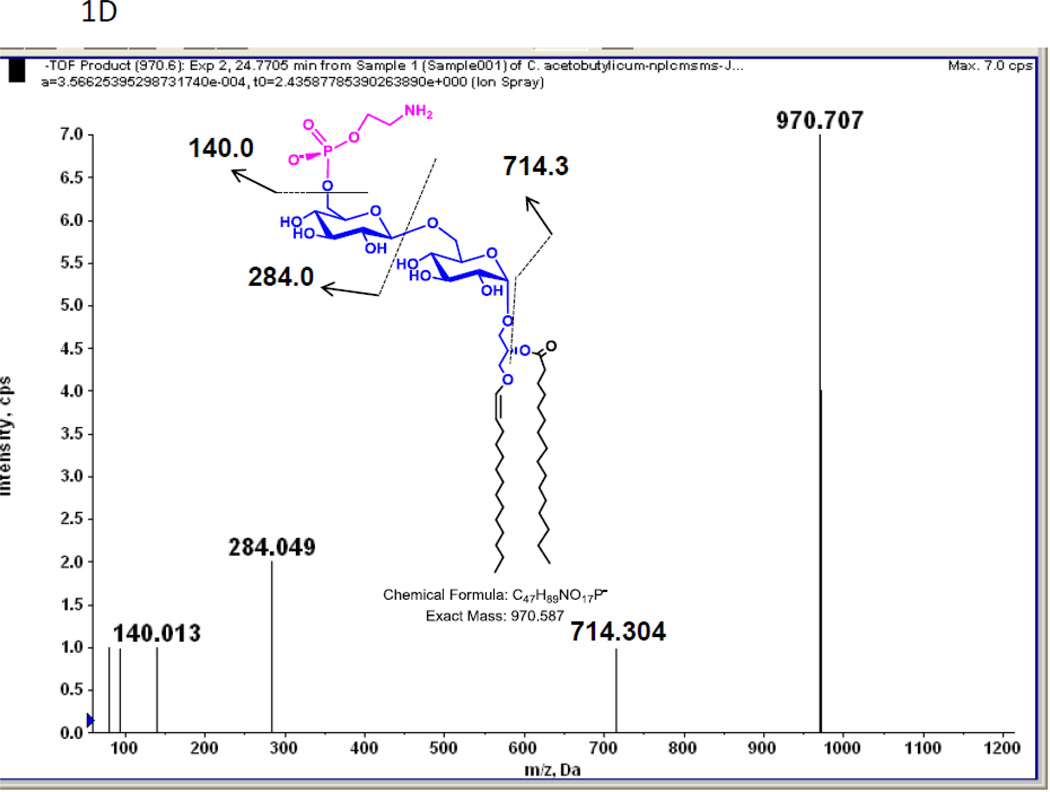
In a previous study we found two previously unreported phosphoglycolipids (PGL) in C. acetobutylicum ATCC 4259 which together represented 16.8% of total lipid phosphorus [7]. 2D-TLC of total lipids from C. acetobutylicum ATCC 824 showed the presence of two PGL with similar migration and staining properties to those seen previously (not shown). Mass spectrometry of total lipids from strain ATCC 824 revealed two very polar lipids, the less polar of which had several molecular species one of which M-H]− m/z 834.58 (Fig. 1A) produced a fragment at 140.010 m/z upon collision-induced dissociation MS/MS (Fig. 1B). This fragment corresponds to ethanolamine-phosphate, previously observed upon fragmentation of Etn-P-GlcNAcdiradylglycerol from Clostridium tetani [16]. Other fragments observed are consistent with the designation of the less polar phosphoglycolipid as Etn-P-glycosyldiradylglycerol. The masses of the more polar phosphoglycolipid exactly matched that of Etn-P-diglycosyldiradylglycerol molecular species (Fig. 1C). Collision-induced dissociation MS/MS of this lipid produced fragments consistent with the designation Etn-P-diglycosyldiradylglycerol (Fig. 1D).
Figure 1.
