…irrespective of the definition of AKI, and validation of the presence of AKI and its severity, we need to apply these definitions in a systematic way to both research and clinical care to determine the ultimate utility of them…
Keywords: acute kidney injury, chronic kidney disease, health services, hospital mortality
Abstract
Background
The Kidney Disease: Improving Global Outcomes (KDIGO) system for classification of acute kidney injury (AKI) severity utilizes a staging schema based on relative changes in serum creatinine (sCr) concentration and urine output. This study compares the in-hospital mortality associated with KDIGO-defined AKI stages and AKI stages defined by absolute sCr increases (‘Delta-Creatinine’).
Methods
The study included an analysis of hospital discharge and laboratory data from an urban academic medical center over a 1-year period. Including adult in-patients undergoing two or more sCr measurements, the study classified AKI stages using the KDIGO consensus standards as well as absolute increases in sCr (‘Delta-Creatinine’); Stage 0, sCr increase <0.3 mg/dL, Stage 1, sCr increase 0.3–0.69 mg/dL, Stage 2, sCr increase 0.7–1.19 mg/dL and Stage 3, sCr increase ≥1.2 mg/dL or initiation of renal replacement therapy. The Delta-Creatinine cut-points were defined to optimize discrimination of in-patient mortality between AKI stages. The associations between KDIGO and Delta-Creatinine AKI stages and in-hospital mortality were compared using the time-dependent hazard ratios (HRs) and the net reclassification improvement (NRI).
Results
Of the 19 878 hospitalizations included in the analysis, the prevalence of AKI was 23.4% as defined by the KDIGO criteria. The Delta-Creatinine system discriminated the differences between adjacent AKI stages (i.e. 1 versus 0, 2 versus 1, 3 versus 3) earlier than the KDIGO system. The NRI between Delta-Creatinine and KDIGO for the prediction of mortality was 9.7% [95% confidence interval (CI) 6.2–13.2%]. Stratification by age, sex, race and history of chronic kidney disease (CKD) resulted in similar NRI values.
Conclusion
The Delta-Creatinine system, based on the absolute increases in sCr, provides a promising alternative to the KDIGO system for characterizing the severity of AKI and its associations with in-patient mortality.
INTRODUCTION
Acute kidney injury (AKI) is a syndrome of diverse etiology that is characterized by a rapid decline in the glomerular filtration rate (GFR). The syndrome ranges from minimal asymptomatic elevations in serum creatinine (sCr) concentration to complete anuric kidney failure [1]. AKI is a leading cause of in-hospital mortality, and the high incidence and prevalence rates have persisted over the past decade [2]. Different studies have estimated the prevalence rates of AKI as 3.2–20% in non-intensive care unit (ICU) patients, and as 22–67% in critically ill ICU patients. Multiple studies have shown an increased mortality in hospitalized patients who develop AKI [3]. In a systematic review of studies classifying AKI based on the Risk, Injury, Failure, Loss and End-stage renal disease (RIFLE) criteria, the mortality of patients with AKI was 31.2% compared with 6.9% in patients without AKI [4]. The effects of AKI on mortality and morbidity may persist even after discharge from the hospital, with increased mortality rates at 90 days as well as 1 year after the episode of AKI [3, 5, 6].
The risk of in-patient mortality and the need for renal replacement therapy are directly related to the severity of AKI, an observation that supports the use of a categorical staging system rather than a simple binary descriptor. The severity of AKI is commonly characterized using the relative changes in sCr and urine output. Existing widely used AKI staging systems include RIFLE and the Acute Kidney Injury Network (AKIN), which stage AKI according to the relative changes in sCr. While these systems are consistent in their identification of AKI, their application may assign individual patients to different AKI stages.
