Abstract
Background
The implications of chemical hyperparathyroidism on bone and mineral metabolism measures in maintenance hemodialysis (MHD) are not well known. We hypothesized that a higher serum intact parathyroid hormone (iPTH) level is associated with the higher likelihood of hyperphosphatemia, hyperphosphatasemia [high serum alkaline phosphatase (ALP) levels] and hypercalcemia.
Methods
Over an 8-year period (July 2001–June 2009), we identified 106 760 MHD patients with iPTH and calcium (Ca), phosphorous (P) and ALP data from a large dialysis clinic. Logistic regression models were examined to assess the association between serum iPTH increments and the likelihood of hyperphosphatemia (P ≥5.5 mg/dL), hypercalcemia (Ca ≥10.2 mg/dL) and hyperphosphatasemia (ALP ≥120 U/L).
Results
Patients were 61 ± 16 years old and included 45% women, 59% diabetics and 33% Blacks. Compared with an iPTH level of 100 to <200 pg/mL, patients with an iPTH level of 600–700, 700 to <800 and ≥800 pg/mL had 122% (OR: 2.22, 95% CI: 2.04–2.41), 153% (OR: 2.53, 95% CI: 2.29–2.80) and 243% (OR: 3.43, 95% CI: 3.22–3.66) higher risk of hyperphosphatemia, respectively, and had 109% (OR: 2.09, 95% CI: 1.93–2.26), 130% (OR: 2.30, 95% CI: 2.10–2.52) and 376% (OR: 4.76, 95% CI: 4.50–5.04) higher risk of hyperphosphatasemia, respectively. Compared with an iPTH level of 100 to <200 pg/mL, both the low iPTH (<100 pg/mL, OR: 2.45, 95% CI: 2.27–2.64) and the high iPTH (≥800 pg/mL: OR: 2.13, 95% CI: 1.95–2.33) levels were associated with hypercalcemia.
Conclusions
Higher levels of iPTH are incremental correlates of hyperphosphatemia and hyperphosphatasemia, whereas both very low and high PTH levels are linked to hypercalcemia. If these associations are causal, correction of hyperparathyroidism may have overarching implications on bone and mineral disorders in MHD patients.
Keywords: hemodialysis, serum alkaline phosphatase, serum calcium, serum intact parathyroid hormone, serum phosphorous
INTRODUCTION
A number of reports have described an increased risk of all-cause and cardiovascular mortality in patients with disorders of mineral metabolism [1–6]. Mineral and bone disorders (MBDs) are associated with accelerated atherosclerosis [7], which is an important cause of cardiovascular death in long-term dialysis patients [8], in patients with chronic kidney disease (CKD) [9–11] and in kidney transplant recipients [12, 13]. However, treatment of MBD improves the survival of patients on maintenance dialysis [14].
The Kidney Disease Outcome Quality Initiative (K/DOQI) and the Kidney Disease: Improving Global Outcomes guidelines suggest that serum parathyroid hormone (PTH) should be maintained at >150 or 130 pg/mL, respectively [15], in chronic dialysis patients in order to avoid adynamic bone disease, especially in African–American patients [16]. Since PTH levels correlate with bone histology, most clinical studies use intact parathyroid hormone (iPTH) to designate the state of bone turnover, especially since bone biopsies are not commonly used and easily obtained. Previous studies have demonstrated that higher turnover bone disorders are associated with serum levels of iPTH >400 pg/mL [15]. However, significant discrepancies may exist between the histopathologic diagnosis of the adynamic bone and the biochemical detection by means of ‘low PTH’ [17], which might be a marker of protein–energy waste syndrome in these patients [18].
The primary response to PTH by the healthy kidney is to increase renal calcium resorption and phosphate excretion. In the kidney, PTH blocks reabsorption of phosphate in the proximal tubule while promoting calcium reabsorption in the ascending loop of Henle, distal tubule and collecting tubule. However, in patients with end-stage renal failure, the predictors of serum PTH level might be different.
Thus, there is limited knowledge on the predictors of serum iPTH level in maintenance hemodialysis (MHD) patients. We hypothesized that higher serum phosphorous, alkaline phosphatase (ALP) levels and lower serum calcium level were associated with higher serum iPTH levels in a large representative national cohort of MHD patients across the USA.
MATERIALS AND METHODS
Patients
We extracted, refined and examined data from all individuals with end-stage renal failure who underwent MHD treatment from July 2001 through June 2009 in any one of the outpatient dialysis facilities of a large dialysis organization (LDO) in the USA (excluding the acquisition of units owned by Gambro). The study was approved by relevant Institutional Review Committees. Inclusion criteria were patients who had been undergoing dialysis for at least 90 days, were being treated with MHD at the time of entry into the cohort and had serum phosphorus and iPTH measurements at the baseline quarter.
