Abstract
STUDY QUESTION
Do uric acid levels across the menstrual cycle show associations with endogenous estradiol (E2) and reproductive hormone concentrations in regularly menstruating women?
SUMMARY ANSWER
Mean uric acid concentrations were highest during the follicular phase, and were inversely associated with E2 and progesterone, and positively associated with FSH.
WHAT IS KNOWN ALREADY
E2 may decrease serum levels of uric acid in post-menopausal women; however, the interplay between endogenous reproductive hormones and uric acid levels among regularly menstruating women has not been elucidated.
STUDY DESIGN, SIZE, DURATION
The BioCycle study was a prospective cohort study conducted at the University at Buffalo research centre from 2005 to 2007, which followed healthy women for one (n = 9) or 2 (n = 250) menstrual cycle(s).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were healthy women aged 18–44 years. Hormones and uric acid were measured in serum eight times each cycle for up to two cycles. Marginal structural models with inverse probability of exposure weights were used to evaluate the associations between endogenous hormones and uric acid concentrations.
MAIN RESULTS AND THE ROLE OF CHANCE
Uric acid levels were observed to vary across the menstrual cycle, with the lowest levels observed during the luteal phase. Every log-unit increase in E2 was associated with a decrease in uric acid of 1.1% (β = −0.011; 95% confidence interval (CI): −0.019, −0.004; persistent-effects model), and for every log-unit increase in progesterone, uric acid decreased by ∼0.8% (β = −0.008; 95% CI: −0.012, −0.004; persistent-effects model). FSH was positively associated with uric acid concentrations, such that each log-unit increase was associated with a 1.6% increase in uric acid (β = 0.016; 95% CI: 0.005, 0.026; persistent-effects model). Progesterone and FSH were also associated with uric acid levels in acute-effects models. Of 509 cycles, 42 were anovulatory (8.3%). Higher uric acid levels were associated with increased odds of anovulation (odds ratio 2.39, 95% CI: 1.25, 4.56).
LIMITATIONS, REASONS FOR CAUTION
The change in uric acid levels among this cohort of healthy women was modest, and analysis was limited to two menstrual cycles. The women in this study were healthy and regularly menstruating, and as such there were few women with high uric acid levels and anovulatory cycles.
WIDER IMPLICATIONS OF THE FINDINGS
These findings demonstrate the importance of taking menstrual cycle phase into account when measuring uric acid in premenopausal women, and confirm the hypothesized beneficial lowering effects of endogenous E2 on uric acid levels. These findings suggest that there could be an underlying association affecting both sporadic anovulation and high uric acid levels among young, regularly menstruating women. Further studies are needed to confirm these findings and elucidate the connection between uric acid and reproductive and later cardiovascular health.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract # HHSN275200403394C). No competing interests declared.
Keywords: anovulation, estradiol, menstrual cycle, premenopausal women, uric acid
Introduction
Uric acid is a heterocyclic compound that is created when the body breaks down purine nucleotides. About 70% of daily uric acid excretion occurs via the kidneys, and in 5–25% of humans impaired renal excretion leads to hyperuricemia. Hyperuricemia has been associated with acute and chronic diseases, including gout, cardiovascular disease and type 2 diabetes, though these associations are typically observed among men and post-menopausal women (Hayden and Tyagi, 2004; Goodson and Solomon, 2007; Darmawan, 2008; Dehghan et al., 2008; Feig et al., 2008). It has been hypothesized that estradiol (E2) may affect serum levels of uric acid through mechanisms involving renal clearance, secretion and reabsorption (Puig et al., 1986; Yahyaoui et al., 2008). However, the interplay between endogenous reproductive hormones and uric acid levels in premenopausal women has not been elucidated.
During the normal menstrual cycle, there are predictable and marked changes in the hormones produced by the pituitary (LH and FSH) and the ovary (E2 and progesterone) due to a complex system of positive and negative feedback, which in turn affect the process of ovulation (Hall, 2011). Endogenous sex hormones contribute to the overall physiological health of premenopausal women and fluctuations in these hormones beyond the normal range have been linked to variations in several known risk factors for cardiovascular disease, including cholesterol, insulin, F2-isoprostanes and high sensitivity C-reactive protein (Gaskins et al., 2010; Mumford et al., 2010; Schisterman et al., 2010; Yeung et al., 2010). Elevated uric acid is another known risk factor for cardiovascular disease among men and post-menopausal women, though its variability across the menstrual cycle in premenopausal women has not been established (Hayden and Tyagi 2004; Goodson and Solomon, 2007; Darmawan, 2008; Feig et al., 2008).
The objectives of this study were to characterize variability of uric acid levels across the menstrual cycle and evaluate the associations between endogenous serum hormone concentrations and ovulation with uric acid levels in a cohort of young, regularly menstruating women. Based on prior research in similar cohorts (Pucher et al., 1933; Adamopoulos et al., 1977; Wingrove et al., 1998), our primary hypothesis was that E2 would decrease both acute (measured on the same day) and persistent (measured on the previous visit) uric acid levels.
