Abstract
The regenerative potential for adult bone marrow-derived mesenchymal stromal cells (MSCs) has been extensively investigated in the setting of arthritic disease and focal cartilage defects. In vitro chondrogenic differentiation of MSCs is regularly accomplished by the widely used pellet culture system where MSCs are maintained in high-density pellets to mimic mesenchymal condensation during development. Supplementation of chondrogenic MSC pellet cultures with growth differentiation factor-5 (GDF-5), a highly regulated gene in the chondrogenic phase of endochondral ossification (EO), was investigated here under the hypothesis that GDF-5 will enhance the chondrogenic differentiation of MSCs, thereby supporting their entry into ossification. The supplementation of chondrogenic MSC pellets with the recombinant human GDF-5 protein significantly enhanced MSC chondrogenic differentiation, as demonstrated by enhanced collagen type II and sulfated glycosaminoglycan (GAG) incorporation into the extracellular matrix. Increased P-SMADs 1-5-8 were observed in pellets treated with GDF-5 and transforming growth factor (TGF)-β 3 when compared to the pellets treated with TGF-β 3 alone, demonstrated by immunostaining and western blot analysis of the chondrogenic pellet extract. A concurrent increase in alkaline phosphatase, collagen types I and X, and osteopontin secretion indicated a transition of these cultures to hypertrophy. Together, these data support the application of GDF-5 to enhance MSC chondrogenic differentiation and hypertrophy as a precursor to EO.
Introduction
The regenerative potential for adult bone marrow-derived mesenchymal stromal cells (MSCs) has been extensively investigated in the setting of arthritic disease [1,2], focal cartilage defects [3], and bone repair [4]. After transplantation to the damaged knees, MSCs slow joint degradation and contribute to repair of arthritic tissue [2,5] and cartilage defects [6]. Similar beneficial effects are observed when bone fractures are treated with MSCs [7]. The widespread demonstration of an effective outcome in preclinical models of disease has lead to a series of clinical studies currently underway to test MSCs in cartilage repair [8–10], osteoarthritis [11–13], and long-bone fracture repair [14].
MSCs undergo chondrogenic differentiation in high-density pellet cultures [15,16] that mimic the mesenchymal condensation formed during limb development. When transplanted subcutaneously, chondrogenically primed MSCs develop along a pathway toward endochondral ossification (EO), exhibiting features such as development of a bony collar, vascularization, osteoclastic resorbtion of the cartilage, and the presence of the hematopoietic foci [17,18]. In fact, MSCs in pellet cultures express collagen type I, indicating the development of osseous tissue, and markers of chondrocyte hypertrophy such as collagen type X, osteopontin (OPN), and MMP-13 expression, followed by alkaline phosphatase (ALP) activity and mineralization [17,19–25]. Interestingly, when MSCs are directly placed in an articular cartilage defect, their spontaneous differentiation results in expression of collagen type II without collagen type X, indicating the development of an articular-like phenotype [26].
The expression profile of growth differentiation factor-5 (GDF-5) points to its role in the early phase of EO, specifically during prechondrogenic condensation [27]. During mesenchymal cell condensation, the presence of GDF-5 increases the cell–cell adherence, thereby promoting condensation. Overexpression studies in vivo have shown that exposure to GDF-5 increases the length and width of bones of the limb, perhaps due to this enhanced cellular ability to condense [28–31]. Later in organogenesis, GDF-5 mRNA expression localizes to the future joint spaces, directly correlating with the position, number, and timing of developing joints [31,32]. Chondrogenic differentiation of human embryonic stem cells [33], fetal human MSCs [34], or embryonic chick mesenchymal cultures with recombinant GDF-5 protein [35] or adenovirally expressed GDF-5 in adipose-derived progenitor chondrogenesis [36] reliably stimulated an upregulation of the chondrogenic marker genes such as collagen type II and aggrecan. However, concurrent with this simulation was the increased transcription of collagen type X and ALP, the indicators of chondrocyte hypertrophy [36,37], the precursor to EO.
Therefore, this investigation assessed the in vitro supplementation of human bone marrow-derived MSCs with the recombinant human GDF-5 protein under the hypothesis that GDF-5 will enhance the chondrogenic differentiation of MSCs, therefore supporting their entry into EO. This study advanced the previous investigations by using clinically relevant adult bone marrow-derived MSCs instead of fetal progenitors and significantly lower doses of GDF-5 to detect a beneficial effect [34].
Materials and Methods
MSC isolation and culture
The bone marrow was obtained from the iliac crest of healthy donors. In each assay, 3–5 biologic replicates were performed from donors aged 18–35 years. All procedures were performed with informed consent and approved by the Clinical Research Ethics Committee at the University College Hospital, Galway. The marrow was diluted 1:10 in phosphate-buffered saline (PBS; Gibco) and centrifuged at 900 g for 10 min. The spun cells were washed and plated at a density of 235,000 to 340,000 cells/cm2. A complete MSC expansion medium [alpha-MEM with Glutamax (Gibco), 10% selected Hyclone fetal bovine serum (FBS; Thermo Scientific), 1% penicillin/streptomycin (Sigma), 1% nonessential amino acids (Sigma), and 5 ng/mL fibroblast growth factor (FGF-2; Pepro-Tech, London, England or R&D Systems)] was added to the MSC culture in support of cellular proliferation. Cells were incubated at 37°C with 5% CO2 in a humidified chamber. After 5 days, the culture medium was replaced, and the cells were fed twice weekly thereafter. Upon confluence, the MSCs were subcultured and passaged every 5–7 days as required to passage 3 for differentiation.