Clinical and demographic measures
The creation of the 5-year cohort (1 July 2001 to 30 June 2006) with 20 calendar quarters has been described previously [4, 19–23]. The current 8-year cohort includes three additional years of repeated measures during 12 more calendar quarters (up to 30 June 2009). To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, i.e. over a 13-week interval, were averaged values. Average values were obtained from up to 32 calendar quarters (q1–q32) for each laboratory and clinical measure for each patient over the 8-year cohort period. The first (baseline) studied quarter for each patient was that calendar quarter, in which patient's vintage reached >90 days. Histories of tobacco smoking and pre-existing comorbid conditions were obtained by linking the LDO's database to the Medical Evidence Form 2728 of the United States Renal Data System (USRDS), and the latter was categorized into 11 comorbid conditions: AIDS, HIV-positive status, atherosclerotic heart disease, congestive heart failure, other cardiac disease, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, history of hypertension, inability to ambulate and cancer. The presence or absence of diabetes at baseline was obtained from the LDO and USRDS.
Laboratory measures
Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, within 24 h. All laboratory values were measured by automated and standardized methods. Most laboratory values were measured monthly. Serum phosphorus and iPTH were usually measured quarterly. We divided patients into nine a priori categories based on iPTH values: <100, ≥800 pg/mL and seven increments of 100 pg/mL in-between to examine the ‘dose–response’ association between iPTH categories and odds of hyperphosphatemia [serum phosphorous (P) >5.5 mg/dL], hyperphosphatasemia [high ALP level >120 U/L] and hypercalecmia [serum calcium >10.5 mg/dL].
Epidemiologic and statistical methods
Data were summarized using proportions and means [±standard deviation (SD)]. We examined P-values for trends across pre-transplant serum iPTH categories. Linear regression was used to examine the strength of association between iPTH and the three other relevant MBD markers (Ca, P and ALP). All analyses were also performed using repeated-measures fixed-effect models. We also performed logistic regression to assess the association of iPTH increments with hypophosphatemia [serum phosphorous (P) >5.5 mg/dL], high ALP (ALP level >120 U/L) and hypercalcemia [serum Ca >10.5 mg/dL]. For each analysis, three models were examined: (i) unadjusted model; (ii) case-mix-adjusted model that included age, gender, race/ethnicity (African-Americans and other self-categorized Blacks, non-Hispanic Caucasians or Whites, Asians, Hispanics and others), categories of dialysis vintage (<6 months, 6 months to <2 years, 2–<5 years and ≥5 years), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced and widowed), dialysis dose as indicated by Kt/V (single pool), residual renal function during the entry quarter, i.e. urinary urea clearance, types of vascular access (catheter, graft, fistula and others/unknown) and pre-existing comorbidities (as listed above) and (iii) case-mix plus malnutrition–inflammation complex syndrome (MICS)-adjusted model which included all of the covariates in the case-mix model as well as markers of nutritional status and inflammation, including body mass index (BMI), total nitrogen appearance (also known as normalized protein catabolic rate) and nine laboratory surrogates with known association with clinical outcomes in hemodialysis patients including serum levels of albumin, total iron-binding capacity, ferritin, creatinine, calcium, bicarbonate, white blood cell count, lymphocyte percentage and hemoglobin. Missing covariate data of continuous and binary variables were imputed by the mean of patient or population as appropriate; a category of missing covariate data was created for each categorical variable with more than two categories. All statistical analyses were carried out with SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline data and correlations
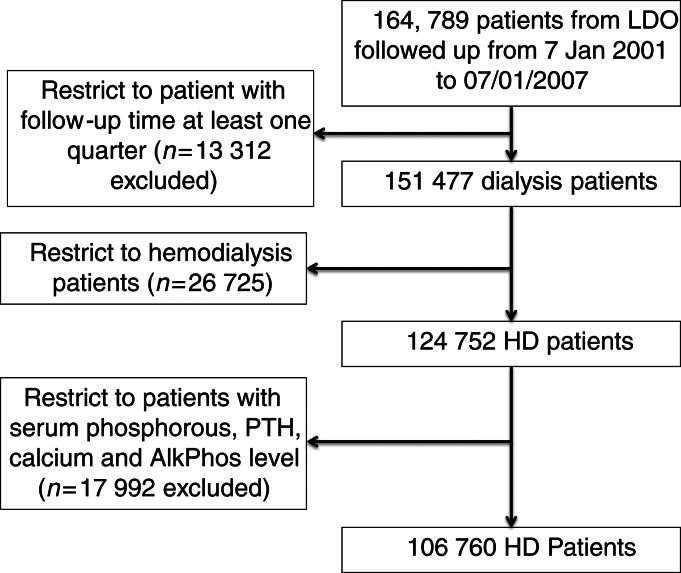
Over the 6-year period (July 2001–June 2007), 164 789 adult subjects received dialysis treatment in units owned by an LDO (Figure 1); of these, 124 752 patients were undergoing MHD at the time of entry into the cohort. The study cohort of 106 760 MHD patients was identified after excluding individuals with no data on serum phosphorous, iPTH, calcium and ALP (n = 17 992).
FIGURE 1:
Flow chart of patient selection.
Table 1 shows baseline demographic, clinical and laboratory characteristics of the studied MHD patients according to four a priori categories based on baseline iPTH—higher iPTH levels were associated with younger age, less White and more Black patients, and higher BMI and higher serum phosphorous level.
Table 1.