More recently, a Kidney Disease: Improving Global Outcomes (KDIGO) workgroup developed a consensus-based AKI staging system drawing consolidating elements of both RIFLE and AKIN [7]. However, the KDIGO system is complex and requires a baseline pre-hospitalization sCr (as measured or estimated from the existing formulas utilizing age, gender and ancestry) as well as urine output measurements during hospitalization. These measures are not routinely available for all hospitalized patients, especially if they have not been treated in a critical care setting. KDIGO AKI staging also depends upon the magnitude of the index sCr value. The cut-points for KDIGO AKI staging were determined by consensus, and have not been rigorously evaluated in a prospective manner.
Some studies have classified and staged AKI using absolute rather than relative increases in sCr creatinine from an index value (‘Delta-Creatinine’) [3, 8, 9]. Although simpler and more amenable to use with administrative data sets, this approach has not previously been formally compared with any of the AKI staging systems. Our aim was to compare the performance of the KDIGO and Delta-Creatinine approaches to staging AKI, using the net reclassification improvement (NRI) analysis [8,10], and to select cut-points for AKI stages that optimize the discrimination of risk prediction of in-patient mortality in adults.
MATERIALS AND METHODS
Design, setting, and source of data
The Institutional Review Board of the University of Alabama at Birmingham (UAB) approved the study. We used hospital discharge data from the UAB Hospital, a 908-bed urban academic tertiary care referral medical center in Birmingham, Alabama, USA. The institution receives 64 000 visits at its emergency department per year, it is the only level I trauma center in Alabama and has eight ICUs with more than 180 critical care beds.
We used the UAB Hospital standard UB-04 discharge data set, consisting of patient demographics and diagnostic and financial information. Data analysts linked the hospital data set with sCr obtained for each hospitalization. Commercial software systems manage discharge and laboratory data (HealthQuest Data Systems, Highland, CA, and Cerner PathNet, Cerner, Inc., Kansas City, MO). We previously used these data to describe the association of in-patient mortality with increasing severity of AKI using the AKIN staging criteria [10].
Selection of subjects
Adult (≥18 years old) patients hospitalized between 1 October 2009 and 30 September 2010 were included in the study. Prisoners, patients admitted to the psychiatry service, labor and delivery service and patients transferred from other hospitals were excluded from the analysis. ‘Bedded outpatients,’ a term denoting individuals admitted after scheduled surgical or other procedures were also excluded. Individuals with a history of kidney transplant [discharge diagnosis international classification of diseases (ICD)-9 V42.0] or end-stage renal disease with maintenance dialysis (ICD-9 V45.1, V45.11, V45.12, V56, V56.0, V56.8) were not included in the analysis. Because the analysis relied upon the comparison of serial sCr, only patients undergoing two or more sCr measurements during the course of their hospitalization were included.
Methods of measurement
Implausible sCr values (<0.3 or >20 mg/dL) were eliminated, resulting in exclusion of 212 of 150 269 (0.14%) sCr measurements from the analysis. We limited the study to the first 60 sCr measurements for each admission to exclude AKI associated with prolonged hospitalizations, preserving 89.2% of the sCr and 99.5% of the peak sCr values in the data set.
AKI stages were calculated based upon increases from an index sCr value. The index sCr value was defined as the lowest of the first three sCr values reported for each hospitalization [8]. The peak sCr value was defined as the highest measured sCr during hospitalization, and the Delta-Creatinine was defined as the difference between the index sCr and the peak sCr value. Pre-hospitalization sCr and urine output rates during hospitalization were not available.
We defined AKI stages using two methods: (i) the current KDIGO consensus definitions and (2) an empiric ‘Delta-Creatinine’ system developed for this study. The KDIGO bases AKI stages upon ‘relative’ sCr increases or initiation of renal replacement therapy (RRT) [7]. (Table 1) We devised the Delta-Creatinine system based upon the ‘absolute’ changes in the sCr or initiation of RRT (Table 1). Using the Kaplan–Meier survival curves, we defined Delta-Creatinine cut-points to optimize the distribution of hazard ratios (HRs) across time (e.g. in-patient mortality and length of stay) for each stage. The initiation of RRT was determined through the discharge diagnoses ICD-9p 39.95 or 54.98.