Materials and Methods
Study design
The BioCycle study was a prospective cohort of 259 regularly menstruating, healthy female volunteers, aged 18–44 years, recruited from the western region of NY, USA. Details of the study design and screening process are described elsewhere (Wactawski-Wende et al., 2009). In brief, exclusion criteria included the current use of oral contraceptives (or use during the previous three months) and diagnosis of certain chronic conditions, including history of menstrual and ovulation disorders or clinical signs or history of gynecological problems. Further, women with a history of polycystic ovary syndrome (PCOS) were also excluded. Women with a self-reported BMI of <18 or >35 kg/m2 at screening were also excluded. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study, and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Participants were followed for one or two menstrual cycles with blood samples collected for hormonal and uric acid assessment during the following phases: second day of menstruation; mid-follicular; late follicular; LH/FSH surge; predicted ovulation; early luteal; mid-luteal and late luteal phase. Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA, USA) were used to assist with the timing of specimen collection (Howards et al., 2009). Fertility monitors measured estrone-3-glucuronide and LH in urine, starting on calendar day 6 after menses and continuing for 10–20 days, depending on whether the woman reached peak levels on the monitor. Monitor indications of low, high and peak fertility were used to time mid-cycle visits, with other visits scheduled according to an algorithm that took each woman's reported cycle length into consideration (Mumford et al., 2011a). Women were highly compliant with the study protocol, with 94% of women completing at least seven of eight clinic visits per cycle.
Hormone, uric acid and ovulation assessment
Reproductive hormones and uric acid were measured in serum using fasting blood samples collected at each cycle visit (eight visits per cycle). All samples were frozen at −80°C and sent as complete participant cycle batches for hormone analysis to reduce batch variability (Kaleida Health Center for Laboratory Medicine, Buffalo, NY, USA). E2, FSH, LH and progesterone were measured using solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories, Inc. (Valencia, CA, USA) on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA). Uric acid was measured using a Beckman LX20 autoanalyzer and employing a uricase methodology coupled to the production of hydrogen peroxide. Across the study period the inter-assay coefficient of variation for these tests reported by the laboratory was <10% for E2, <5% for LH and FSH, <14% for progesterone and <3% for uric acid. Cycles were defined as anovulatory if peak progesterone concentrations were ≤ 5 ng/ml (Legro et al. 2007; Diamanti-Kandarakis et al., 2009) across the cycle and no serum LH peak was observed during the later cycle visits (42 of 509 cycles (8.3%)).
Covariate assessment
At study enrollment, BMI was obtained using standardized protocols by trained study staff, while age, race, smoking, physical activity, lifestyle, health and reproductive history were obtained using validated questionnaires (Craig et al., 2003; Wactawski-Wende et al., 2009). Cycle length was defined as the number of days from the first day of bleeding (menstruating by 1600 h) until the day before the next onset of bleeding after confirming 2 consecutive days of bleeding, to differentiate from spotting. Day of ovulation in ovulatory women was assigned based on dates and levels of the LH peak using the fertility monitor compared with the observed LH maximum value in serum and the first day of progesterone rise. Follicular phase length was defined as Day 1 of bleeding to the day of ovulation. Luteal phase length was defined as ovulation through the last day of the cycle. No covariate was missing for >5% of women.
Dietary intake was assessed using a 24-h dietary recall conducted four times/cycle corresponding to menstruation, mid-follicular phase, ovulation and mid-luteal phase (Nutrition Data System for Research software version 2005, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA). The majority of cycles had complete dietary recall information (87% of cycles with four recalls per cycle).
Statistical analysis
Descriptive statistics were calculated for demographic characteristics and dietary intake. Fisher's exact test and analysis of variance were used to test for associations between demographic variables and tertiles of mean uric acid levels (mean of uric acid levels across up to 16 visits per woman). Based on previous studies, we further defined a woman as hyperuricemic if her uric acid levels were >6 mg/dl (Nagahama et al., 2004; Freid and Smith, 2007; Darmawan, 2008; Feig et al., 2008). Cycle length, luteal phase length, percentage of women with short luteal phase lengths (<12 days) and mean hormone levels across the cycle were compared between tertiles of mean uric acid levels (mean of uric acid levels across up to eight visits per woman per cycle). Uric acid levels were also compared between women with luteal phase lengths <12 versus ≥12 days. In addition, changes in uric acid, E2 and progesterone concentrations were calculated separately for ovulatory and anovulatory cycles, and were subsequently analyzed and compared across all phases of the menstrual cycle, as well as collapsed by follicular and luteal phases. P-values were calculated for each pairwise between-visit comparison.