Demographic, clinical and laboratory values in 106 760 MHD patients (from July 2001 to June 2009) according to four categories of iPTH
| Variable | Total (n = 106 760) | Intact parathyroid hormone (pg/mL) |
P-value for trend | |||
|---|---|---|---|---|---|---|
| iPTH < 150 (n = 28 048) | 150 to <300 (n = 36 615) | 300 to <600 (n = 27 468) | ≥600 (n = 14 629) | |||
| Age (years) | 61 ± 16 | 64 ± 15 | 63 ± 15 | 59 ± 16 | 53 ± 17 | <0.001 |
| Gender (% female) | 45 | 47 | 44 | 45 | 46 | 0.03 |
| Diabetes mellitus (%) | 59 | 59 | 63 | 60 | 47 | <0.001 |
| Race (%) | ||||||
| White | 42 | 51 | 45 | 36 | 28 | <0.001 |
| Black | 33 | 22 | 29 | 38 | 51 | <0.001 |
| Hispanic | 15 | 16 | 16 | 16 | 13 | <0.001 |
| Asian | 3 | 4 | 3 | 3 | 2 | <0.001 |
| Other | 7 | 7 | 7 | 7 | 6 | <0.001 |
| Vintage (time on dialysis, %) | ||||||
| 0–6 months | 14 | 16 | 14 | 14 | 11 | <0.001 |
| 6–24 months | 31 | 31 | 34 | 31 | 25 | <0.001 |
| 2–5 years | 34 | 35 | 35 | 35 | 32 | <0.001 |
| >5 years | 21 | 18 | 17 | 20 | 32 | <0.001 |
| Primary insurance (%) | ||||||
| Medicare | 62 | 63 | 63 | 61 | 60 | <0.001 |
| Medicaid | 6 | 5 | 5 | 7 | 9 | <0.001 |
| Private Insurance | 10 | 12 | 10 | 10 | 9 | <0.001 |
| Other | 13 | 11 | 14 | 15 | 14 | <0.001 |
| Marital status (%) | ||||||
| Married | 38 | 40 | 41 | 37 | 32 | <0.001 |
| Divorced | 7 | 6 | 6 | 7 | 8 | <0.001 |
| Single | 23 | 18 | 20 | 25 | 34 | <0.001 |
| Widowed | 14 | 15 | 15 | 13 | 10 | <0.001 |
| Vascular access (%) | ||||||
| Catheter | 35 | 37 | 35 | 36 | 33 | <0.001 |
| AVF | 22 | 21 | 22 | 22 | 23 | <0.001 |
| Graft | 25 | 26 | 25 | 25 | 25 | 0.01 |
| Other | 1 | 1 | 1 | 1 | 1 | 0.65 |
| Kt/V (single pool) | 1.53 ± 0.35 | 1.56 ± 0.36 | 1.54 ± 0.35 | 1.50 ± 0.35 | 1.47 ± 0.34 | <0.001 |
| Residual renal function (Kru) | 2.50 ± 2.49 | 2.60 ± 2.69 | 2.61 ± 2.55 | 2.41 ± 2.28 | 2.11 ± 2.16 | <0.001 |
| Pre-existing comorbidities (%) | ||||||
| AIDS | 0.5 | 0.6 | 0.4 | 0.4 | 0.7 | 0.09 |
| HIV+ | 0.8 | 0.8 | 0.7 | 0.8 | 1.2 | <0.001 |
| Cancer | 4.4 | 5.5 | 4.5 | 3.7 | 3.1 | <0.001 |
| Atherosclerotic heart disease | 20.7 | 23.5 | 22.9 | 19.1 | 13.3 | <0.001 |
| Heart failure | 27.5 | 29.5 | 29.7 | 26.2 | 20.4 | <0.001 |
| Other cardiac disease | 5.6 | 6.4 | 6.3 | 4.9 | 3.6 | <0.001 |
| Cerebrovascular disease | 7.4 | 8.4 | 8.0 | 6.7 | 5.3 | <0.001 |
| Peripheral vascular disease | 11.3 | 13.1 | 12.5 | 10.1 | 6.9 | <0.001 |
| History of hypertension | 76.5 | 75.4 | 77.9 | 77.7 | 73.1 | <0.001 |
| Non-ambulatory | 3.1 | 4.3 | 3.0 | 2.6 | 2.0 | <0.001 |
| Pulmonary disease | 5.7 | 6.8 | 6.1 | 5.3 | 3.4 | <0.001 |
| Smoker | 4.7 | 4.1 | 4.6 | 5.0 | 5.3 | <0.001 |
| Laboratory values | ||||||
| Intact parathyroid hormone (pg/mL) | 350 ± 371 | 87 ± 40 | 220 ± 42 | 416 ± 83 | 1059 ± 547 | <0.001 |
| Phosphorus (mg/dL) | 5.56 ± 1.48 | 5.04 ± 1.33 | 5.39 ± 1.33 | 5.83 ± 1.45 | 6.49 ± 1.65 | <0.001 |
| Alkaline phosphatase (U/L) | 119 ± 87 | 113 ± 87 | 112 ± 74 | 117 ± 77 | 152 ± 120 | <0.001 |
| Albumin (g/dL) | 3.67 ± 0.47 | 3.59 ± 0.50 | 3.67 ± 0.46 | 3.71 ± 0.45 | 3.78 ± 0.43 | <0.001 |
| Creatinine (mg/dL) | 7.94 ± 3.27 | 7.14 ± 3.03 | 7.49 ± 3.02 | 8.35 ± 3.23 | 9.85 ± 3.54 | <0.001 |
| Total iron-binding capacity (mg/dL) | 209 ± 46 | 203 ± 48 | 211 ± 46 | 212 ± 45 | 208 ± 44 | <0.001 |