Table 1.
AKI staging systems
| Stage | KDIGO | Delta-Creatinine |
|---|---|---|
| 0 | No AKI | No AKI |
| 1 | (sCr increase to 1.5–1.9 times index) or (≥0.3 mg/dL sCr increase) | (sCr increase 0.3–0.69 mg/dL over index) |
| 2 | (sCr increase to 2.0–2.9 times index) | (sCr increase 0.7–1.19 mg/dL over index) |
| 3 | (sCr increase to ≥3.0 times index) or (sCr increase to ≥4.0 mg/dL) or (initiation of RRT) or (in patients <18 years, decrease in eGFR to <35 mL/min per 1.73 m2) | (sCr increase ≥1.2 mg/dL over index) or (initiationof RRT) |
AKI stages defined using KDIGO and Delta-Creatinine systems. sCr = serum creatinine. RRT = renal replacement therapy. index = lowest of first three sCr values.
Data Analysis
We compared KDIGO and Delta-Creatinine staging using the Kaplan–Meier survival curves and Cox multivariable regression models. Analysis of the Schoenfeld residuals showed that the relationships between AKI stages and time to death violated the proportional hazards assumption of the Cox model. Therefore, we analyzed the association between time-to-hospital death and AKI stages using Cox regression models with time-dependent HRs. We defined time to death as elapsed time from index sCr to in-hospital death. We censored cases surviving to hospital discharge. We fit the models using the natural-log transformation of hospital days as the exposure time, adjusting for median age, gender and black or non-black race. We calculated the relative HRs for in-patient mortality between the adjacent KDIGO and Delta-Creatinine defined AKI stages [11].
We determined the NRI between KDIGO and Delta-Creatinine, defining KDIGO stages as the classification variable and Delta-Creatinine stages as the reclassification variable [12, 13]. To determine the stability of the reclassification, in a sensitivity analysis, we stratified the NRI by the following subsets: ≥60 years versus <60 years, male versus females, black versus whites and chronic kidney disease (CKD) versus no CKD. The CKD status was based on the index sCr and the CKD Epidemiology Collaboration equation for the estimated GFR (eGFR), with CKD defined as an eGFR <60 mL/min per 1.73 m2 [14].
All analyses were carried out using Stata version 12.0 (Stata Corp, College Station, TX) and Microsoft Excel (Microsoft, Inc, Redmond, WA).
Role of the funding source
The funders had no role in the study design, data collection, analysis or interpretation of data, in the writing of the report or the decision to submit the paper for publication.
RESULTS
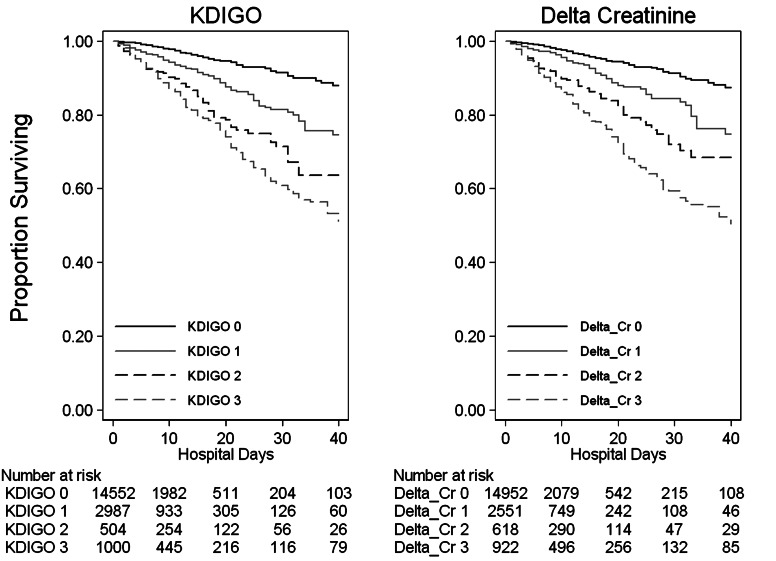
We included 19 249 hospitalizations in the present analysis, consisting of 15 096 individual patients; repeat hospitalizations occurred for 1612 individuals (Table 2). Defining AKI as KDIGO Stages 1–3, the prevalence of AKI was found to be 23.4%. For each KDIGO AKI stage (1, 2 and 3), the median times to peak sCr were 2.0, 3.8 and 2.0 days, respectively. For each Delta-Creatinine AKI stage, the median times to peak sCr were 1.7, 2.5 and 3.5 days, respectively. The Kaplan–Meier rates of survival to hospital discharge varied with KDIGO and Delta-Creatinine stages (Figure 1). We selected cut-points for Delta-Creatinine AKI stages to optimally differentiate survival functions for each stage (Table 1).