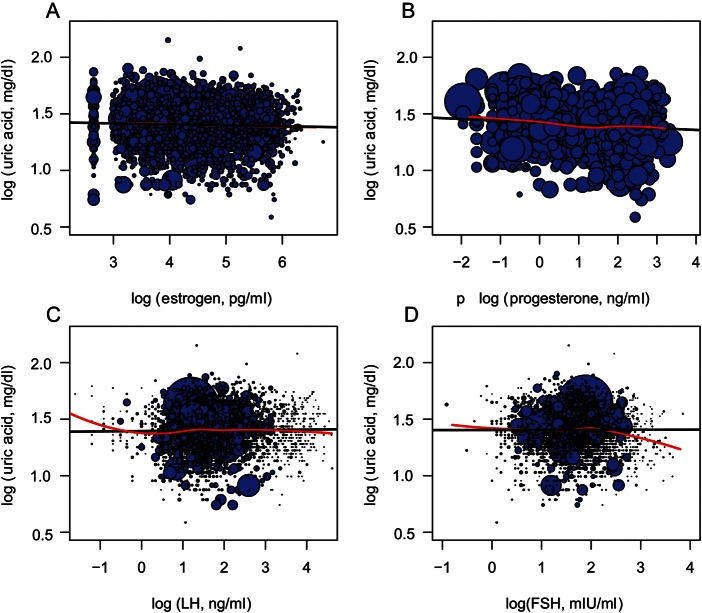
Marginal structural models with inverse probability of exposure weights were used to estimate associations between log hormone levels and log uric acid levels measured on the same day (acute effects) or between log hormone levels at one visit predicting log uric acid levels at the next visit (persistent effects) (Robins et al., 2000; Cole and Hernan, 2008). Persistent-effects models represent prolonged exposure to hormones (∼2 days; range 1–5 days) and were used to establish temporality of effects. Weighted linear mixed effect models with random intercepts were used to estimate the parameters of the marginal structural model, allowing both uric acid and hormone levels to vary over time (Robins et al., 2000; Cole and Hernan, 2008). Random-intercept models were used to account for the variation in baseline hormone concentrations between women and the correlation between visits of the same woman. Stabilized inverse probability of exposure weights for each cycle visit were obtained by ordinal logistic regression with the exposure categorized in deciles to decrease unnecessary variability in the weights, and were adjusted for age, BMI, race and other reproductive hormones. For example, models to calculate the weights for E2 included age, BMI, race, FSH, LH and progesterone, as well as past measurements of E2. Weights for the other hormones were calculated in a similar manner. Additional measures of dietary intake, physical activity, smoking, alcohol use and race were considered as potential covariates but did not appreciably alter the estimates. The mean (range) of the stabilized weights for the acute models were 1.00 (0.13, 10.92) for E2, 0.99 (0.13, 3.55) for progesterone, 1.00 (0.03, 27.64) for LH and 0.99 (0.01, 35.99) for FSH (values were similar for persistent models). Results were similar when truncating the weights at the 1st and 99th percentile. Scatter plots of log transformed hormone and uric acid concentrations, with data points sized proportionally to the inverse probability weights are shown to display the relative contributions of each individual in the study, along with the weighted regression line from the unadjusted acute effects models and a lowess smoothed regression line to demonstrate linearity of associations (results correspond to regression model results). Uric acid and hormone levels were allowed to vary over time, and all models included hormone and uric acid concentrations throughout the cycle, including up to eight measurements per cycle. As changes in hormones represent cyclic changes and are the result of complex feedback mechanisms, we utilized this marginal structural modeling approach to specifically address this type of time-varying confounding. This approach enables evaluation of the change in the uric acid level for each increase in E2, after adjustment for progesterone and FSH levels, for example.
Generalized estimating equations were used to model the association between mean uric acid levels across each cycle and the odds of anovulation while adjusting for age, race and BMI. Models were additionally run to evaluate the association between uric acid levels during each phase of the menstrual cycle and the probability of anovulation. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. A P < 0.05 was considered significant.
Results
Demographics
Overall, women in the BioCycle study were young (mean age: 27.3 years), of healthy weight (mean BMI: 24.1), physically active (moderate to high physical activity: 90%), non-smokers (96%) and of white (59%) and black (20%) race (Table I). Mean levels of uric acid varied significantly according to age and BMI, with lower mean levels of uric acid observed among older and thinner women within this cohort of young healthy women of reproductive age. In addition, women with higher uric acid levels tended to have experienced at least one anovulatory cycle. Physical activity, dietary animal protein and average alcohol intake were not significantly associated with uric acid. Only a small number of women were hyperuricemic at any given point in a cycle (uric acid ≥6 mg/dl; n = 20 women). Only 10 of these women had high levels on more than one visit. In particular, seven women had high levels on Day 2 (1.4%), five during the mid-follicular phase (1.0%), two during late follicular (0.4%), three during the LH/FSH peak visit (0.6%), four during predicted ovulation (0.8%), six during early luteal (1.2%), six during mid-luteal (1.2%) and three during the late luteal phase (0.6%) (women had high levels at more than one visit).
Table I.
Characteristics of women participating in the BioCycle study (259 women) according to the mean serum uric acid level, BioCycle study, Buffalo, NY 2005–2007.
| Mean serum uric acid (tertiles)a |
P-valueb | ||||
|---|---|---|---|---|---|
| Total cohort | Low (UA ≤3.83 mg/dl) | Medium (3.83 mg/dl <UA, ≤4.44 mg/dl) | High (UA >4.44 mg/dl) | ||
| n (number of women)c | 259 | 83 | 91 | 85 | |
| Demographics | |||||
| Mean (SD) | |||||
| Age, years | 27.3 (8.2) | 29.6 (8.6) | 27.2 (8.3) | 25.1 (7.2) | 0.002 |
| Age at menarche, years | 12.5 (1.2) | 12.4 (1.2) | 12.4 (1.1) | 12.5 (1.4) | 0.97 |