A. A C. acetobutylicum lipid eluting at 20–21 min in normal phase liquid chromatography has the exact masses of several molecular species of an Etn-P derivative of a monoglycosyldiradylglycerol. The predominant forms are 1-O-alk-1’-enyl, acyl species (plasmalogens) indicated with a p. B. Collision-induced dissociation MS/MS of the [M-H]− m/z 834.58 molecular ion species yields a product ion at the m/z 140.01, corresponding to Etn-P. Other fragments consistent with the designated structure are shown. The location of attachment of Etn-P to the sugar has not been determined. C. The lipid eluting at 24–25 min has the exact masses of several molecular species of Etn-P derivative of a DGDRG. Both diacyl and alk-1’-enyl species are evident. Fig. 1D. Collision-induced dissociation MS/MS of the [M-H]− m/z 970.587 also shows the product ion at the m/z 140.01 and other fragments consistent with the structure shown.
3.2. Regulation by addition of solvent in the growth medium
C. butyricum responds to both increased unsaturation of the membrane lipids and to addition of solvents to the growth medium by a reciprocal increase in the content of the glycerol acetal of plasmenylethanolamine and a decrease in PE and PlaE [12,17,18]. C. acetobutylicum responded to increased unsaturation of the membrane lipids by increasing the relative amounts of DGDG and Etn-P-MGDG compared to MGDG [7]. We therefore asked whether DGDG and Etn-P-MGDG also increase in response to the addition of octanol to the growth medium. Addition of octanol to the medium of growing cells of Clostridium beijerinckii (formerly C. acetobutylicum) IFP 903; ATCC 39057 caused essentially identical changes in the ratios of unsaturated to saturated fatty acids as observed during the production of acetone-butanol by this organism [3].
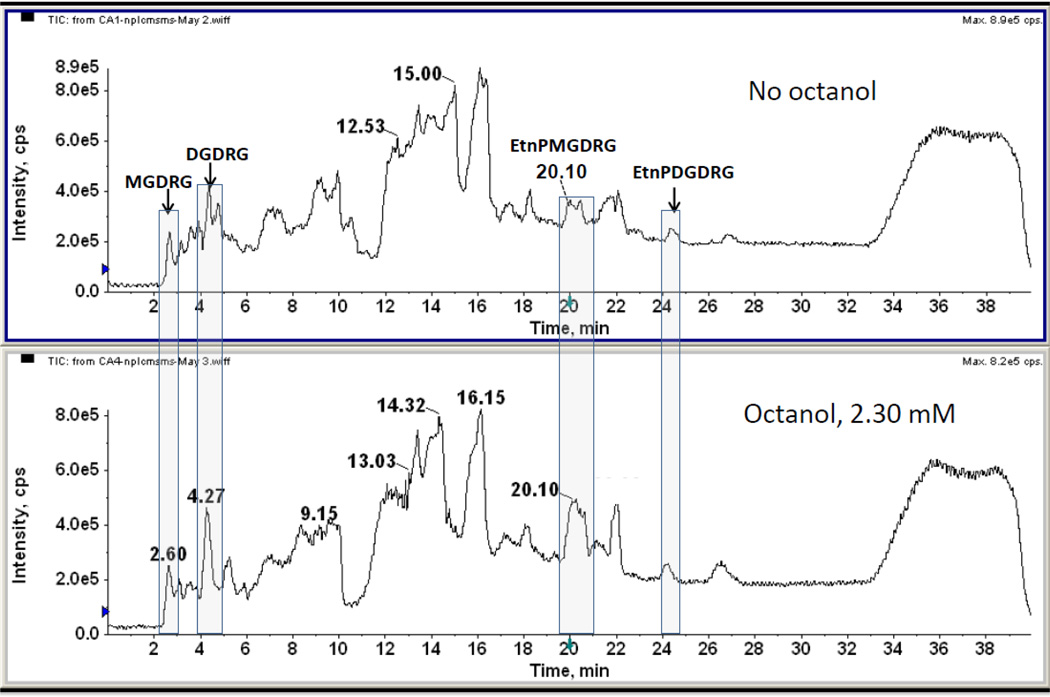
Cells were grown in the presence of 0.765 and 2.30 mM octanol, a concentration range that has been shown to affect the fatty acid composition of C. acetobutylicum [3], and the lipid class composition of C. butyricum [18]. The total lipids were subjected to LC/MS (Fig. 2). The lipids eluting at 2.60 and 4.27 min. were shown by MS to be a MGDRG and a DGDRG, respectively (data not shown) and the lipids eluting at 20 and 24–25 min corresponded to the Etn-P derivatives of MGDRG and a DGDRG, respectively (see Fig. 1). Both DGDRG and Etn-P-MGDRG, but not Etn-P-DGDRG, appeared to increase relative to other polar lipids when cells are grown in the presence of octanol (Fig. 2). Similar results were obtained when cells were grown in the presence of 0.765 mM octanol (data not shown).