| Bicarbonate (mg/dL) | 22.4 ± 3.0 | 22.8 ± 3.0 | 22.5 ± 2.9 | 22.1 ± 3.0 | 21.7 ± 3.1 | <0.001 |
| Calcium (mg/dL) | 9.19 ± 0.71 | 9.31 ± 0.70 | 9.13 ± 0.63 | 9.12 ± 0.72 | 9.21 ± 0.85 | <0.001 |
| Ferritin (ng/mL) | 514 ± 487 | 555 ± 531 | 497 ± 455 | 486 ± 459 | 527 ± 518 | <0.001 |
| Normalized protein catabolic rate (g/kg/day) | 0.95 ± 0.26 | 0.92 ± 0.26 | 0.95 ± 0.25 | 0.96 ± 0.25 | 0.99 ± 0.25 | <0.001 |
| Blood hemoglobin (g/dL) | 12.03 ± 1.37 | 12.04 ± 1.38 | 12.14 ± 1.33 | 12.02 ± 1.37 | 11.79 ± 1.42 | <0.001 |
| White blood cells (×103/μL) | 7.5 ± 2.5 | 7.8 ± 2.8 | 7.5 ± 2.5 | 7.3 ± 2.4 | 7.1 ± 2.3 | <0.001 |
| Lymphocyte (% of total WBC) | 20.0 ± 7.9 | 19.8 ± 7.8 | 20.3 ± 7.8 | 20.9 ± 7.9 | 21.6 ± 8.1 | <0.001 |
| Body mass index (kg/m2) | 26.7 ± 6.9 | 25.7 ± 6.4 | 26.7 ± 6.7 | 27.4 ± 7.3 | 27.7 ± 7.5 | <0.001 |
Data are presented in mean ± standard deviation (SD).
Predictors of serum iPTH level
Table 2 shows the predictors of high-level (≥600 pg/mL) iPTH in 106 760 MHD patients. Female gender was associated with an 18% higher risk of high iPTH level. Compared with Whites, Blacks have a 2-fold higher risk of high iPTH level in a fully adjusted model (Table 2). Similar results were found in incident MHD patients in our sensitivity analysis (not shown).
Table 2.
Odds ratio for a high level [PTH ≥ 600 versus <600 (ref.)] intact parathyroid hormone (pg/mL) in 106 760 MHD patients in the baseline calendar quarter
| Variable | Unadjusted |
Case-mix adjusteda |
Case-mix and MICS adjustedb |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (+10 year) | 0.70 | (0.69, 0.71) | 0.78 | (0.77, 0.79) | 0.94 | (0.93, 0.96) |
| Gender (female) | 1.05 | (1.02, 1.09) | 1.17 | (1.13, 1.22) | 1.18 | (1.13, 1.24) |
| Race | ||||||
| White (reference) | 1 | 1 | 1 | |||
| Black | 2.79 | (2.68, 2.91) | 1.96 | (1.88, 2.05) | 2.09 | (1.99, 2.20) |
| Hispanic | 1.27 | (1.20, 1.35) | 1.01 | (0.95, 1.08) | 1.02 | (0.96, 1.09) |
| Asian | 1.01 | (0.89, 1.15) | 0.88 | (0.77, 1.00) | 0.90 | (0.79, 1.03) |
| Other | 1.49 | (1.38, 1.61) | 1.24 | (1.14, 1.34) | 1.23 | (1.13, 1.34) |
| Vintage (time on dialysis) | ||||||
| 0–6 months (reference) | 1 | 1 | 1 | |||
| 6–24 months | 1.01 | (0.95, 1.08) | 0.99 | (0.93, 1.06) | 0.89 | (0.83, 0.95) |
| 2–5 years | 1.23 | (1.16, 1.30) | 1.20 | (1.12, 1.28) | 0.93 | (0.87, 1.00) |
| >5 years | 2.26 | (2.13, 2.40) | 1.72 | (1.60, 1.84) | 1.19 | (1.10, 1.29) |
| Primary insurance | ||||||
| Medicare (reference) | 1 | 1 | 1 | |||
| Medicaid | 1.66 | (1.55, 1.77) | 1.17 | (1.09, 1.26) | 1.17 | (1.09, 1.26) |
| Private insurance | 0.93 | (0.88, 0.99) | 0.89 | (0.83, 0.95) | 0.89 | (0.83, 0.95) |
| Other | 1.10 | (1.05, 1.16) | 1.15 | (1.09, 1.22) | 1.23 | (1.16, 1.31) |
| Marital status | ||||||
| Married (reference) | 1 | 1 | 1 | |||
| Divorced | 1.48 | (1.38, 1.59) | 1.21 | (1.13, 1.30) | 1.16 | (1.08, 1.26) |
| Single | 2.00 | (1.92, 2.09) | 1.16 | (1.11, 1.22) | 1.16 | (1.10, 1.22) |
| Widowed | 0.86 | (0.81, 0.92) | 1.09 | (1.02, 1.16) | 1.08 | (1.01, 1.16) |
| Vascular access | ||||||
| Catheter (reference) | 1 | 1 | 1 | |||
| AVF | 1.16 | (1.11, 1.22) | 0.94 | (0.89, 0.99) | 0.89 | (0.84, 0.94) |
| Graft | 1.06 | (1.01, 1.11) | 0.86 | (0.81, 0.90) | 0.81 | (0.77, 0.86) |