Chondrogenic differentiation
MSCs were trypsinized using 0.5% trypsin EDTA (Sigma) from the monolayer and centrifuged, and the expansion medium was removed. After suspension in an incomplete chondrogenic medium [ICM; DMEM high-glucose (Sigma), 100 nM dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, 40 μg/mL L-proline, 1% ITS Premix supplement (Becton Dickinson), 1 mM sodium pyruvate (Sigma), and 1% penicillin/streptomycin (Sigma)], MSCs were pelleted by centrifugation at 500 g for 5 min. MSCs were resuspended in a complete chondrogenic medium [CCM; ICM with 10 ng/mL transforming growth factor β 3 (TGF-β 3; R&D Systems or Pepro-Tech)] or a GDF-5 containing medium (CCM with 100, 150 or 200 ng/mL recombinant human GDF-5; Biopharm), such that 250,000 cells were included in each pellet. Again, the cultures were centrifuged at 500 g for 5 min to create high-density cultures. Upon identifying 150 ng/mL as the optimal dose, pellets received 150 ng/mL GDF-5 for the entire culture period (0–21 days) or from 0 to 7 days or 7 to 21 days of culture. The pellets were incubated at 37°C with 5% CO2 in a humidified chamber, and the medium was replaced three times a week.
The glycosaminoglycan (GAG) content was assessed by the 1,9-dimethylmethylene blue (DMMB) analysis. Chondroitin sulfate (Sigma) standards were prepared using a dilution buffer [50 mM sodium phosphate (Sigma), 2 mM N-acetyl cysteine (Sigma), and 2 mM EDTA (Sigma), adjusted to pH 6.5] to achieve concentrations ranging from 0 to 2 μg of GAG. Individual pellets were digested [papain (Sigma) in a dilution buffer at 25 μg/mL] overnight at 60°C and combined with DMMB (adjusted to pH 3). The resultant samples were evaluated on a Wallac Victor plate reader at 595 nm. The DNA content was assessed using the Quant-iT Pico Green dsDNA assay kit from Invitrogen according to the manufacturer's protocol. Together, the results from the DMMB and DNA analyses allowed expression of GAG normalized to DNA content. Statistically, TGF-β 3-treated cultures were compared to individual GDF-5-treated cultures at day 21.
Quantitative reverse transcriptase-polymerase chain reaction analysis
Chondrogenic pellets were removed from culture and washed in PBS, and RNA was isolated using TriReagent (Invitrogen) according to the manufacturer's protocol. Combinatorial retrotranscription and amplification QIAGEN SYBR Green qRT-PCR kits and QIAGEN QuantiTect primers were used to amplify aggrecan and collagen type I transcripts, while primers for collagen type II were designed: 5′–3′ TCCCTTTGGTCCTGGTTGCCC and ATCTGCCCAACTGACCTCGCCA (Sigma) and amplified with QIAGEN reagents according to the manufacturer's protocol. Similarly, primers for drosha, a consistently expressed transcript regardless of time point or treatment, were designed to normalize sample loading: 5′–3′ TCCATGCACCAGATTCTCTG and TACGGACAGAGCTTGGTTTCG (Sigma) and amplified using QIAGEN reagents and protocols. Reactions were performed at 50°C for 30 min and 95°C for 15 min, followed by 40 cycles at 94°C for 15 s and 60°C for 1 min. ABI Prism 7000 (Applied Biosystems) was used for amplification, and the resultant data were analyzed using the ΔΔCt method [38], normalizing to both the drosha loading control and the day-0 CCM-treated pellets to reflect the basal transcript levels. At each time point, the fold change in the transcript level of TGF-β 3-treated cultures was evaluated with regard to the GDF-5-treated cultures.
Immunohistochemical analysis
Staining for collagen type I or II was accomplished after rehydrating paraffin-embedded sections in a decreasing alcohol series and a pepsin antigen retrieval. Sections were blocked both for peroxidase and nonspecific binding, followed by incubation with an anti-collagen type I antibody or a collagen type II antibody (Abcam), and developed using DakoCytomation EnVision HRP+3,3′-Diaminobenzidine (DAB; DAKO) according to the manufacturer's instructions.
Phosphorylated SMADs 1-5-8 and 2–3 were stained by initially rehydrating the paraffin-embedded sections in a decreasing alcohol series. Antigens were retried in a warm (60°C) 10 mM sodium citrate buffer (pH 6.0) before heating to 97°C for 20 min before finally allowing the solution to cool for 40 min. Sections were blocked for both peroxidase and nonspecific binding. Primary antibodies for P-SMADs 2–3 and P-SMADs 1-5-8 (Cell Signaling Technology) were separately diluted 1:100 in 10% equine serum and incubated with the samples for 1 h at room temperature. Staining was developed using DAB+Chromogen (DAKO) according to the manufacturer's instructions, and then counterstained with hematoxylin (Sigma) before dehydration and mounting.
ALP activity
To quantify the relative level of soluble ALP activity in a pellet-conditioned medium, samples were collected at days 14 and 21 of culture and stored at −20°C. To assay, the pellet-conditioned medium was diluted 1:13 in p-Nitrophenyl phosphate (pNPP; Sigma), and the assay performed as indicated by the manufacturer. The absorbance of each sample was analyzed at 405 nm on a Wallac Victor plate reader, and then converted to μM concentration using the equation: Absorbance=(extinction coefficient) (concentration) (path length). At each individual time point, the concentration of ALP product was statistically evaluated by comparing TGF-β 3-treated pellets with GDF-5-treated pellets.
Western blotting
Pellets were washed in cold PBS, snap-frozen, and manually crushed. Upon crushing, protein lysates were immediately prepared by the addition of 0.1% nonyl phenoxypolyethoxylethanol (Sigma). The protein was loaded and separated on a 10% polyacrylamide gel, followed by transfer to a polyvinylidene fluoride membrane (Millipore) by electroblotting. The type X collagen western blot contained 35 μg of protein per lane, whereas the P-SMAD 1-5-8 western blot contained 20 μg of protein per lane. Membranes were blocked for nonspecific binding in 5% bovine serum albumin followed by incubation with a 1:1,000 diluted anti-type X collagen antibody (Sigma) or P-SMAD 1-5-8 antibody (Cell Signaling Technologies) at 4°C overnight. After subsequent washing the membranes, blots were probed with an appropriate horseradish peroxidase-conjugated secondary antibody (Cal Biochem or Santa Cruz Biotechnology) diluted 1:3,000 and visualized by an enhanced chemiluminescence system (Amersham Biosciences) according to the manufacturer's instructions. An anti-beta actin (Abcam) or anti-GAPDH (Abcam) antibody diluted 1:1,000 was used to ensure equal protein loading between samples.