Table 2.
Characteristics of hospitalized patients with AKI
| Characteristic | AKI (n = 4116) | No AKI (n = 15 130) | P-value AKIversus No-AKI |
|---|---|---|---|
| Age, mean years (SD) | 56.9 (16.8) | 54.5 (17.7) | <0.001 |
| Sex, men; n (%) | 2322 (51.5%) | 7554 (51.3%) | 0.06 |
| Race/ethnicity - n (%) | |||
| White | 2581 (57.2%) | 9555 (64.9%) | <0.001 |
| Black | 1821 (40.4%) | 4865 (33.0%) | |
| Other | 99 (2.1%) | 278 (1.9%) | |
| Unknown | 8 (0.2%) | 31 (0.2%) | |
| CKD, n (%)* | 1261 (28.0%) | 1358 (9.2%) | <0.001 |
| Admission source | |||
| Emergency department, n (%) | 2458 (54.5) | 8861 (60.2) | <0.001 |
| Other, n (%) | 2051 (45.5) | 5868 (39.8) | |
| Admitted to ICU, n (%) | 163 (3.6%) | 336 (2.3%) | <0.001 |
| Any hospital days in ICU, n (%) | 1861 (41.3%) | 3256 (22.1%) | <0.001 |
| sCr measurements | |||
| Number of sCr, median n (IQR) | 8 (5–15) | 4 (3–7) | <0.001 |
| Baseline sCr, mean mg/dL (SD) | 1.7 (2.2) | 0.9 (0.5) | <0.001 |
| Peak sCr, mean mg/dL (SD) | 2.5 (2.4) | 1.0 (0.5) | <0.001 |
| Maximum sCr increase, mean mg/dL (SD) | 0.8 (1.0) | 0.1 (0.1) | <0.001 |
| Time to peak sCr, median days (IQR) | 2.6 (1.4–5.6) | 1.2 (0.6–2.4) | <0.001 |
| Hospital length of stay, days, median (IQR) | 7 (4–13) | 4 (2–7) | <0.001 |
| Hospital mortality, n (%) | 482 (10.7%) | 210 (1.4%) | <0.001 |
AKI defined using the KDIGO criteria [sCr increase ≥0.3 mg/dL from the index sCr (lowest of the first three sCr values), sCr increase to ≥150% of baseline, or initiation of dialysis].
*CKD determined from the eGFR, calculated from the baseline sCr using the CKD Epidemiology Collaboration (CKD-EPI) equation [14]. AKI = acute kidney injury. CKD = chronic kidney disease. sCr = creatinine. LOS = length of stay. ICU = intensive care unit.
FIGURE 1:
Kaplan–Meier graphs for hospital survival, stratified by KDIGO (left) and Delta-Creatinine (right) stages of AKI. Highest survival curves represent KDIGO and Delta-Creatinine Stage 0. Lowest survival curves represent KDIGO and Delta-Creatinine Stage 3.