| Other | 1.11 | (0.79, 1.55) | 1.28 | (0.90, 1.81) | 1.27 | (0.88, 1.83) |
| Other variables | ||||||
| Kt/V (single pool) (+1) | 0.60 | (0.57, 0.63) | 0.72 | (0.68, 0.76) | 0.97 | (0.91, 1.04) |
| Residual renal function (Kru) (+1 mL/min) | 0.88 | (0.86, 0.89) | 0.95 | (0.94, 0.97) | 1.00 | (0.98, 1.01) |
| Pre-existing comorbidities | ||||||
| Diabetes mellitus | 0.56 | (0.54, 0.58) | 0.69 | (0.66, 0.71) | 0.71 | (0.68, 0.74) |
| AIDS | 1.58 | (1.27, 1.95) | 1.01 | (0.78, 1.32) | 1.06 | (0.80, 1.41) |
| HIV+ | 1.68 | (1.42, 1.98) | 0.82 | (0.66, 1.00) | 0.78 | (0.63, 0.98) |
| Cancer | 0.71 | (0.65, 0.78) | 1.02 | (0.92, 1.13) | 1.00 | (0.90, 1.11) |
| Atherosclerotic heart disease | 0.60 | (0.57, 0.63) | 1.00 | (0.94, 1.05) | 1.03 | (0.97, 1.09) |
| Heart failure | 0.70 | (0.67, 0.73) | 0.96 | (0.91, 1.00) | 0.95 | (0.90, 0.99) |
| Other cardiac disease | 0.65 | (0.60, 0.71) | 0.96 | (0.87, 1.06) | 0.99 | (0.90, 1.09) |
| Cerebrovascular disease | 0.72 | (0.67, 0.78) | 0.96 | (0.89, 1.04) | 1.06 | (0.97, 1.15) |
| Peripheral vascular disease | 0.59 | (0.56, 0.63) | 0.90 | (0.84, 0.97) | 0.94 | (0.87, 1.01) |
| History of hypertension | 1.01 | (0.97, 1.06) | 1.08 | (1.03, 1.13) | 1.05 | (1.00, 1.11) |
| Non-ambulatory | 0.65 | (0.58, 0.73) | 0.86 | (0.76, 0.98) | 0.95 | (0.83, 1.08) |
| Pulmonary Disease | 0.60 | (0.54, 0.65) | 0.87 | (0.79, 0.96) | 0.90 | (0.81, 0.99) |
| Smoker | 1.23 | (1.14, 1.33) | 1.02 | (0.94, 1.11) | 1.01 | (0.93, 1.11) |
| Laboratory values | ||||||
| Phosphorus (+1 mg/dL) | 1.57 | (1.56, 1.59) | 1.49 | (1.47, 1.51) | 1.48 | (1.46, 1.51) |
| ALP (+10 U/L) | 1.04 | (1.04, 1.04) | 1.04 | (1.03, 1.04) | 1.05 | (1.04, 1.05) |
| Albumin (+0.1 g/dL) | 1.06 | (1.06, 1.07) | 1.03 | (1.03, 1.04) | 1.05 | (1.04, 1.05) |
| Total iron-binding capacity (+10 mg/dL) | 0.99 | (0.99, 1.00) | 1.01 | (1.00, 1.01) | 0.98 | (0.98, 0.99) |
| Creatinine (+1 mg/dL) | 1.21 | (1.20, 1.21) | 1.13 | (1.12, 1.14) | 1.05 | (1.04, 1.06) |
| Bicarbonate (+1 mg/dL) | 0.91 | (0.91, 0.92) | 0.93 | (0.92, 0.93) | 0.98 | (0.97, 0.99) |
| Ferritin (+100 ng/mL) | 1.01 | (1.00, 1.01) | 0.99 | (0.99, 1.00) | 0.98 | (0.98, 0.99) |
| Calcium (+1 mg/dL) | 1.05 | (1.02, 1.07) | 0.96 | (0.94, 0.98) | 1.08 | (1.05, 1.11) |
| Normalized protein catabolic rate (+0.1 g/kg/day) | 1.06 | (1.05, 1.07) | 1.09 | (1.09, 1.10) | 0.99 | (0.98, 1.00) |
| Blood hemoglobin (+1 g/dL) | 0.86 | (0.85, 0.87) | 0.90 | (0.89, 0.91) | 0.85 | (0.83, 0.86) |
| White blood cells (+1 × 103/μL) | 0.92 | (0.91, 0.93) | 0.96 | (0.96, 0.97) | 0.94 | (0.93, 0.95) |
| Lymphocyte (+10% of total WBC) | 1.21 | (1.19, 1.24) | 0.95 | (0.93, 0.98) | 0.96 | (0.94, 0.99) |
| Body mass index (+1 kg/m2) | 1.02 | (1.02, 1.02) | 1.02 | (1.01, 1.02) | 1.02 | (1.01, 1.02) |
aCase-mix model is adjusted for age, gender, race/ethnicity, categories of dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), residual renal function during the entry quarter, vascular access types, tobacco use, pre-existing comorbidities including diabetes mellitus, AIDS, HIV positive status, atherosclerotic heart disease, congestive heart failure, other cardiac disease, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, history of hypertension, inability to ambulate and cancer.