Osteopontin enzyme-linked immunosorbent assay
OPN content in a pellet-conditioned medium was determined with R&D Systems Enzyme-linked immunosorbent assay (ELISA), after antibody coating, according to the manufacturer's instructions. The conditioned medium was diluted 1:4 in an alpha-MEM before evaluation. The resulting plate was evaluated at 450 nm on a Perkin and Elmer Victory 3 plate reader. The resultant data were statistically evaluated by comparing TGF-β 3-treated cultures with GDF-5-treated cultures within each time point.
Histological analysis
Pellets were washed twice in PBS and fixed in 10% neutral buffered formalin (Sigma) for 1 h after 21 days in culture. Samples were placed in tissue cassettes (Simport) and dehydrated with an increasing series of industrial methylated spirits (Lennox) to Histoclear (National Diagnostics). After embedding in paraffin, the samples were cut at a thickness of 5 μm using a Leica (Ashbourne) RM2235 microtome. Safranin O (Sigma) and fast green (Sigma) staining was used to histologically analyze sulfated proteoglycan deposition in the extracellular matrix (ECM). The paraffin-embedded, sectioned samples were deparaffinized, rehydrated, incubated in hematoxylin (Sigma), followed by fast green and Safranin O, then dehydrated, cover-slipped, and photographed on an Olympus IX71 microscope.
Statistical analysis
Data presented are either an average of at minimum three MSC donors±the standard deviation of replicates or as a fold change over the control±standard error of the mean, with the exception of western blotting and immunostaining analysis displaying one donor. To determine statistical significance, GraphPad Prism 5 or Microsoft Excel was utilized to perform appropriate data analysis. To evaluate the GAG:DNA content (Fig. 1A, B), nonparametric Mann–Whitney analysis was performed with Gaussian approximation using GraphPad Prism. Quantitative reverse transcriptase (qRT)-polymerase chain reaction (PCR) data were analyzed with GraphPad Prism through two-way ANOVA with a Bonferroni post-test. Comparison of SMAD phosphorylation was accomplished with nonparametric one-way ANOVA with a Kruskal-Wallis post-test on GraphPad Prism. ALP activity was evaluated by two-sample equal variance t-test with Microsoft Excel. Analysis of OPN levels by ELISA were compared statistically by one-way ANOVA with a Tukey post-test. Statistically significant changes are marked as *=P<0.05; **=P<0.01; ***=P<0.001.
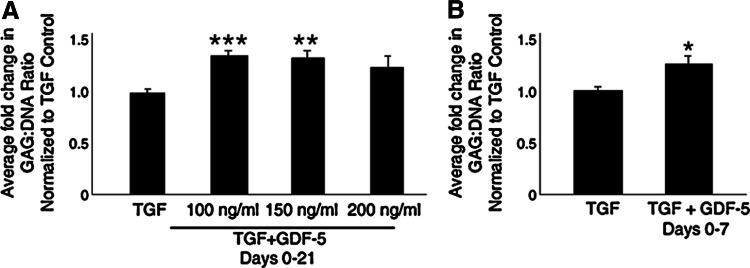
FIG. 1.
Growth differentiation factor-5 (GDF-5) enhances glycosaminoglycan (GAG) accumulation. (A) After performing a dose–response study incubating control pellets with transforming growth factor (TGF)-β 3 alone or in combination with GDF-5 at 100, 150, or 200 ng/mL for 21 days, a 1,9-dimethylmethylene blue (DMMB) analysis was performed to quantify GAG content. Displayed as average fold change of four donors with three replicates each and normalized to both total DNA content and control samples, a statistically significant enhancement of GAG deposition was identified in 100 and 150 ng/mL treated cultures. A trend toward enhancing sulfated GAG incorporation was identified in 200 ng/mL treated pellets, but this was not statistically significant. (B) To assess specifically the effect of GDF-5 treatment on early-condensing pellet cultures, cultures were treated with TGF- β 3 for 21 days with 150 ng/mL GDF-5 on days 0–7. GAG content of five donors with two replicates each was quantified and normalized to total DNA content and expressed relative to control pellet levels. A statistically significant increase in GAG incorporation into the pellet extracellular matrix (ECM) was again identified in GDF-5-treated cultures. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001.
Results
GDF-5 enhances MSC chondrogenesis
To investigate the chondrostimulatory effect of GDF-5 on MSCs, GAG production was quantified and normalized to the total DNA content in the chondrogenic pellets. A statistically significant increase in GAG production per cell was observed after supplementation of chondrogenic pellets with 100 (P=0.0002) or 150 (P=0.0015) ng/mL GDF-5 from days 0–21 of culture as compared to the control cultures (Fig. 1A). No change in DNA content was observed as a result of GDF-5 supplementation (data not shown). Although the mean GAG content of 100 ng/mL treated cultures was significantly increased over control levels, this was only reproducible in two of the four donors assessed. However, 150 ng/mL treatment of MSCs from every donor indicated a statistically significant increase over control, making this the optimal treatment regime for the remainder of this investigation. While a trend to increased GAG production was observed in cultures treated with 200 ng/mL for 21 days, the increase was not statistically significant.
As GDF-5 is hypothesized to influence prechondrogenic condensation and thereby stimulate later GAG incorporation, chondrogenic pellets were treated with GDF-5 on days 0–7 (Fig. 1B) to determine whether the effect of GDF-5 is predominantly on MSC condensation. A moderate, yet significant (P=0.0146), increase in GAG production per cell was identified when cultures were treated with GDF-5 through condensation (days 0–7; Fig. 1B). However, the effect of GDF-5 treatment through days 0–21 had a greater statistical validity (Fig. 1A). Therefore, the three-week treatment protocol was utilized throughout the remaining investigations.