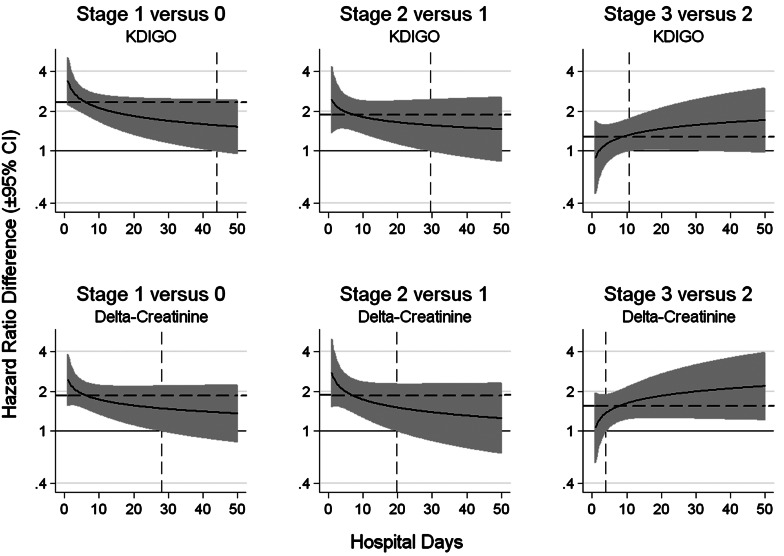
The HRs for successive AKI stages are presented in Figure 2 for the KDIGO (upper panels) and Delta-Creatinine (lower panels) stages. The solid horizontal line at 1.00 represents the reference where the hazard ratios of death are identical for the two stages. The dashed horizontal line in each panel represents the time-invariant HR obtained using Cox multiple regression models under the proportional hazards assumption. The time-invariant HRs for the KDIGO stages were 2.37, 1.85 and 1.34, with an overall HR of 1.76. The time-invariant HRs for the Delta-Creatinine stages were 1.89, 1.85 and 1.58, with an overall HR of 1.77 (Supplementary data, Appendix 1).
FIGURE 2:
Comparison of HRs of death for successive AKI stages using KDIGO and Delta-Creatinine systems. Each panel depicts the time-dependent HRs (solid lines ± 95% confidence interval) comparing successive AKI stages with the KDIGO stages in the top panels and the Delta-Creatinine categories shown in the bottom panels. The horizontal lines at HR = 1 represents equality between AKI stages. The vertical dashed line represents the time point where the 95% CI of the difference in HRs falls below or rises above HR = 1. The dashed horizontal lines indicate time-invariant HRs obtained using Cox proportional hazard multivariable regression models, adjusted for age, race and gender.
The Delta-Creatinine system discriminated the differences between adjacent AKI stages (i.e. 1 versus 0, 2 versus 1, 3 versus 3) at different time points compared with the KDIGO system (Figure 2; dashed vertical lines). For example, for Stage 1 versus Stage 0 AKI, the lower 95% confidence interval (CI) of the HR intersected with HR 1.00 at 43.9 days for KDIGO and at 28.1 days for Delta-Creatinine, thereafter there was no significant difference in the HRs between Stage 1 and Stage 0. For Stage 2 versus Stage 1 AKI, the intersection occurred at 29.6 days for KDIGO and 19.7 days for Delta-Creatinine, thereafter there was no significant difference in the HRs between Stage 2 and Stage 1. For Stage 3 versus Stage 2 AKI, the intersection occurred at 10.6 days for KDIGO and at 4.0 days for Delta-Creatinine, suggesting that the discrimination between moderate (Stage 2) and severe (Stage 3) AKI could be made sooner with the Delta-Creatinine system than with KDIGO AKI stages.