Figure 2.
Total ion chromatograms of normal phase liquid chromatography/MS of the lipids of C. acetobutylicum grown without and with 2.3 mM 1-octanol. Relative increases in DGDRG eluting at 4.27 min and Etn-P-MGDRG eluting at 20.1 min are evident in cells grown in octanol-containing medium. There is essentially no change in the Etn-P-DGDRG eluting at 24–25 min.
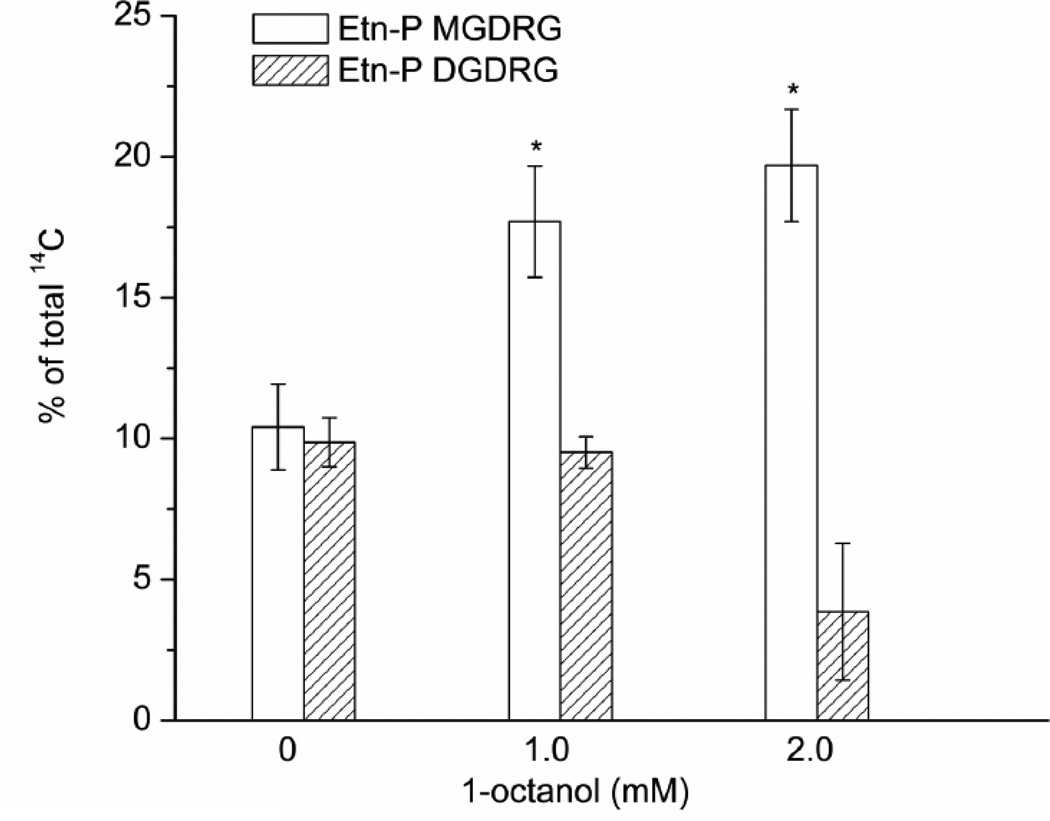
In order to quantify the effect of octanol addition on the relative amounts of Etn-P-MGDRG, cells were grown in the presence of [1-14C] acetate overnight, and the lipids were extracted and separated by TLC. The 14C-labeled lipids were quantified with a phosphoimager (Fig. 3). Etn-P-MGDRG increased 1.75-fold and 1.9-fold, in the presence of 1.0 mM and 2.0 mM octanol. No change was seen in the amounts of Etn-P-DGDRG. At the higher concentration of octanol, there was an approximately 60% reduction in the relative amounts of Etn-P-DGDRG.