GDF-5 stimulates collagen type II and GAG incorporation in the ECM
The effect of GDF-5 treatment on expression of collagen type II and aggrecan was assessed by qRT-PCR, histological staining, and immunohistochemistry. Consistent enhancement of collagen type II transcripts was observed in GDF-5-treated cultures as compared to TGF-β 3-treated controls beginning 2 days after the initiation of chondrogenesis and continuing to day 21 (Fig. 2A). A statistically significant (P=0.0172) increase in collagen type II content was observed at day 21 as compared to control pellets at the same time point. A similar increase in the aggrecan mRNA transcripts were observed in GDF-5-treated cultures beginning 4 days after chondrogenic pellet and continuing through day 14. However, after 21 days, the level of aggrecan transcript was comparable in both treatment groups (Fig. 2B).
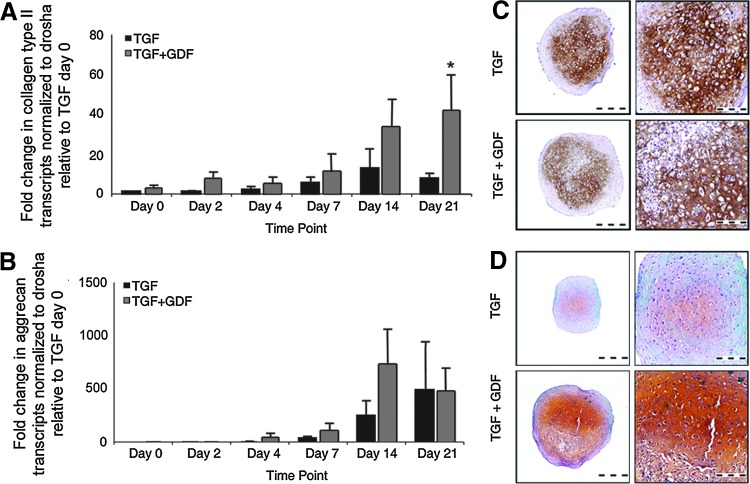
FIG. 2.
GDF-5 supports collagen type II and GAG incorporation into the ECM. mRNA was isolated from TGF-β 3- and GDF-5-treated cultures of three donors with 5–6 pellets harvested at days 0, 2, 4, 7, 14, and 21 of the chondrogenic pellet culture. (A) qRT-polymerase chain reaction (PCR) analysis for collagen type II transcripts revealed a trend to increased transcript levels in GDF-5-treated cultures as compared to TGF-β 3-treated controls, beginning at day 2 and continuing to day 21 of culture. (B) qRT-PCR analysis for aggrecan transcripts in days 0, 2, 4, 7, 14, and 21 pellet cultures treated with TGF-β 3 alone or TGF-β 3 in combination with GDF-5 revealed increased levels of aggrecan beginning at day 4 and continuing through day 21. Increased levels of aggrecan transcripts were identified in GDF-5-treated cultures. All qRT-PCR analyses were normalized to the drosha transcript levels and expressed relative to day 0, TGF-β 3-treated levels. Immunohistochemical analysis of five donors, including two replicates per donor for (C) collagen type II protein in the ECM, revealed a similar distribution in TGF-β 3- and GDF-5-treated cultures with a concentrated deposition in the center of the pellet. (D) Safranin O staining of day-21 paraffin-embedded pellets from six donors with two replicates per donors indicates the presence of sulfated GAG in the ECM. Enhanced quantities of GAG were associated with GDF-5 treatment as indicated by the more intense staining levels and a wider distribution of positive staining to the periphery of the pellet. qRT, quantitative reverse transcriptase. *P<0.05; **P<0.01; ***P<0.001. Color images available online at www.liebertpub.com/scd
Despite the increase in mRNA content, the presence of collagen type II in the chondrogenic ECM was not qualitatively confirmed by immunohistochemistry (Fig. 2C). Assessment of day-21 chondrogenic pellets in the presence or absence of GDF-5 demonstrated a similar pattern of collagen type II protein deposition in the center of the pellet; however, a greater intensity of staining was observed in control pellets as compared to GDF-5-treated cultures.
Histological evaluation of the control and GDF-5-treated pellets with Safranin O and Fast Green illustrates the regions of sulfated GAG deposition in the cartilaginous pellet, of which aggrecan would be a primary constituent. Supporting the DMMB results demonstrating greater quantities of GAG (Fig. 1), an enhanced Safranin O staining intensity in GDF-5-treated cultures indicated increased quantities and breadth of GAG incorporation into the ECM (Fig. 2D), suggesting a more chondrogenic pellet. Although a concurrent increase in aggrecan mRNA was not observed (Fig. 2B), the enhanced GAG deposition observed by Safranin O staining (Fig. 2D) is possible by increased ECM retention in GDF-5-treated cultures, the incorporation of greater quantities of chondroitin sulfate, and/or an increase in its sulfation levels, or a greater protein production from existing transcript levels.