bMalnutrition inflammation complex syndrome (MICS)-adjusted model includes all of the case-mix covariates as well as body mass index, nPCR, serum levels of albumin, total iron-binding capacity, ferritin, creatinine, calcium, bicarbonate, white blood cell count, lymphocyte percentage and hemoglobin.
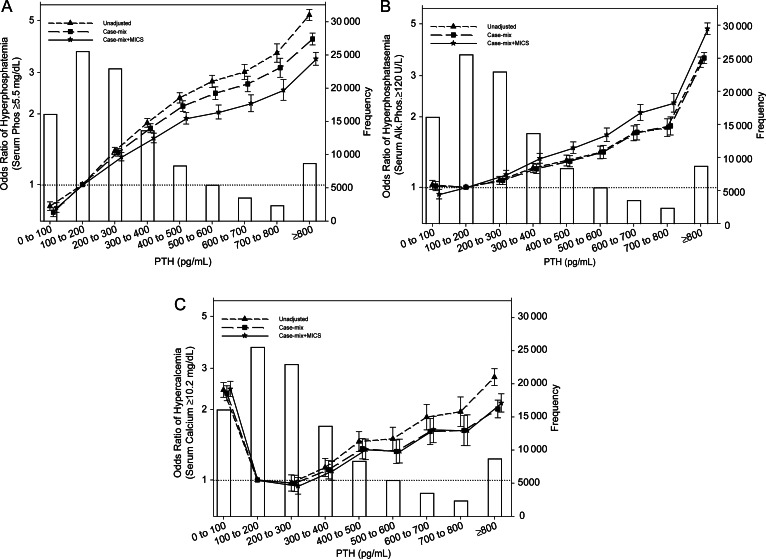
Figure 2A shows the odds ratios for hyperphosphatemia (serum phosphorus ≥5.5 mg/dL) in 106 760 MHD patients in different iPTH categories. The association between serum iPTH level and risk of hyperphosphatemia was strictly linear and incremental in the entire serum iPTH range. Compared with an iPTH level of 100 to <200 pg/mL, patients with an iPTH level of 300 to <400, 400 to <500, 500 to <600, 600 to <700, 700 to <800 and ≥800 pg/mL have 58% (OR: 1.58, 95% CI: 1.51–1.66), 92% (OR: 1.92, 95% CI: 1.81–2.03), 104% (OR: 2.04, 95% CI: 1.90–2.18), 122% (OR: 2.22, 95% CI: 2.04–2.41), 153% (OR: 2.53, 95% CI: 2.29–2.80) and 243% (OR: 3.43, 95% CI: 3.22–3.66) higher risk of hyperphosphatemia, respectively (Figure 2A).
FIGURE 2:
Odds ratios for hyperphosphatemia (serum phosphorus ≥5.5 mg/dL) (A), high ALP level (≥120 U/L) (B) and high calcium level (≥10.2 mg/dL) (C) in 106 760 MHD patients in the baseline calendar quarter of the cohort for selected ranges of PTH. The case-mix model is adjusted for age, gender, race/ethnicity, categories of dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), residual renal function during the entry quarter, vascular access types, tobacco use, pre-existing of comorbidities including diabetes mellitus, AIDS, HIV-positive status, atherosclerotic heart disease, congestive heart failure, other cardiac disease, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, history of hypertension, inability to ambulate and cancer. Malnutrition-inflammation complex syndrome (MICS)-adjusted model includes all of the case-mix covariates as well as body mass index, nPCR, serum levels of albumin, total iron-binding capacity, ferritin, creatinine, calcium, bicarbonate, white blood cell count, lymphocyte percentage and hemoglobin. Error bars represent SD.
Figure 2B shows the odds ratios for high serum ALP (≥120 U/L) in 106 760 MHD patients in different iPTH categories. The association between serum iPTH level and the risk of high serum ALP was also strictly linear and incremental in the entire serum iPTH range. Compared with an iPTH level of 100 to <200 pg/mL, patients with an iPTH level of 300 to <400, 400 to <500, 500 to <600, 600 to <700, 700 to <800 and ≥800 pg/mL have 33% (OR: 1.33, 95% CI: 1.27–1.40), 48% (OR: 1.48, 95% CI: 1.40–1.56), 68% (OR: 1.68, 95% CI: 1.57–1.79), 109% (OR: 2.09, 95% CI: 1.93–2.26), 130% (OR: 2.30, 95% CI: 2.10–2.52) and 376% (OR: 4.76, 95% CI: 4.50–5.04) higher risk of high serum ALP level, respectively (Figure 2B).