Figure 3.
C. acetobutylicum was grown with increasing concentrations of 1-octanol and 14C-acetate. The Etn-P-modified glycolipids were separated by one-dimensional TLC and quantified with a Phosphoimager. Significant increases in Etn-P-MGDRG are seen at 1.0 and 2.0 mM 1-octanol. No significant changes were seen in the content of Etn-P-DGDRG. Shown are the means ± S.D. of results from three cultures.
4. Discussion
The historical importance of C. acetobutylicum in the production of industrial chemicals such as acetone and butanol, and contemporary interest in the use of butanol as a biofuel emphasize the need for understanding the cellular responses to the production of solvents. Previous studies have considered the effects of solvents on both hydrocarbon chain and lipid class compositions [3,5,19]. In the only study that considered lipid class composition [3], the C. acetobutylicum strain investigated, IFP 903; ATCC 39057, has been reclassified as Clostridium beijerinckii. In this study large changes in membrane lipids were seen during the acetone-butanol fermentation. Cardiolipin and the glycerol acetals of plasmenylethanolamine and plasmenyl-N-monomethylethanolamine increased and phosphatidylglycerol and the sum of phosphatidylethanolamine, phosphatidyl N-monomethylethanolamine (which included their respective plasmalogen forms) decreased. The ratio of unsaturated to saturated fatty acids decreased in parallel. Addition of solvents such butanol, hexanol, octanol and acetone produced similar changes in the ratios of unsaturated to saturated fatty acids with somewhat larger changes seen with the longer chain alcohols. It is important to note that the ratios of unsaturated to saturated fatty acids (U/S) during the acetone-butanol fermentation were compared to changes in U/S upon addition of various solvents including octanol. During fermentation the ratios of unsaturated to saturated fatty acids (U/S) decreased from 0.98 to 0.69 as the amount of solvent increased from 0 to 13.5 g/L. When octanol was added U/S decreased from 1.03 to 0.66, an almost identical change. Based on this finding, octanol was used in our study to reflect changes that occur during formation of solvents. In a study with C. butyricum with defined hydrocarbon chain compositions, the glycerol acetal of PlaE increased markedly in cells grown with octanol [18]. This lipid has been shown to help to sustain the bilayer phase when cellular lipids were enriched in unsaturated fatty acids [12].
In a previous paper in which we examined the effects of changes in the degree of membrane lipid unsaturation on polar lipid composition of C. acetobutylicum ATCC 4259, we observed two previously unreported phosphorus-containing glycolipids. The less polar of the two was shown to increase markedly as the ratio of unsaturated to saturated fatty acids increased in the cellular lipids [7]. Here we show that these glycolipids are ethanolamine-P (Etn-P) derivatives of a monoglycosyldiradylglycerol (the less polar lipid) and a diglycosyldiradylglycerol. Ethanolamine-P was recently shown to modify an N-acetylglucosaminydiradylglycerol in C. tetani and the attachment was shown to be to the 6’-position of the sugar [16]. The same lipid has now been found in Clostridium sporogenes and in strains of Clostridium botulinum related to C. sporogenes [20]. Thus it appears that an Etn-P modification of membrane glycolipids is a recurrent theme among clostridia. We have not investigated the identities of the sugars in EtnP-MGDRG and EtnP-DGDRG, but we assume they are derivatives of the previously reported galactosyl-DRG and glucosyl-galactosyl-DRG of C. acetobutylicum [21,22].