GDF-5 stimulation increases SMAD phosphorylation
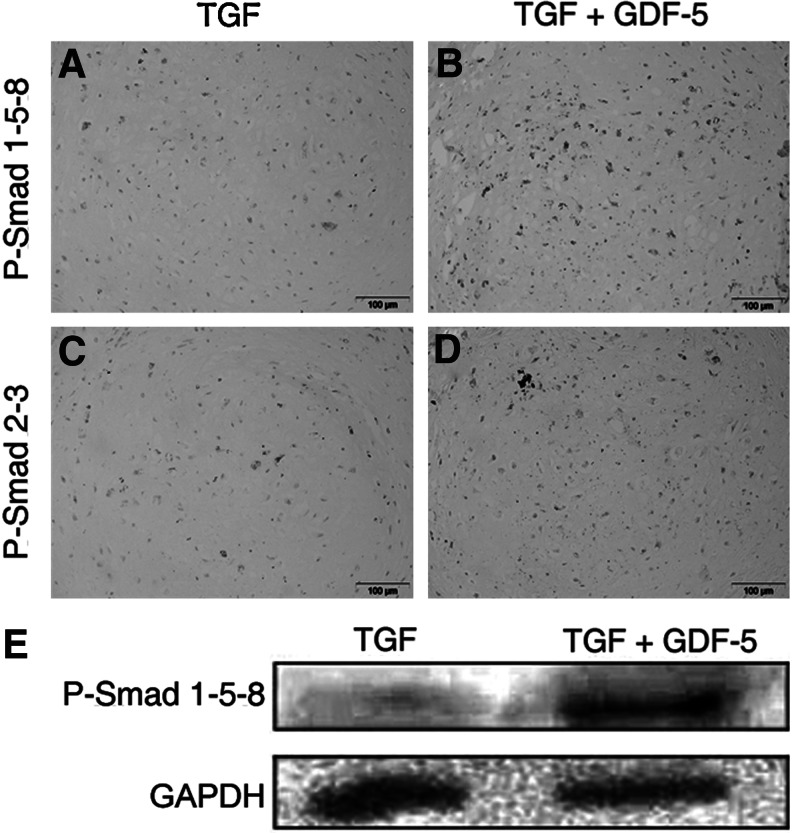
To investigate the mechanism of GDF-5-enhanced chondrogenesis, day-21 chondrogenic pellets were immunostained to identify phosphorylated, active SMAD proteins. An increase in phosphorylated SMADs 1-5-8 signaling in chondrogenic pellets have been demonstrated by others to be directly associated with expression of hypertrophic markers such as collagen type X and ALP, as well as mineralization [39]. Trends of increased staining for P-SMADs 1-5-8 and P-SMADs 2–3 were observed in pellets treated with GDF-5 (Fig. 3B, D) when compared to the pellets treated with TGF-β 3 alone (Fig. 3A, C). As signaling through P-SMADs 1-5-8 is of particular interest, western blotting analysis of the chondrogenic pellet extract after 21 days of in vitro culture demonstrated minimal levels of P-SMADs 1-5-8 in TGF-β 3-treated cultures. This basal level of SMAD signaling was greatly enhanced upon the addition of GDF-5 to the pellet culture medium (Fig. 3E). Comparable protein loading was confirmed by probing similarly with an anti-GAPDH antibody.
FIG. 3.
GDF-5 stimulation increases SMADs 2–3 and SMADs 1-5-8 phosphorylation. Representative images of 21-day chondrogenic pellets treated with TGF-β 3 alone (A and C) or in combination with GDF-5 (B and D). Immunohistochemistry was performed on pellets from three donors with 3 to 4 replicates per donor to observe the phosphorylation of both SMADs 1-5-8 (A–B) and SMADs 2–3 (C–D). The DAB staining indicates a positive stain for the relevant P-SMADs as indicated. The sections were counterstained with hematoxylin. The scale bars represent 100 μm. (E) These data were supported by increased levels of P-SMADs 1-5-8 identified in GDF-5-treated pellets as assayed by western blot analysis at day 21 of culture, while TGF-β 3-treated chondrogenic pellets expressed low levels of P-SMADs 1-5-8. The western blot displayed was generated with 6–8 chondrogenic pellets per treatment group from one bone marrow donor.
GDF-5 increases the presence of hypertrophic markers
As hypertrophic differentiation of MSCs would indicate the initiation of EO, the effect of GDF-5 treatment on the transition of chondrogenic MSCs into hypertrophy was evaluated by profiling the traditional markers of ALP activity, collagen type X and I deposition, and OPN secretion.
Evaluation of a chondrogenic pellet-conditioned medium for ALP activity at 14 and 21 days of culture demonstrated a statistically significant increase (P=6.08×10−5 and P=0.003, respectively) in ALP activity in GDF-5-treated cultures as compared to controls at the same time point. Although minimal, ALP activity was also observed in control cultures (Fig. 4A).
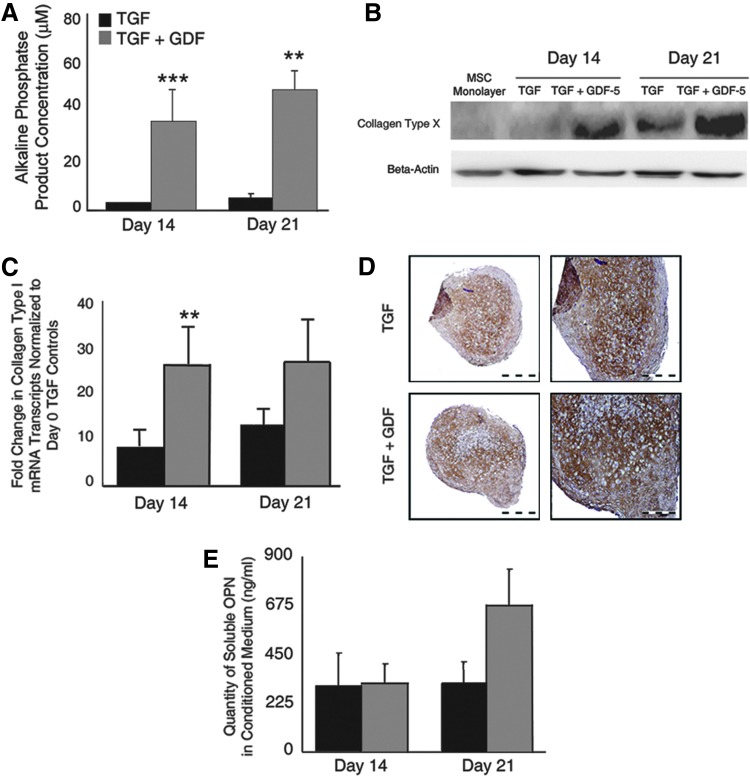
FIG. 4.