| BMI, kg/m2 | 24.1 (3.9) | 23.2 (3.4) | 23.7 (3.7) | 25.4 (4.2) | 0.0004 |
| n (%) | |||||
| Race | |||||
| White | 154 (59) | 46 (55) | 55 (60) | 53 (62) | 0.71 |
| Black | 51 (20) | 20 (24) | 18 (20) | 13 (15) | |
| Other | 54 (21) | 17 (21) | 18 (20) | 19 (22) | |
| ≤High school education | 33 (13) | 12 (14) | 9 (10) | 12 (14) | 0.60 |
| Married | 66 (25) | 26 (31) | 22 (24) | 18 (21) | 0.31 |
| Nulliparousd | 187 (74) | 55 (67) | 69 (78) | 63 (77) | 0.26 |
| Current smoker | 10 (4) | 1 (1) | 4 (4) | 5 (6) | 0.31 |
| Physical activity | |||||
| Low | 25 (10) | 10 (12) | 6 (7) | 9 (11) | 0.28 |
| Moderate | 92 (35) | 35 (42) | 31 (34) | 26 (31) | |
| High | 142 (55) | 38 (46) | 54 (59) | 50 (59) | |
| Ever sexually actived | 192 (75) | 69 (84) | 63 (70) | 60 (72) | 0.07 |
| Currently sexually activee | 135 (70) | 47 (68) | 47 (75) | 41 (68) | 0.66 |
| Past OC used | 140 (55) | 53 (65) | 48 (53) | 39 (47) | 0.07 |
| At least one anovulatory cyclef | 35 (14) | 7 (8) | 8 (9) | 20 (24) | 0.007 |
| Ovulatory cycle groupg | |||||
| Two ovulatory cycles | 219 (88) | 75 (93) | 81 (91) | 63 (79) | 0.03 |
| One ovulatory and one anovulatory cycle | 24 (10) | 6 (7) | 5 (6) | 13 (16) | |
| Two anovulatory cycles | 7 (3) | 0 (0) | 3 (3) | 4 (5) | |
| Dietary variablesh | |||||
| Mean (SD) | |||||
| % Total energy from carbohydrates | 50.8 (7.1) | 51.6 (6.8) | 50.8 (7.7) | 50.1 (6.8) | 0.38 |
| % Total energy from protein | 15. (2.9) | 15.5 (2.8) | 15.8 (3.1) | 15.9 (3.0) | 0.62 |
| % Total energy from fat | 33.9 (5.4) | 33.5 (5.0) | 33.7 (5.9) | 34.5 (5.3) | 0.45 |
| % Total energy from alcohol | 1.0 (2.0) | 1.0 (2.0) | 1.1 (2.2) | 1.0 (1.6) | 0.86 |
| Total energy, kcal | 1607.4 (354.3) | 1625.7 (396.9) | 1575.4 (305.1) | 1624.0 (361.1) | 0.56 |
| Total fat, g | 62.1 (18.5) | 61.9 (18.6) | 60.9 (18.1) | 63.7 (19.1) | 0.59 |
| Alcohol, g | 2.7 (5.2) | 2.7 (5.6) | 2.8 (5.7) | 2.5 (4.0) | 0.96 |
| Fructose, g | 16.9 (9.0) | 17.9 (10.1) | 15.7 (8.1) | 17.3 (8.8) | 0.24 |
| Total protein, g | 62.2 (15.6) | 61.7 (17.6) | 61.6 (14.2) | 63.4 (15.1) | 0.69 |
| Animal protein, g | 40.7 (14.4) | 39.4 (15.3) | 40.3 (13.4) | 42.3 (14.7) | 0.39 |
| Total cholesterol, mg | 209.8 (98.0) | 204.1 (95.3) | 205.5 (92.3) | 220.1 (106.6) | 0.50 |
OC, oral contraceptives; UA, uric acid.
aWomen were categorized according to tertile of mean uric acid levels across all 16 study visits.
bTwo-sided P-values for continuous variables calculated using analysis of variance (ANOVA), and for categorical variables using Fisher's exact test.
c250 women in the BioCycle study were followed for two cycles, nine women were followed for one cycle, for a total of 509 cycles.
dFor six women information was missing on parity, and for four women information was missing on past OC use and if ever sexually active.
ePercentages calculated from the 192 women reporting ever being sexually active.
fTwenty-eight women in the study had only one anovulatory cycle, and seven women in the study had two anovulatory cycles for a total of 35 women with at least one anovulatory cycle. There were a total of 42 anovulatory cycles out of a total 509 in the BioCycle study.
gOnly includes 250 women with two cycles under study.
hIndicates the mean of the within-woman average of all 24-h recall measurements across the study.
Cycle length and luteal phase length were not significantly associated with uric acid levels (Table II). However, cycles with high mean uric acid concentrations across the cycle were more likely to be anovulatory (Tertile 3 versus Tertile 1: 14.0 versus 4.7%; P= 0.007), and more likely to have shorter luteal phases though this was not statistically significant (Tertile 3 versus Tertile 1: 13.7 versus 6.4; P= 0.11). Mean E2 and progesterone levels were lower among women in the highest tertile of mean uric acid levels (Table II).
Table II.
Characteristics of menstrual cycles according to the mean serum uric acid levels.
| Mean serum uric acid (tertiles)a |
P-valueb | ||||
|---|---|---|---|---|---|
| Total cohort | Low (UA ≤3.83 mg/dl) | Medium (3.83 mg/dl <UA, ≤4.44 mg/dl) | High (UA >4.44 mg/dl) | ||
| n (number of cycles)c | 509 | 171 | 173 | 165 | |
| Anovulatoryd | 42 (8.3) | 8 (4.7) | 11 (6.4) | 23 (14.0) | 0.007 |
| Cycle length, days | 28.8 ± 4.1 | 28.4 ± 4.1 | 29.1 ± 3.7 | 28.9 ± 4.5 | 0.32 |
| Luteal phase length, days | 13.9 ± 2.4 | 13.8 ± 1.7 | 14.2 ± 2.3 | 13.9 ± 3.0 | 0.31 |
| Luteal phase <12 d, n (%) | 41 (9.3) | 10 (6.4) | 13 (8.3) | 18 (13.7) | 0.11 |
| Uric acid (mg/dl) levels for luteal phase <12 days | 4.35 ± 0.64 | 3.51 ± 0.31 | 4.18 ± 0.18 | 4.93 ± 0.30 | 0.97 |
| Uric acid (mg/dl) levels for luteal phase ≥12 days | 4.08 ± 0.65 | 3.44 ± 0.35 | 4.10 ± 0.18 | 4.88 ± 0.36 | |
| Mean estradiole (pg/ml) | 112.0 ± 43.4 | 114.0 ± 41.4 | 116.4 ± 44.5 | 105.4 ± 43.6 | 0.05 |
| Mean progesterone (ng/ml) | 3.4 ± 1.8 | 3.7 ± 1.7 | 3.6 ± 1.7 | 3.0 ± 1.8 | 0.0004 |
| Mean LH (ng/ml) | 9.5 ± 3.6 | 9.1 ± 3.5 | 9.7 ± 3.6 | 9.8 ± 3.8 | 0.21 |
| Mean FSH (mIU/ml) | 6.4 ± 2.4 | 6.7 ± 2.8 | 6.2 ± 1.8 | 6.3 ± 2.4 | 0.09 |
aWomen were categorized according to tertile of mean uric acid levels across all 16 study visits.