Pioneering work on the polar lipids of Acholeplasma laidlawii has shown that one mechanism used by this organism to regulate the phase behavior of it membrane lipids is to add a second sugar to monoglucosyldiacylglycerol (MGDG) in response to either increased membrane lipid unsaturation or the presence of solvents in the growth medium [8,11,23]; reviewed in [10]. An increase in the effective polar head group size compensates for the perturbations caused by the presence of solvents or the change in the cross-section area of the acyl chains upon increases in unsaturation, which can lead to the formation of non-lamellar lipid phases and disruption of the barrier properties of the membrane. McElhaney and colleagues have reported that the concentration of a normally minor lipid of A. laidlawii, glycerylphosphoryldiglucosyldiacylglcyerol increases from 3 mol% to more than 20 mol% upon enrichment of the lipids with unsaturated or short chain iso-branched fatty acid [24]. This lipid prefers the normal micellar phase in isolation and strongly counteracts the formation of the reversed hexagonal phase when mixed with lipids that in isolation prefer the reversed hexagon phase [25]. Thus addition of the glycerol-P group to a diglycosyldiacylglycerol serves to provide even greater protection to cell membranes to the adverse affects of enrichment with certain fatty acids than the diglycosyldiacylglycerol itself.
In C. acetobutylicum it appears that a second modification of MGDRG through addition of Etn-P is important in regulating membrane homeostasis. We know that the presence of an alk-1’-enyl bond in the plasmalogen form of PE increases the already high tendency of PE to form the reversed hexagonal phase [26]. Similar studies have not been carried out on the plasmalogen forms of the glycolipids of C. acetobutylicum. Based on the work of the McElhaney group with A. laidlawii discussed above, we can argue that the addition of the charged Etn-P would have a similar effect to that of the addition of glycerol-P to a glycolipid, but the effect could differ because of the differences in size and degree of hydration of these polar groups. Further biophysical studies on these novel Etn-P-containing glycolipids of C. acetobutylicum and the effects of the addition of Etn-P to MGDRG on resistance to the high levels of acetone and butanol produced by this organism would be of great interest.
BBA Highlights.
Clostridium acetobutylicum is an important producer of industrial solvents
Novel ethanolamine-phosphate glycosyldiradylglycerols are characterized
Lipids respond to membrane stress
Potential involvement in solvent resistance
ACKNOWLEDGEMENTS
B.T. is supported by the grant from the National Natural Science Foundation of China (31170079), the program for New Century Excellent Talents in University, and the State Scholarship Fund of China. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 from NIH.
Abbreviations
- DGDRG
diglycoslydiradylglycerol
- Etn-P
ethanolamine-phosphate
- MGDG
monoglycosyldiacylglycerol
- MGDRG
monoglycoslydiradylglycerol. In designations of the molecular species the number before the colon is the sum of the chain lengths and the number after the colon is the number of double bonds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jones DT, Woods DR. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986;50:484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones DT, Keis S. Origins and Relationships of Industrial Solvent-Producing Clostridial Strains. FEMS Microbiol. Rev. 1995;17:223–232. [Google Scholar]
- 3.Lepage C, Fayolle F, Hermann M, Vandecasteele J-P. Changes in membrane lipid composition of Clostridium acetobutylicum during acetone-butanol fermentation: Effects of solvents, growth temperature and pH. J. Gen. Microbiol. 1987;133:103–110. [Google Scholar]
- 4.Bowles LK, Ellefson WL. Effects of butanol on Clostridium acetobutylicum. Appl. Environ. Microbiol. 1985;50:1165–1170. doi: 10.1128/aem.50.5.1165-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vollherbst-Schneck K, Sands JA, Montenecourt BS. Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 1984;47:193–194. doi: 10.1128/aem.47.1.193-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingram LO. Adaptation of membrane lipids to alcohols. J. Bacteriol. 1976;125:670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston NC, Goldfine H. Replacement of the aliphatic chains of Clostridium acetobutylicum by exogenous fatty acids: Regulation of phospholipid and glycolipid composition. J. Bacteriol. 1992;174:1848–1853. doi: 10.1128/jb.174.6.1848-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieslander Å, Rilfors L, Lindblom G. Metabolic changes of membrane lipid composition in Acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: arguments for effects on lipid packing. Biochemistry. 1986;25:7511–7517. doi: 10.1021/bi00371a038. [DOI] [PubMed] [Google Scholar]