GDF-5 treatment enhances mesenchymal stromal cell (MSC) hypertrophy. (A) As an indication of hypertrophy, soluble alkaline phosphatase (ALP) activity was assessed with pNPP substrate and the concentration of the resultant product calculated from the conditioned medium of four donors, evaluated in duplicate. At both days 14 and 21, a statistically significant increase in ALP activity was identified in GDF-5-treated cultures as compared to the TGF-β 3-treated controls. (B) Similarly, increased levels of collagen type X protein were identified in GDF-5-treated pellets as assayed by western blot analysis at days 14 and 21 of culture. The data presented are representative of three comparable donors with five to six pellets homogenized at each time point. MSC monolayer cultures and TGF-β 3-treated cultures remained negative for collagen type X on day 14, while GDF-5 treatment stimulated collagen type X incorporation into the ECM. This trend continued at day 21 where TGF-β 3-treated cultures expressed a basal level of collagen type X protein, but GDF-5-treated pellets contained enhanced quantities. (C) qRT-PCR analysis of samples from three donors was assayed for collagen type I transcripts in duplicate. qRT-PCR analysis indicated the continual presence of mRNA through the 21 days in culture with a rapid increase in quantity at days 14 and 21 of the culture. Greater quantities of collagen type I were observed in the GDF-5-treated pellets. (D) Immunohistochemical staining of collagen type I protein after 21 days in culture indicated the presence of collagen type I in both control and GDF-5-treated pellets in similar quantities, but with a wider breadth of expression in GDF-5-treated pellets. The images presented are representative of four donors, each with two biologic replicates. (E) Enzyme-linked immunosorbent assay analysis of osteopontin content in a pellet-conditioned medium indicated a nonsignificant increase in GDF-5-treated pellets over control levels at day 21. Data are presented as ng/mL+SEM of seven donors at day 14 and eight donors at day 21 assayed in triplicate. *P<0.05; **P<0.01; ***P<0.001. Color images available online at www.liebertpub.com/scd
Western blotting for collagen type X protein indicated the absence of collagen type X in monolayer MSCs and the day-14 control pellet ECM (Fig. 4B). However, at 14 days of culture, GDF-5-treated pellets displayed the deposition of collagen type X in the ECM. After 21 days of pellet culture, collagen type X protein was present in both the control and GDF-5-treated pellets, with greater quantities in pellets treated with GDF-5. Comparable protein loading was confirmed by probing similarly with an anti-beta actin antibody (Fig. 4B).
Similar regulation of collage type I mRNA transcripts was observed by qRT-PCR (Fig. 4C). A consistent increase in collagen type I transcripts was observed in GDF-5-treated cultures, significantly more in day-14 pellets treated with GDF-5 as compared to day-14 controls (P=0.0012). A notable increase in the transcript was observed at days 14 and 21 in both the control and GDF-5-treated cultures, with over double the quantity of transcripts in GDF-5-treated cultures. Immunohistochemical localization of collagen type I protein identified a broader extent of protein distribution toward the periphery of the pellet as compared to control samples, potentially indicating a greater number of cells expressing collagen type I protein. A similar intensity of staining was observed (Fig. 4D).
A nonsignificant trend to increased OPN secretion was observed as a result of GDF-5 supplementation to chondrogenic pellets. An ELISA for OPN content in the pellet-conditioned medium demonstrated that the addition of GDF-5 resulted in secretion of over double the amount of OPN in TGF-β 3-treated cultures at 21 days (Fig. 4E).
Discussion
Here, human bone marrow-derived MSCs were supplemented with recombinant human GDF-5 protein in vitro to evaluate its potential to augment of MSC chondrogenic differentiation and transition into EO. Together, these data demonstrated enhanced MSC chondrogenesis in the presence of GDF-5, as well as a resultant increase in the markers of hypertrophic differentiation.
The application of the recombinant human GDF-5 protein to chondrogenic MSC pellets was investigated first as a means of enhancing chondrogenic differentiation. As observed through increased GAG deposition and a considerable increase in collagen type II and aggrecan transcripts, supplementation of pellet cultures with GDF-5 positively stimulated chondrogenic differentiation. This stimulation was observed both when applied for the full culture duration or specifically during prechondrogenic condensation, insinuating again that the mechanism of GDF-5 is to support condensation and therefore differentiation [29,35]. Perhaps the continued exposure of MSCs to GDF-5 is in fact inhibitory, while a temporal or transient exposure, as was demonstrated to be beneficial with other TGF-β superfamily members [40–42], would result in even greater chondrogenic differentiation. Most importantly, enhanced differentiation of MSCs in the presence of GDF-5 might be extrapolated clinically as a therapeutic for chondral repair or regeneration of healthy cartilage in conditions such as arthritis.
The phosphorylation of SMADs 1-5-8, intracellular signaling molecules stimulated as a result of binding to its receptor [43], is associated with chondrocyte terminal differentiation. An increase in phosphorylated SMADs 1-5-8 signaling in chondrogenic pellets have been demonstrated by others to be directly associated with expression of hypertrophic markers such as collagen type X and ALP, as well as mineralization [39]. To investigate the mechanism of GDF-5-enhanced chondrogenesis in this study, immunostaining and western blotting for phosphorylated active SMAD proteins were performed on day-21 chondrogenic pellets of MSCs. GDF-5 treatment resulted in an increase in phosphorylated SMADs 1-5-8 in the cytoplasm and nucleus, suggesting that these SMADs are involved in the chondrogenic differentiation of these cultures. Simultaneously, SMADs 1-5-8 are potentially stimulating their hypertrophic differentiation.
Because of their expression profiles and functions, collagen type I and X [44,45], OPN, and ALP [46] are hypothesized to be markers of cartilaginous transformation into ossified tissue [47]. Here an increase in hypertrophic markers, such as ALP activity, deposition of collagen types I and X, and OPN secretion, was observed in 21-day pellet cultures as a result of GDF-5 treatment. Notably, the control cultures indicate low levels of ALP activity, and expression of collagens type I and X and OPN. GDF-5 has previously been shown to enhance the hypertrophic differentiation of chick limb mesenchyme [37], and appears to modulate the duration of chondrocyte hypertrophy in the growth plate [48]. Here, GDF-5 was demonstrated to similarly induce chondrocyte hypertrophy in adult human MSC-derived cultures in vitro, an indicator that progenitor cells cultured in these conditions might serve as a model of early EO, specifically during the transition between cartilage and ossified bone.