Figure 2C shows the odds ratios for hypercalcemia (serum calcium ≥10.2 mg/dL) in 106 760 MHD patients in different iPTH categories. The association between serum iPTH level and risk of hypercalcemia was U-shaped, both low iPTH and high iPTH levels were associated with hypercalcemia. Compared with an iPTH level of 100 to <200 pg/mL, patients with an iPTH level of <100, 400 to <500, 500 to <600, 600 to <700, 700 to <800 and ≥800 pg/mL have 145% (OR: 2.45, 95% CI: 2.27–2.64), 35% (OR: 1.35, 95% CI: 1.22–1.50), 33% (OR: 1.33, 95% CI: 1.18–1.49), 64% (OR: 1.64, 95% CI: 1.44–1.87), 63% (OR: 1.63, 95% CI: 1.40–1.90) and 113% (OR: 2.13, 95% CI: 1.95–2.33) higher risk of hypercalcemia, respectively (Figure 2C).
DISCUSSION
In this large-scale and contemporary cohort of 106 760 MHD patients, we report that serum phosphorous and ALP levels were a strong and significant predictor of the serum iPTH level. In addition, the serum PTH level showed a strong, incremental and linear association with increased risk of hyperphosphatemia and high serum ALP level. Moreover, there was a U-shaped association between the serum PTH level and risk of hypercalcemia.
A strictly linear and incremental association was found between serum iPTH level and risk of hyperphosphatemia. It has been recognized for more than four decades that high serum phosphorous is important in the development and progression of secondary hyperparathyroidism [24]; therefore, it is not surprising that the association was strong and linear. However, our study was one of the first studies to show this association in MHD patients using such a large population. Previous studies showed increased all-cause mortality, cardiovascular, and infectious mortality risk in MHD patients with high phosphorous and iPTH levels [4, 25–27]. In the Netherlands Cooperative Study on the Adequacy of Dialysis, a study carried out in Noordzij et al. [28] reported that the presence of plasma phosphorous concentrations greater than the K/DOQI targets increased all-cause mortality risk in incident hemodialysis and peritoneal dialysis patients. In addition, a recent meta-analysis in CKD patients indicated that only high serum phosphorous level was a strong and independent predictor of mortality [29].
Similarly to hyperphosphatemia, the association between the serum iPTH level and risk of high ALP level was strictly linear and incremental, indicative of the relation between the high-turnover bone disease and serum iPTH level. There is a physiological explanation for the association between high-turnover bone disease and serum iPTH level. Serum iPTH binds to osteoblasts via PTH receptor-1, and stimulates the osteoblasts to increase their expression of receptor activator of nuclear factor-kB ligand (RANKL) [30]. In addition, serum iPTH has an indirect effect on osteoclast activity too. The binding of RANKL to its receptors stimulates these osteoclast precursors to fuse, forming new osteoclasts, which ultimately augments bone resorption [31]. It is important to know the predictors of high ALP level, as high serum ALP level, as a marker of high-turnover bone disease [32], was associated with increased mortality risk in MHD patients [4, 33].
A U-shaped association was found between the serum PTH level and risk of hypercalcemia. Compared with patients with an iPTH level of 100 to <200 pg/mL, patients with an iPTH level of <100 pg/mL have more than two times higher risk of hypercalcemia. There is a physiological explanation for this observation. The low iPTH level can be a marker of adynamic bone disease [34], which is associated with a higher serum calcium level [35]. In adynamic bone disease, there is a low capacity of the bone to buffer calcium and consequent inability to handle extra calcium load [36]. This can easily result in hypercalcemia after minimal calcium loading. The high iPTH level was also associated with a high risk of hypercalcemia. One of the potential explanations of this association is that the side effect of almost all medication (such as vitamin D derivates and vitamin D receptor agonists) against hyperparathyroidism is hypercalcemia. Previously, we showed similar U-shaped associations between serum calcium level and mortality in MHD patients [4, 37]. However, this association was independent of the serum iPTH level, consequently the mortality association cannot be explained only with the iPTH level [37].
The strength of our paper is that we used a large, contemporary and national representative database from an LDO' clinic. The large number of patients allowed us to adjust for several important confounders such as inflammation and pre-existing comorbidities including diabetes mellitus.
Our study should be qualified for several potential limitations. We did not have data about 25(OH) vitamin D, 1.25(OH)2 vitamin D, fibroblast growth factor-23 and osteocalcin levels. In addition, medications (such as vitamin D and phosphate binders) [38] were not available in our database. Patients who did not have measured serum iPTH, ALP and calcium levels were excluded from the analyses. The excluded patients may have been different from those included in our study, which may have biased our results. Another potential limitation is that we did not have bone-specific ALP data.
CONCLUSIONS
The association of PTH level with hyperphosphatemia and a high ALP level was linear and incremental, whereas the association with the high level of serum calcium is U-shaped, in that both very low levels and high levels of PTH are associated with hypercalcemia. In addition, we report here that serum phosphorous and ALP levels were a strong and significant predictor of the serum iPTH level.