bTwo-sided P-values for continuous variables calculated using repeated measures ANOVA, and for categorical variables using Fisher's exact test. All comparisons take repeated measures and correlations between cycles into account.
c250 women in the BioCycle study were followed for two cycles, nine women were followed for one cycle, for a total of 509 cycles.
dTwenty-eight women in the study had only one anovulatory cycle, and seven women in the study had two anovulatory cycles for a total of 35 women with at least one anovulatory cycle. There were a total of 42 anovulatory cycles out of a total 509 in the BioCycle study.
eIndicates the mean of the within-woman average of all hormone measurements.
Uric acid variability across the menstrual cycle
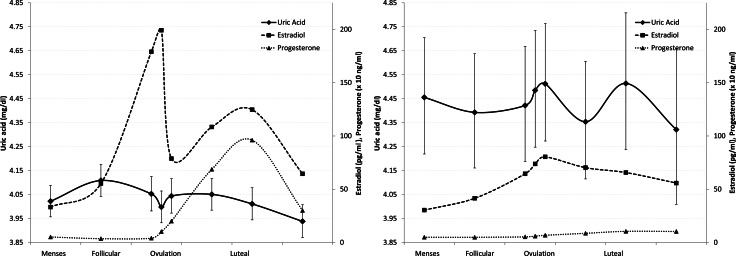
Uric acid levels peaked during the follicular phase, dropped around ovulation and further declined during the luteal phase (Fig. 1). Specifically, mean uric acid levels decreased by 1.9% (means: 4.21 versus 4.14 mg/dl; P= 0.09) from the mid-follicular phase to around ovulation, by 2.4% (means: 4.21 versus 4.11 mg/dl;P= 0.04) at the mid-luteal phase and by 3.9% (means: 4.21 versus 4.05 mg/dl; P= 0.001) at the late luteal phase. However, the mean change within a cycle was much greater [30% change; range, 6–139%; mean change (SD), 1.1 (0.5) mg/dl]. In addition, uric acid levels were higher among anovulatory than ovulatory cycles during both the follicular (P= 0.0008) and luteal phases (P= 0.0002), and tended to be higher during the follicular than the luteal phase of ovulatory cycles, although this difference was not statistically significant (P= 0.38) (Table III and Fig. 1). These changes correspond to higher E2 and progesterone levels during ovulatory cycles, especially during the luteal phase (Table III).
Figure 1.
Geometric mean uric acid levels with 95% CIs, and geometric mean estradiol and progesterone levels across phases of the human menstrual cycle by ovulatory status (left: ovulatory; right: anovulatory).
Table III.
Hormone and uric acid levels by cycle phase overall and by ovulatory status.
| Total cohort | Ovulatory cycles | Anovulatory cycles | P (ovulatory versus anovulatory) | |
|---|---|---|---|---|
| n (number of cycles) | 509 | 467 | 42 | |
| Uric acida (mg/dl) | ||||
| Follicular phase | 4.16 ± 0.69 | 4.13 ± 0.68 | 4.50 ± 0.62 | 0.0008 |
| Luteal/premenstrual phase | 4.12 ± 0.65 | 4.09 ± 0.64 | 4.47 ± 0.58 | 0.0002 |
| P (follicular versus luteal) | 0.37 | 0.38 | 0.86 | |
| Estradiol (pg/ml) | ||||
| Follicular phase | 102.5 ± 51.7 | 105.9 ± 51.8 | 64.4 ± 32.4 | <0.0001 |
| Luteal/premenstrual phase | 122.1 ± 54.7 | 125.2 ± 53.9 | 87.5 ± 51.3 | <0.0001 |
| P (follicular versus luteal) | <0.0001 | <0.0001 | 0.02 | |
| Progesterone (ng/ml) | ||||
| Follicular phase | 0.9 ± 1.0 | 1.0 ± 1.0 | 0.7 ± 0.4 | 0.04 |
| Luteal/premenstrual phase | 6.1 ± 3.2 | 6.6 ± 3.0 | 1.3 ± 0.8 | <0.0001 |
| P (follicular versus luteal) | <0.0001 | <0.0001 | <0.0001 | |
aThe mean uric acid, estradiol and progesterone levels were calculated for each woman overall and by phase for each cycle under study.
Sex hormones
Overall, E2 and progesterone were inversely associated with uric acid levels across the menstrual cycle, with the persistent effects being stronger (Table IV, Fig. 2). In acute effects models, after adjusting for age, race, BMI and other hormones, E2 was not significantly associated with uric acid levels (β = −0.005, 95% confidence interval (CI): −0.012, 0.002). In persistent-effects models, for every log-unit increase in E2, uric acid decreased by 1.1% (β = −0.011, 95% CI: −0.019, −0.004). For every log-unit increase in progesterone (an increase of ∼2 log units was observed between the follicular and luteal phases), uric acid decreased by ∼0.6% (β = −0.006, 95% CI: −0.010, −0.002) in acute-effects models and by 0.8% (β = −0.008, 95% CI: −0.012, −0.004) in persistent-effects models.