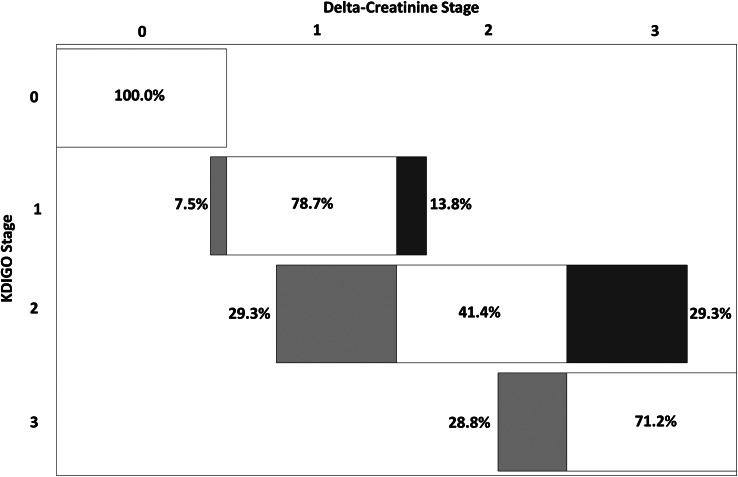
The NRI analysis is presented in Table 3. Compared with the original classification by the KDIGO staging system, the Delta-Creatinine staging system reclassified 562 of 19 238 episodes to higher AKI stages, and 663 episodes to lower AKI stages (Table 3). The largest proportion of reclassifications occurred with KDIGO Stage 2 and 3 patients (Figure 3). Among KDIGO Stage 2 patients, Delta-Creatinine incorrectly down-reclassified 9 of 83 deaths (10.8%), and incorrectly up-reclassified 105 of 423 survivors (24.8%). Among KDIGO Stage 3 patients, Delta-Creatinine incorrectly down-reclassified 28 of 213 deaths (13.1%). The rate of clinically correct reclassification was 18.5% (Table 3). The rate of clinically incorrect reclassification was 9.0%. The NRI was 9.7% (95% CI 6.2–13.2%), favoring the Delta-Creatinine approach to AKI staging. Stratification by age, sex, race and history of CKD resulted in similar NRI values (Supplementary data, Appendix 2).
Table 3.
NRI for staging of AKI
| Delta-Creatinine stage | ||||
|---|---|---|---|---|
| KDIGO stage | 0 | 1 | 2 | 3 |
| Deaths | ||||
| 0 | 210 | 0 | 0 | 0 |
| 1 | 9 | 115 | 48 | 15 |
| 2 | 0 | 9 | 31 | 43 |
| 3 | 14 | 9 | 5 | 185 |
| Survivors | ||||
| 0 | 14 517 | 0 | 0 | 0 |
| 1 | 217 | 2246 | 305 | 46 |
| 2 | 0 | 140 | 178 | 105 |
| 3 | 163 | 45 | 53 | 529 |
| Clinically correct reclassification | ||||
| Proportion reclassified up: survivors | 3.3% | |||
| Proportion reclassified down: deaths | 15.3% | |||
| Clinically incorrect reclassification | ||||
| Proportion reclassified up: deaths | 6.5% | |||
| Proportion reclassified down: survivors | 2.5% | |||
| NRI (±95% CI) | 9.7% (6.2–13.2%) | |||
Staging of AKI using the KDIGO (classification categories) and Delta-Creatinine (reclassification categories) systems. Includes a total of 19 238 hospitalizations for patients with ≥2 creatinine measurements.
FIGURE 3:
Net reclassification of AKI from KDIGO to the Delta-Creatinine-based staging system. The percentages of each KDIGO stage reclassified upwards (rightwards) to higher or downwards (leftwards) to the lower Delta-Creatinine stage are shown in the figure. For example, for KDIGO Stage 1 AKI, while most would be reclassified as Delta-Creatinine Stage 1, 7.5% would be reclassified to the lower Delta-Creatinine Stage 0, and 13.8% would be reclassified to the higher Delta-Creatinine Stage 2. NRI 9.5% (95% CI 6.0–13.0%). Figure adapted from the CKD Prognosis Consortium (http://www.jhsph.edu/ckdpc).