- 9.Goldfine H. Membrane Biogenesis. In: Timmis KN, editor. Hydrocarbons, Oils and Lipids: Diversity, Properties and Formation, vol. 1, Handbook of Hydrocarbon and Lipid Microbiology. Berlin: Springer; 2010. pp. 417–424. [Google Scholar]
- 10.Rilfors L, Lindblom G. Regulation of lipid composition in biological membranes - biophysical studies of lipids and lipid synthesizing enzymes. Colloids and Surfaces B-Biointerfaces. 2002;26:112–124. [Google Scholar]
- 11.Wieslander Å, Christiansson A, Rilfors L, Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980;19:3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]
- 12.Goldfine H, Johnston NC, Mattai J, Shipley GG. The regulation of bilayer stability in Clostridium butyricum: Studies on the polymorphic phase behavior of the ether lipids. Biochemistry. 1987;26:2814–2822. doi: 10.1021/bi00384a024. [DOI] [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Kates M. Isolation, Analysis and Identification of Lipids. Amsterdam: North-Holland Publishing Company; 1986. Techniques of Lipidology. [Google Scholar]
- 15.Guan Z, Johnston NC, Aygun-Sunar S, Daldal F, Raetz CR, Goldfine H. Structural characterization of the polar lipids of Clostridium novyi NT. Further evidence for a novel anaerobic biosynthetic pathway to plasmalogens. Biochim. Biophys. Acta. 2011;1811:186–193. doi: 10.1016/j.bbalip.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston NC, Aygun-Sunar S, Guan Z, Ribeiro AA, Daldal F, Raetz CR, Goldfine H. A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J. Lipid Res. 2010;51:1953–1961. doi: 10.1194/jlr.M004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston NC, Goldfine H. Phospholipid aliphatic chain composition modulates lipid class composition, but not lipid asymmetry in Clostridium butyricum. Biochim. Biophys. Acta. 1985;813:10–18. doi: 10.1016/0005-2736(85)90339-6. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald DL, Goldfine H. Effects of solvents and alcohols on the polar lipid composition of Clostridium butyricum under conditions of controlled lipid chain composition. Appl. Environ. Microbiol. 1991;57:3517–3521. doi: 10.1128/aem.57.12.3517-3521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baer SH, Blaschek HP, Smith TL. Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl. Environ. Microbiol. 1987;53:2854–2861. doi: 10.1128/aem.53.12.2854-2861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan Z, Johnston NC, Raetz CRH, Johnson EA, Goldfine H. Lipid diversity among botulinum neurotoxin-producing clostridia. Microbiology. 2012;158:2577–2584. doi: 10.1099/mic.0.060707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oulevey J, Bahl H, Thiele OW. Novel alk-1-enyl ether lipids isolated from Clostridium acetobutylicum. Arch. Microbiol. 1986;144:166–168. [Google Scholar]
- 22.Thiele OW, Oulevey J, Bahl H. Neuartige Alkenylether-lipide aus anaeroben Bakterien. Fette Seifen Anstrichm. 1985;87:551–556. [Google Scholar]
- 23.Wieslander Å, Rilfors L. Qualitative and quantitative variations of membrane lipid species in Acholeplasma laidlawii A. Biochim. Biophys. Acta. 1977;466:336–346. doi: 10.1016/0005-2736(77)90229-2. [DOI] [PubMed] [Google Scholar]
- 24.Lewis RNAH, McElhaney RN. Acholeplasma laidlawii B membranes contain a lipid (glycerylphosphoryldiglucosyldiacylglycerol) which forms micelles rather than lamellar or reversed phases when dispersed in water. Biochemistry. 1995;34:13818–13824. doi: 10.1021/bi00042a013. [DOI] [PubMed] [Google Scholar]
- 25.Foht PJ, Tran QM, Lewis RNAH, McElhaney RN. Quantitation of the phase preferences of the major lipids of the Acholeplasma-Laidlawii B-membrane. Biochemistry. 1995;34:13811–13817. doi: 10.1021/bi00042a012. [DOI] [PubMed] [Google Scholar]
- 26.Lohner K, Hermetter A, Paltauf F. Phase behavior of ethanolamine plasmalogen. Chem. Phys. Lipids. 1984;34:163–170. [Google Scholar]