Table IV.
Associations between serum concentrations of menstrual hormones and uric acid levels.
| Log hormone | Modela | Acute: uric acid (mg/dl) |
Persistent: uric acid (mg/dl) |
||
|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | ||
| Estradiol (pg/ml) | Model 1 | −0.012 | −0.020, −0.005* | −0.017 | −0.025, −0.010** |
| Model 2 | −0.006 | −0.013, 0.0008 | −0.011 | −0.018, −0.004* | |
| Model 3 | −0.005 | −0.012, 0.002 | −0.011 | −0.019, −0.004* | |
| Progesterone (ng/ml) | Model 1 | −0.007 | −0.011, −0.003* | −0.008 | −0.013, −0.004* |
| Model 2 | −0.006 | −0.010, −0.002* | −0.008 | −0.012, −0.003* | |
| Model 3 | −0.006 | −0.010, −0.002* | −0.008 | −0.012, −0.004* | |
| FSH (mIU/ml) | Model 1 | −0.004 | −0.014, 0.006 | 0.0009 | −0.009, 0.011 |
| Model 2 | 0.016 | 0.006, 0.025* | 0.021 | 0.011, 0.031** | |
| Model 3 | 0.017 | 0.007, 0.028* | 0.016 | 0.005, 0.026* | |
| LH (ng/ml) | Model 1 | 0.006 | −0.0004, 0.013 | 0.007 | 0.0002, 0.014* |
| Model 2 | 0.004 | −0.002, 0.010 | 0.004 | −0.002, 0.011 | |
| Model 3 | 0.002 | −0.006, 0.010 | 0.005 | −0.002, 0.012 | |
| Odds ratio | 95% CI | ||||
| Anovulatory | Model 1 | 2.42 | 1.47, 4.00* | ||
| Model 2 | 2.39 | 1.25, 4.56* | |||
β, effect estimate; CI, confidence interval.
aModel 1: unadjusted; Model 2: adjusted for age, race, and body mass index; Model 3: adjusted for age, race, BMI, and other reproductive hormones through the use of inverse probability of exposure weights. Models for hormones obtained using weighted linear mixed models and for anovulation using generalized linear mixed models.
*P-value <0.05, **P-value <0.0001.
Figure 2.
Scatter plot of log transformed hormone [(A) estrogen, (B) progesterone, (C) LH and (D) FSH] and uric acid concentrations, with data points sized proportionally to the inverse probability weights. The black line represents the weighted regression line from the unadjusted acute effects models (beta coefficients and 95% CIs for these models are presented in Table IV) and the red line a lowess smoothed regression line.
FSH concentrations were associated with uric acid levels in both acute and persistent models, where each log-unit increase in FSH was associated with a 1.7% increase in uric acid in both models (Table IV, Fig. 2). No associations were observed between LH concentrations and uric acid.
Anovulation
Results from a generalized estimating equations model adjusting for age, race and BMI showed that for each mg/dl increase in mean uric acid levels over the cycle, the adjusted odds ratio of anovulation was 2.39 (95% CI: 1.25, 4.56; Table IV). Thus, each mg/dl increase in uric acid was associated with a 139% elevation in the odds of anovulation (less likely to ovulate). Uric acid levels during each phase of the menstrual cycle were associated with increased odds of anovulation (data not shown).
Discussion
Uric acid levels were highest during the follicular phase and declined during the luteal phase. Increased levels of endogenous E2 were associated with decreased uric acid concentrations in persistent-effects models only, and progesterone was associated with decreased uric acid concentrations in both acute- and persistent-effects models. In addition, increased uric acid levels were associated with increased odds of sporadic anovulation in this cohort of young, regularly menstruating women. This is the first study to use multiple measurements, timed to key periods of hormonal variability, over the course of the menstrual cycle to evaluate both the acute and persistent effects of reproductive hormones on uric acid. Previous studies among premenopausal women were not well timed to menstrual cycle phase (Adamopoulos et al., 1977; Anton et al., 1986; Wingrove et al., 1998). This novel finding of an association between uric acid and anovulation raises a potentially important biological pathway relating metabolic disturbances and normal hormonal function. Thus, this study provides important insight into the associations between sex hormones, ovulation and uric acid among healthy women of reproductive age.
Although the mean uric acid changes observed across the cycle were modest (only 2–4% on average), the within-woman variability was much greater (∼30% on average). Previous studies are limited, though Pucher et al. (1933) assessed the variability of uric acid across the menstrual cycle and observed similar results in two young women; serum uric acid levels were highest during menstruation and continuously fell thereafter. Of note, in the present study the variability across the cycle was observed among healthy women of reproductive age, further emphasizing the importance of considering menstrual cycle variability, as greater changes may be observed among other at-risk populations (overweight/obese, women with PCOS, etc.).