DISCUSSION
We describe a novel approach to classification and staging of AKI using ‘absolute’ rather than relative increases in sCr. Compared with the current KDIGO consensus AKI staging, Delta-Creatinine staging is relatively simple and has better utility for large-scale epidemiologic studies [7]. Furthermore, Delta-Creatinine allows for use of an index creatinine derived from in-patient samples rather than referring to pre-hospitalization baseline values, which may not be readily available. The Delta-Creatinine system showed superior performance by NRI analysis for prediction of in-patient mortality, even without the use of urine output data, and the improvement in classification was robust for a range of disease subsets and demographic strata. Because detailed urine output data are not systematically collected outside of the ICU, the Delta-Creatinine system may have broader utility than KDIGO staging, particularly with patients in non-critical care settings.
The findings of this analysis should not be surprising. The Delta-Creatinine system is based upon absolute rather than relative increases in sCr. Thus, for most individuals, the Delta-Creatinine system will be more sensitive to small sCr increases. This finding also signals that the absolute sCr increases may have greater clinical importance than the relative increases. In addition, compared with KDIGO staging, Delta-Creatinine consistently identified AKI and transitions to more severe AKI stages at earlier time points. This approach could be important in the development of more sensitive methods for characterizing risks associated with AKI. This approach could also prove important if AKI were utilized in dynamic models of hospital mortality assessment, prognostication and targeted interventions.
A number of prior studies have similarly characterized AKI in terms of absolute sCr increases. Chertow et al. examined AKI among 9000 in-patients in Boston, MA, and associated in-patient mortality with absolute sCr increases [8]. Using data from over 87 000 patients in the Cooperative Cardiovascular Project, Newsome et al. characterized AKI in terms of quartiles of absolute sCr increases following admission for acute myocardial infarction among Medicare beneficiaries [3]. Using data from almost 60 000 subjects in the the Acute Coronary Treatment and Intervention Outcomes Network Registry, Fox et al. characterized AKI and subsequent mortality in patients with acute myocardial infarction [9]. These prior studies and the current one support this general approach to characterizing AKI. This simplified approach to AKI detection allows for broad application to a range of hospital data sets. Our selection of sCr cut-points based upon optimal mortality discrimination between AKI stages is a novel approach compared with prior studies that used arbitrarily defined thresholds to define AKI stages.
Our study used the NRI for quantifying the diagnostic improvement of Delta-Creatinine over KDIGO. The NRI analysis [12, 13] has been used in a variety of applications. For example, NRI has been used to clarify the definitions for CKD stages, [15] to assess the added importance of cystatin C in CKD [16], and to evaluate the value of B-type natriuretic peptide and coronary calcification scores for the Framingham Risk Score for coronary heart disease [17] and evaluating the role of lipid-related biomarkers in cardiovascular disease prediction [18]. Compared with traditional receiver operator curves for sensitivity and specificity, this approach is more robust for evaluating the added benefit of additional biomarkers or comparing staging systems [19–21].
LIMITATIONS
These data originated from a single medical center and merit external validation using data from other settings. We selected cut-points for each Delta-Creatinine stage based upon the graphical assessment of survival functions. Whether these cut-points perform well with larger, independent validation sets and whether they work well for outcomes other than in-patient mortality require prospective validation. Even if the specific empirically derived cut-points used in the current analysis prove not to be generally applicable, the approach described herein does provide an approach for optimizing risk prediction strategies, and even targeted interventions that could impact on the course and outcome of AKI in hospitalized patients.
The change in sCr could well be viewed as a continuous variable; efforts to categorize continuous variables can lead to loss of power and residual confounding [22]. Furthermore, the use of data-derived ‘optimal’ cut-points can lead to serious bias, again emphasizing the importance of evaluating the approach described herein with independent validation data sets [22].
The peak Delta-Creatinine was calculated in a post-hoc analysis, using the index or baseline sCr and the highest sCr value recorded during the hospital stay. For any prospective or dynamic evaluation of peak Delta-Creatinine, the value recorded on any given hospital day could be revised if the subsequent measurement of sCr was higher. Nevertheless, the recorded Delta-Creatinine would give a minimal estimate of the risk associated with AKI, subject to upward revision. If the subsequent sCr were lower than that which defined the peak Delta-Creatinine, then there would not be any decrease in the risk ascribed to AKI. Whether or not this will be useful in a dynamic context will require prospective evaluation.