Chondrogenically differentiated MSCs regularly display the markers of chondrocyte hypertrophy such as collagen type X and ALP activity in vitro [45,49]. This undesirable outcome results in terminal differentiation of chondrocytes, followed by ossification when transplanted in vivo subcutaneously [24,25] where they are exposed to high levels of vascularization, unlike articular cartilage. Recent studies argue that the observed terminal differentiation of in vitro differentiated MSCs is a direct result of the in vitro culture conditions, not of the natural differentiation process [50,51] inherent in these progenitor cells. Mueller and Tuan performed an in-depth study investigating the contribution of TGF- β and dexamethasone, both constituents of the in vitro culture medium employed in the current study, concluding that the resultant MSC-derived chondrogenic pellets recapitulate EO in vitro [21]. More recently, Cals et al. have demonstrated that individual TGF-β subtypes orchestrate in vitro terminal differentiation [52]. Exploitation of these observations has lead to the in vitro chondrogenic priming of MSCs, followed by subsequent implantation and natural ossification, as an improved method to engineer bone for regenerative applications [24,53].
In conclusion, combining MSCs with GDF-5 significantly enhances MSC chondrogenic differentiation as well as MSC entry to hypertrophy. This study is the first to demonstrate that the embryonic phenomenon of GDF-5-enhanced mesenchymal cell chondrogenesis may be recapitulated in vitro with adult progenitor cells at a clinically relevant dose of recombinant protein [34]. Together, GDF-5 and MSCs might therefore be used therapeutically in nonunion fracture repair to regenerate damaged bone where further pro-osteogenic or angiogenic factors may be present to support MSC entry into EO. Ongoing investigations are considering the in vitro and in vivo potential of MSCs in the presence of GDF-5 to ossify, thereby creating high quality de novo bone through EO.
Acknowledgments
The recombinant human GDF-5 employed through this investigation was kindly provided by Biopharm GmbH in Heidelberg, Germany. The authors thank Nicole Scully, MSc, and Keith Reidy for their technical contribution to this investigation, and Dr. Wiltrud Richter for the Alizarin red staining protocol. Science Foundation Ireland (09/SRC/B1794), European Framework 7 (HEALTH-F5-2010-241719-ADIPOA).
Author Disclosure Statement
C. Coleman, E. Vaughan, D. Browe, E. Mooney, L. Howard, and F. Barry declare that no competing financial interests exist.
References
- 1.Coleman CM. Curtin C. Barry FP. O'Flatharta C. Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;21:1239–1250. doi: 10.1089/hum.2010.138. [DOI] [PubMed] [Google Scholar]
- 2.Murphy JM. Fink DJ. Hunziker EB. Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 3.Marquass B. Schulz R. Hepp P. Zscharnack M. Aigner T. Schmidt S. Stein F. Richter R. Osterhoff G, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39:1401–1412. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- 4.Gawlitta D. Farrell E. Malda J. Creemers LB. Alblas J. Dhert WJ. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng Part B Rev. 2010;16:385–395. doi: 10.1089/ten.TEB.2009.0712. [DOI] [PubMed] [Google Scholar]
- 5.Augello A. Tasso R. Negrini SM. Cancedda R. Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 6.Mokbel A. El-Tookhy O. Shamaa AA. Sabry D. Rashed L. Mostafa A. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 2011;29:275–284. [PubMed] [Google Scholar]
- 7.Khosla S. Westendorf JJ. Modder UI. Concise review: Insights from normal bone remodeling and stem cell-based therapies for bone repair. Stem Cells. 2010;28:2124–2128. doi: 10.1002/stem.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Singergy AA. Haleem AM. Atta HM. United States National Institutes of Health. “The Use of Autologous Bone Marrow Mesenchymal Stem Cells in the Treatment of Articular Cartilage Defects.”. 2012. http://clinicaltrials.gov/ct2/show/NCT00891501?.term=Autologous+Bone+Marrow+Mesenchymal+Stem+Cells+in+the+Treatment+of+Articular+Cartilage+Defects&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT00891501?.term=Autologous+Bone+Marrow+Mesenchymal+Stem+Cells+in+the+Treatment+of+Articular+Cartilage+Defects&rank=1
- 9.Aroen A. United States National Institutes of Health. “ Mesenchymal Stem Cells in a Clinical Trial to Heal Articular Cartilage Defects.”. 2012. http://clinicaltrials.gov/ct2/show/NCT00885729?.term=Mesenchymal+Stem+Cells+in+a+Clinical+Trial+to+Heal+Articular+Cartilage+Defects.&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT00885729?.term=Mesenchymal+Stem+Cells+in+a+Clinical+Trial+to+Heal+Articular+Cartilage+Defects.&rank=1
- 10.Garcia ACM. de Miguel F. United States National Insittutes of Health. “Autologous Mesenchymal Stem Cells vs. Chondrocytes for the Repair of Chondral Knee Defects (ASCROD).”. 2012. http://clinicaltrials.gov/ct2/show/NCT01399749?.term=Autologous+Mesenchymal+Stem+Cells+vs.+Chondrocytes+for+the+Repair+of+Chondral+Knee+Defects&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01399749?.term=Autologous+Mesenchymal+Stem+Cells+vs.+Chondrocytes+for+the+Repair+of+Chondral+Knee+Defects&rank=1
- 11.Orozco L. Sanchez A. United States Naitonal Institutes of Health. “Treatment of Knee Osteoarthritis With Autologous Mesenchymal Stem Cells (KDD&MSV).”. 2012. http://clinicaltrials.gov/ct2/show/NCT01183728?.term=Treatment+of+Knee+Osteoarthritis+With+Autologous+Mesenchymal+Stem+Cells&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01183728?.term=Treatment+of+Knee+Osteoarthritis+With+Autologous+Mesenchymal+Stem+Cells&rank=1
- 12.Gourabi H. Nejad MRBE. Emadeddin M. Aghdami N. United States National Institutes of Health. “Articular Cartilage Resurfacing With Mesenchymal Stem Cells In Osteoarthritis Of Knee Joint.”. 2012. http://clinicaltrials.gov/ct2/show/NCT01183728?.term=Treatment+of+Knee+Osteoarthritis+With+Autologous+Mesenchymal+Stem+Cells&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01183728?.term=Treatment+of+Knee+Osteoarthritis+With+Autologous+Mesenchymal+Stem+Cells&rank=1
- 13.Stiehler M. United States National Institutes of Health. “Mesenchymal Stromal Cells and Osteoarthritis.”. 2012. http://clinicaltrials.gov/ct2/show/NCT01038596?.term=stiehler&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01038596?.term=stiehler&rank=1