The inverse association between E2 and uric acid levels in the persistent-effects models is consistent with past research, which has evaluated the effects of both endogenous and exogenous E2. Nevertheless, the few studies that have evaluated premenopausal women are small and usually compare premenopausal women with men and post-menopausal women, rather than look at the changes in uric acid across phases of the cycle. They also primarily look at the acute effects of E2, and do not include any persistent effects that take temporality into account. In one of the few studies that analyzed the effects of endogenous E2 on uric acid, an inverse relationship between uric acid levels and endogenous E2 was evident in three premenopausal women (Adamopoulos et al., 1977). In a study among 50 premenopausal and 88 post-menopausal non-obese white women, Wingrove et al. (1998) observed that uric acid concentrations were significantly higher in post-menopausal versus premenopausal women, consistent with lower estrogen levels among post-menopausal women. Similarly, sex differences in the renal handling of uric acid have been observed, with women showing a significantly lower serum urate concentration when compared with men (studied among nine premenopausal women and nine healthy age-matched men), although E2 treatments did not have an effect on the renal handling of uric acid or serum urate levels among these women (Anton et al., 1986; Puig et al., 1986). In a study comparing post-menopausal women with and without gout, Marinello et al. (1985) observed a significant decrease in FSH, LH and E2 in patients with gout. Further, some studies have observed elevated uric acid levels among women with PCOS (Anttila et al., 1996; Macut et al, 2011), although these differences were not always statistically significant (Anttila et al., 1996). The findings of the present study extend previous work and evaluate a larger group of healthy, premenopausal women with visits timed to menstrual cycle phase.
Additional studies have focused on the effect of hormone therapy (HT) on uric acid levels in both post-menopausal women and men. In 9 post-menopausal women out of 13, the administration of conjugated and synthetic E2 produced a fall in the plasma uric acid concentration in the same study analyzing the effects of endogenous E2 on uric acid (Adamopoulos et al., 1977). Sumino et al. (1999) assessed 61 post-menopausal women before and during the administration of HT and showed that treatment significantly reduced mean serum uric acid concentrations throughout the 3–12-month study period in post-menopausal women with hyperuricemia. Similarly, using data from the Third National Health and Nutrition Examination Survey, Hak and Choi (2008) observed that menopause was associated with higher serum uric acid levels and current hormone use among post-menopausal women to be associated with a lower serum uric acid level. In addition to the effects of HT on post-menopausal women, E2 therapy has also been associated with decreases in uric acid levels among transsexual men (Nicholls et al., 1973; Yahyaoui et al., 2008). An antiandrogenic contraceptive pill also was shown to significantly reduce uric acid levels among women with PCOS (Luque-Ramirez et al., 2008).
Ours is the only study to date to our knowledge that analyzed the effects of progesterone, FSH and LH on uric acid levels, in addition to E2. An inverse association between progesterone and uric acid levels was observed in both acute and persistent models, which has not been presented in the past literature, especially among women of reproductive age. In this study, E2 levels were taken into account in the marginal structural models when evaluating the associations between progesterone and uric acid levels. The effects of progesterone during the luteal phase did not appear to be reduced because of high levels of endogenous E2 during the luteal phase. Furthermore, uric acid levels were lowest during the luteal phase, which corresponds to a natural state of opposed estrogen and high progesterone concentrations. Marinello et al. (1985) observed low levels of FSH in women with gout, but did not directly model the relationship between FSH and uric acid levels. Thus, further research in this area is warranted to confirm our findings of a positive association between FSH and uric acid levels after adjustment for age, race and BMI.
Biologically, the uricosuric effects of E2 are thought to take place within the nephron. Endogenous E2 appears to decrease uric acid levels by lowering the post-secretory tubular reabsorption of uric acid (Puig et al., 1986; Yahyaoui et al., 2008). Based on previous studies, renal tubular secretion of urate may greatly exceed uric acid excretion and a large fraction of this secreted urate is reabsorbed by the nephron (Diamond and Paolino, 1973). Thus, E2 may be affecting serum levels of uric acid through mechanisms involving the fractional excretion of uric acid (Yahyaoui et al., 2008). However, these mechanisms are not fully understood as one study found that E2 treatments did not have an effect on the renal handling of uric acid or serum urate levels (Anton et al., 1986; Puig et al., 1986).
Although a number of previous studies have evaluated the association between E2 and uric acid, similar relationships to sporadic anovulation have not been widely studied in the past literature. Studies show that women with either high uric acid concentrations or ovulatory disorders, such as PCOS, may be at increased risk for cardiovascular disease (Legro et al., 2003; Randeva et al., 2012) but the pathways connecting uric acid and anovulation remain unclear. The observed association between uric acid and anovulation could be attributed to issues in follicular development. Based on this association, lower levels of E2, which are associated with higher uric acid, may produce an increase in FSH levels needed to initiate feedback mechanisms in order to compensate for impaired follicular development. Further, as uric acid is a purine derivative and other studies have shown that purines may inhibit oocyte maturation, this could potentially explain the association between uric acid and sporadic anovulation, alongside the increased levels of FSH (Lavy et al., 1990; Alexiou and Leese, 1992; Downs, 1993; Wen et al., 2010). High levels of uric acid among anovulatory women may also be indicative of an underlying endocrine or metabolic disturbance, even among women reporting regular menstrual cycles (Mumford et al., 2011b). Although these mechanisms seem plausible, further studies should elucidate these hypotheses more directly in women with more persistent anovulation.
Intensive monitoring of a large number of young women, of black and white ethnicities, throughout two menstrual cycles, with multiple clinic visits timed with fertility monitors, are unique strengths of this study. Multiple measurements of both hormones and uric acid enabled more precise modeling of the association between E2 and uric acid levels and evaluation of both acute and persistent effects. The prospective design and exclusion criteria at baseline of the BioCycle study strengthen the ability to draw inference, having reduced the potential for bias from known risk factors for anovulation, although the generalizability of the findings is strictly limited to regularly cycling women. Adjustment was made for particular dietary factors that affect uric acid levels, such as alcohol and animal protein intake, but these factors had no effect on the results. Collectively, these unique aspects of the study design allowed for improvement and expansion on previous studies of uric acid and reproductive hormones and ovulation.