Only 40% of hospitalizations in this series received two or more sCr measurements. More frequent sCr testing across all patients could increase the incidence of AKI cases, but the clinical decision process that triggers the ordering of baseline and repeated measures of sCr could represent an important source of residual confounding that requires prospective evaluation. Ascertainment bias may also be relevant, as those individuals with more serious underlying diseases may have had more frequent sCr determinations. Nevertheless, the lack of any requirement for a pre-admission value or ‘estimation’ of index sCr, as is true for the KDIGO staging system, is a distinct advantage of the Delta-Creatinine approach. While we describe patients who developed AKI in the hospital setting, we cannot assess those who had already developed AKI prior to hospital admission.
It is also true that the rates of absolute increases in sCr are likely to be modified by the rates of creatinine generation (such as sarcopenia or acute muscle injury) or acute changes in the volume of distribution of creatinine (such as acute dehydration or volume expansion). However, these caveats also apply to the use of relative changes in sCr. This limitation has been recognized in patients following cardiac surgery, who often have aggressive volume administration following surgery [23].
CONCLUSION
The Delta-Creatinine system, based on absolute increases in sCr, provides an alternative to the current KDIGO staging system for characterizing AKI and its associations with in-hospital mortality. The absolute changes in sCr appear to better inform the diagnosis of AKI and predict mortality risk than the relative changes in sCr. Optimization of the categorical cut-points for any AKI staging system needs to be prospectively validated.
AUTHORS' CONTRIBUTIONS
H.E.W., G.J., R.G. and D.W. conceived the study. H.E.W. obtained the dataset. H.E.W. and D.W. performed the analysis. H.E.W. drafted the manuscript and all authors contributed to its critical review and editing. H.E.W. assumes responsibility for the paper as a whole.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
H.E.W. received grant support from the National Institute of Nursing Research (R01-NR012728). D.G.W. is the Hilda B. Anderson Endowed Professor in Nephrology at the University of Alabama at Birmingham. We acknowledge the UAB-UCSD O'Brien Center for Acute Kidney Injury Research (P30 DK079337) for their technical input.
CONFLICT OF INTEREST STATEMENT
D.G.W. is a consultant to Allocure, Inc. concerning the use of mesenchymal stem cells for treatment of AKI. The authors declare no other conflicts. Prior presentation: None. (See related article by Lafrance and Levin. Defining AKI: closer to getting the math right. Nephrol Dial Transplant 2013; 28: 1340–1342.)
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Darlene Green and Stephen Duncan, who provided the dataset and technical guidance.
REFERENCES
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 3.Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 4.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 5.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012 doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 7.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Section 2: AKI definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Muntner P, Chen AY, et al. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. 2012;125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HE, Muntner P, Chertow GM, et al. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royston P, Lambert P. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, Texas: Stata Press; 2011. [Google Scholar]
- 12.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr., et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. J Amer Med Assoc. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. J Am Med Assoc. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavousi M, Elias-Smale S, Rutten JH, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156:438–444. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 18.The Emerging Risk Factors C. LIpid-related markers and cardiovascular disease prediction. J Am Med Assoc. 2012;307:2499–2506. doi: 10.1001/jama.2012.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hlatky MA. Framework for evaluating novel risk markers. Ann Intern Med. 2012;156:468–469. doi: 10.7326/0003-4819-156-6-201203200-00013. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Pencina MJ, Lingsma HF, et al. Assessing the incremental value of diagnostic and prognostic markers: a review and illustration. Eur J Clin Invest. 2012;42:216–228. doi: 10.1111/j.1365-2362.2011.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 23.Robert AM, Kramer RS, Dacey LJ, et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90:1939–1943. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.