CONFLICT OF INTEREST STATEMENT
K.K.Z. was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA, and has received honoraria and/or grants from Abbott, Amgen, Genzyme and Shire.
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research (DCR) for providing the clinical data and review for this research project. The study was supported by K.K.Z.'s research grant from the American Heart Association grant (0655776Y). K.K.Z.'s other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106); a research grant from DaVita Clinical Research and a philanthropic grant from Mr Harold Simmons. M.Z.M. is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012).
REFERENCES
- 1.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. doi:10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 2.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. doi:10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 3.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. doi:10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 4.Lukowsky LR, Molnar MZ, Zaritsky JJ, et al. Mineral and bone disorders and survival in hemodialysis patients with and without polycystic kidney disease. Nephrol Dial Transplant. 2012;27:2899–2907. doi: 10.1093/ndt/gfr747. doi:10.1093/ndt/gfr747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. doi:10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel DM, Raggi P, Smits G, et al. Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant. 2007;22:3568–3572. doi: 10.1093/ndt/gfm424. doi:10.1093/ndt/gfm424. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Giachelli C, Bellasi A. Interaction of vascular and bone disease in patients with normal renal function and patients undergoing dialysis. Nature clinical practice. Cardiovasc Med. 2007;4:26–33. doi: 10.1038/ncpcardio0725. [DOI] [PubMed] [Google Scholar]
- 8.Shoji T, Maekawa K, Emoto M, et al. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis. 2010;210:145–149. doi: 10.1016/j.atherosclerosis.2009.11.013. doi:10.1016/j.atherosclerosis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Shah A, Duong U, et al. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl. 2010;117:S10–S21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Ureche V, Lu JL, et al. Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant. 2010;25:3003–3011. doi: 10.1093/ndt/gfq144. doi:10.1093/ndt/gfq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JC, Kovesdy CP, Duong U, et al. Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int. 2010;14:182–192. doi: 10.1111/j.1542-4758.2009.00430.x. doi:10.1111/j.1542-4758.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molnar MZ, Kovesdy CP, Mucsi I, et al. Association of pre-kidney transplant markers of mineral and bone disorder with post-transplant outcomes. Clin J Am Soc Nephrol. 2012;7:1859–1871. doi: 10.2215/CJN.01910212. doi:10.2215/CJN.01910212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, et al. Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens. 2012;21:389–403. doi: 10.1097/MNH.0b013e3283546ee0. doi:10.1097/MNH.0b013e3283546ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78:578–589. doi: 10.1038/ki.2010.167. doi:10.1038/ki.2010.167. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 16.Moore C, Yee J, Malluche H, et al. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1484–1493. doi: 10.2215/CJN.01770408. doi:10.2215/CJN.01770408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. doi:10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feroze U, Molnar MZ, Dukkipati R, et al. Insights into nutritional and inflammatory aspects of low parathyroid hormone in dialysis patients. J Ren Nutr. 2011;21:100–104. doi: 10.1053/j.jrn.2010.10.006. doi:10.1053/j.jrn.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar MZ, Streja E, Kovesdy CP, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94:945–954. doi: 10.3945/ajcn.111.014639. doi:10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58:574–582. doi: 10.1053/j.ajkd.2011.03.023. doi:10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streja E, Kovesdy CP, Molnar MZ, et al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57:883–893. doi: 10.1053/j.ajkd.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Streja E, Molnar MZ, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175:793–803. doi: 10.1093/aje/kwr384. doi:10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61:708–715. doi: 10.2337/db11-1015. doi:10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slatopolsky E, Caglar S, Pennell JP, et al. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971;50:492–499. doi: 10.1172/JCI106517. doi:10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. doi:10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2724–2734. doi: 10.1002/jbmr.177. doi:10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noordzij M, Korevaar JC, Boeschoten EW, et al. The kidney disease outcomes quality initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46:925–932. doi: 10.1053/j.ajkd.2005.08.013. doi:10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. doi:10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 30.Mannstadt M, Juppner H, Gardella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol. 1999;277:F665–F675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 31.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. doi:10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 32.Qi Q, Monier-Faugere MC, Geng Z, et al. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26:622–631. doi: 10.1016/0272-6386(95)90599-5. doi:10.1016/0272-6386(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 33.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. doi:10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl. 3):S1. [PubMed] [Google Scholar]
- 35.Malluche HH, Monier-Faugere MC. Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl. 1992;38:S62–S67. [PubMed] [Google Scholar]
- 36.Kurz P, Monier-Faugere MC, Bognar B, et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 1994;46:855–861. doi: 10.1038/ki.1994.342. doi:10.1038/ki.1994.342. [DOI] [PubMed] [Google Scholar]
- 37.Miller JE, Kovesdy CP, Norris KC, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol. 2010;32:403–413. doi: 10.1159/000319861. doi:10.1159/000319861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block GA. Therapeutic interventions for chronic kidney disease-mineral and bone disorders: focus on mortality. Curr Opin Nephrol Hypertens. 2011;20:376–381. doi: 10.1097/MNH.0b013e328346f93f. doi:10.1097/MNH.0b013e328346f93f. [DOI] [PubMed] [Google Scholar]