- 14.Gourabi H. Nejad MRBE. Emadeddin M. Aghdami N. Vosough A. United States National Insitutes of Health. “Treatment of Non Union of Long Bone Fractures by Autologous Mesenchymal Stem Cell.”. 2012. http://clinicaltrials.gov/ct2/show/NCT01206179?.term=Treatment+of+Non+Union+of+Long+Bone+Fractures+by+Autologous+Mesenchymal+Stem+Cell&rank=1. [Sep 10;2012 ]. http://clinicaltrials.gov/ct2/show/NCT01206179?.term=Treatment+of+Non+Union+of+Long+Bone+Fractures+by+Autologous+Mesenchymal+Stem+Cell&rank=1
- 15.Mackay AM. Beck SC. Murphy JM. Barry FP. Chichester CO. Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 16.Yoo JU. Barthel TS. Nishimura K. Solchaga L. Caplan AI. Goldberg VM. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs BG. Aigner T. Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 18.Scotti C. Tonnarelli B. Papadimitropoulos A. Scherberich A. Schaeren S. Schauerte A. Lopez-Rios J. Zeller R. Barbero A. Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry F. Boynton RE. Liu B. Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 20.Ichinose S. Yamagata K. Sekiya I. Muneta T. Tagami M. Detailed examination of cartilage formation and endochondral ossification using human mesenchymal stem cells. Clin Exp Pharmacol Physiol. 2005;32:561–570. doi: 10.1111/j.1440-1681.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller MB. Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwale F. Stachura D. Roughley P. Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–1798. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- 23.Sekiya I. Vuoristo JT. Larson BL. Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell E. van der Jagt OP. Koevoet W. Kops N. van Manen CJ. Hellingman CA. Jahr H. O'Brien FJ. Verhaar JA. Weinans H. van Osch GJ. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15:285–295. doi: 10.1089/ten.tec.2008.0297. [DOI] [PubMed] [Google Scholar]
- 25.Jukes JM. Both SK. Leusink A. Sterk LM. van Blitterswijk CA. de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steck E. Fischer J. Lorenz H. Gotterbarm T. Jung M. Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 27.Cameron TL. Belluoccio D. Farlie PG. Brachvogel B. Bateman JF. Global comparative transcriptome analysis of cartilage formation in vivo. BMC Dev Biol. 2009;9:20. doi: 10.1186/1471-213X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxton P. Edwards C. Archer CW. Francis-West P. Growth/differentiation factor-5 (GDF-5) and skeletal development. J Bone Joint Surg Am 83-A Suppl. 2001;1:S23–S30. [PubMed] [Google Scholar]
- 29.Francis-West PH. Abdelfattah A. Chen P. Allen C. Parish J. Ladher R. Allen S. MacPherson S. Luyten FP. Archer CW. Mechanisms of GDF-5 action during skeletal development. Development. 1999;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- 30.Francis-West PH. Parish J. Lee K. Archer CW. BMP/GDF-signalling interactions during synovial joint development. Cell Tissue Res. 1999;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- 31.Storm EE. Huynh TV. Copeland NG. Jenkins NA. Kingsley DM. Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 32.Storm EE. Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- 33.Oldershaw RA. Baxter MA. Lowe ET. Bates N. Grady LM. Soncin F. Brison DR. Hardingham TE. Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 34.Bai X. Xiao Z. Pan Y. Hu J. Pohl J. Wen J. Li L. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325:453–460. doi: 10.1016/j.bbrc.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 35.Coleman CM. Tuan RS. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech Dev. 2003;120:823–836. doi: 10.1016/s0925-4773(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 36.Feng G. Wan Y. Balian G. Laurencin CT. Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman CM. Tuan RS. Growth/differentiation factor 5 enhances chondrocyte maturation. Dev Dyn. 2003;228:208–216. doi: 10.1002/dvdy.10369. [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellingman CA. Davidson EN. Koevoet W. Vitters EL. van den Berg WB. van Osch GJ. van der Kraan PM. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17:1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 40.Buxton AN. Bahney CS. Yoo JU. Johnstone B. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2011;17:371–380. doi: 10.1089/ten.tea.2009.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byers BA. Mauck RL. Chiang IE. Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng KW. O'Conor CJ. Kugler LE. Cook JL. Ateshian GA. Hung CT. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491–2500. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazono K. Kamiya Y. Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 44.von der Mark K. Kirsch T. Nerlich A. Kuss A. Weseloh G. Gluckert K. Stoss H. Type X collagen synthesis in human osteoarthritic cartilage Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 45.Girkontaite I. Frischholz S. Lammi P. Wagner K. Swoboda B. Aigner T. Von der Mark K. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996;15:231–238. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 46.Gerstenfeld LC. Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 1996;62:1–9. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 47.Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011;2011:683970. doi: 10.1155/2011/683970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikic B. Clark RT. Battaglia TC. Gaschen V. Hunziker EB. Altered hypertrophic chondrocyte kinetics in GDF-5 deficient murine tibial growth plates. J Orthop Res. 2004;22:552–556. doi: 10.1016/j.orthres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Gerstenfeld LC. Landis WJ. Gene expression and extracellular matrix ultrastructure of a mineralizing chondrocyte cell culture system. J Cell Biol. 1991;112:501–513. doi: 10.1083/jcb.112.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellingman CA. Koevoet W. van Osch GJ. Can one generate stable hyaline cartilage from adult mesenchymal stem cells?. A developmental approach. J Tissue Eng Regen Med. 2012;6:e1–e11. doi: 10.1002/term.502. [DOI] [PubMed] [Google Scholar]
- 51.Weiss S. Hennig T. Bock R. Steck E. Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 52.Cals FL. Hellingman CA. Koevoet W. Baatenburg de Jong RJ. van Osch GJ. Effects of transforming growth factor-beta subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2012;6:68–76. doi: 10.1002/term.399. [DOI] [PubMed] [Google Scholar]
- 53.Farrell E. Both SK. Odorfer KI. Koevoet W. Kops N. O'Brien FJ. de Jong RJ. Verhaar JA. Cuijpers V, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]