Nevertheless, the study faced limitations, which included the small number of women with hyperuricemia (≥6 mg/dl; n = 20 women) and the small number of anovulatory cycles (n = 42), which resulted in less precise estimates than would have been ideal. In the absence of a daily transvaginal ultrasound or daily first morning urine measurements, the detection of ovulation has some degree of misclassification. In addition, women were only followed for one or two menstrual cycles. Younger women were more likely to have higher uric acid levels, which is unexpected because of the known increase in uric acid levels with age (Kannel, 1987). This may be attributable to the high percentage of anovulatory cycles and other metabolic disturbances among the young women in this study (Mumford et al., 2011b). Furthermore, overall levels of uric acid were low, which is consistent with the fact that the women in the BioCycle study may not have reached the age where uric acid levels are expected to increase. Lastly, the observed associations between E2 (persistent-effects only) and progesterone (both acute and persistent-effects) and uric acid were small in magnitude. However, given the inter-assay variability for E2 and progesterone the observed associations may be underestimates assuming non-differential, monotonic measurement error.
In conclusion, we observed that uric acid levels varied across the menstrual cycle and were inversely associated with E2 in persistent-effects models and progesterone levels in acute and persistent-effects models among healthy premenopausal women. In addition, regularly menstruating women with higher serum urate levels had a substantially higher probability of anovulatory cycles. These data show, in contrast to the expectation that E2 played the major role, that progesterone as well as E2 levels appear important in suppressing uric acid levels; thus, in terms of uric acid relationships, ovulatory cycles may play an additional role to E2 in protecting women's future cardiovascular health. As both hyperuricemia and anovulation are major risk factors for disease, these findings suggest that there could be an underlying association affecting both sporadic anovulation and high uric acid levels among young, regularly menstruating women (Hayden and Tyagi, 2004; Goodson and Solomon, 2007; Darmawan, 2008; Dehghan et al., 2008; Feig et al., 2008). This study demonstrates that it is important to take menstrual cycle phase into account when measuring uric acid. Further studies are needed to confirm these findings and elucidate the connection between uric acid and reproductive and later cardiovascular health.
Authors' roles
S.L.M. and J.W.-W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
E.F.S. and J.W.-W. were involved in the study concept and design, acquisition of data and study supervision.
S.L.M., S.S.D., A.Z.P., N.J.P., D.R.M., S.R.C., J.W.-W. and E.F.S. were involved in the analysis and interpretation of data.
S.L.M., S.S.D. and E.F.S. were involved in the drafting of the manuscript.
S.L.M., S.S.D., A.Z.P., N.J.P., D.R.M., S.R.C., J.W.-W. and E.F.S. were involved in the critical revision of the manuscript for important intellectual content. S.L.M., S.S.D., S.R.C. and E.F.S. were involved in statistical analysis.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract # HHSN275200403394C).
Conflict of interest
None declared.
Acknowledgements
We are indebted to all the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University at Buffalo, as well as the BioCycle participants for their commitment to the study.
References
- Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh) 1977;85:198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- Alexiou M, Leese HJ. Purine utilisation, de novo synthesis and degradation in mouse preimplantation embryos. Development. 1992;114:185–192. doi: 10.1242/dev.114.1.185. [DOI] [PubMed] [Google Scholar]
- Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35:343–348. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- Anttila L, Rouru J, Penttila T, Irjala K. Normal serum uric acid concentrations in women with polycystic ovary syndrome. Hum Reprod. 1996;11:2405–2407. doi: 10.1093/oxfordjournals.humrep.a019124. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Darmawan J. Disorders of connective tissue, bone, and joints. In: van Boxtel CJ, Santoso B, Edwards IR, editors. Drug Benefits and Risks: International Textbook of Clinical Pharmacology. Amsterdam: IOS Press; 2008. pp. 659–676. [Google Scholar]
- Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piouka A, Livadas S, Piperi C, Katsikis I, Papavassiliou AG, Panidis D. Anti-mullerian hormone is associated with advanced glycosylated end products in lean women with polycystic ovary syndrome. Eur J Endocrinol. 2009;160:847–853. doi: 10.1530/EJE-08-0510. [DOI] [PubMed] [Google Scholar]
- Diamond HS, Paolino JS. Evidence for a postsecretory reabsorptive site for uric acid in man. J Clin Invest. 1973;52:1491–1499. doi: 10.1172/JCI107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM. Purine control of mouse oocyte maturation: evidence that nonmetabolized hypoxanthine maintains meiotic arrest. Mol Reprod Dev. 1993;35:82–94. doi: 10.1002/mrd.1080350114. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freid RM, Smith AD. Chemolysis of urinary calculi. In: Smith AD, Badlani GH, Bagley DH, editors. Smith‘s Textbook of Endourology. Hamilton, ON: BC Decker Inc; 2007,. pp. 149–158. [Google Scholar]
- Gaskins AJ, Mumford SL, Rovner AJ, Zhang C, Chen L, Wactawski-Wende J, Perkins NJ, Schisterman EF BioCycle Study Group. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J Nutr. 2010;140:1669–1676. doi: 10.3945/jn.110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson NJ, Solomon DH. Cardiovascular manifestations of rheumatic diseases. In: Califf RM, Prystowsky EN, Thomas JD, Thompson PD, editors. Textbook of Cardiovascular Medicine. Philadelphia: Lippincott Williams & Wilkins,; 2007,. pp. 638–655. [Google Scholar]
- Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10:R116. doi: 10.1186/ar2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE. The female reproductive system, infertility, and contraception. In: Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison‘s Principles of Internal Medicine. 18th edn. USA: The McGraw-Hill Companies, Inc; 2011. pp. 3028–3039. [Google Scholar]
- Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114:413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- Lavy G, Behrman HR, Polan ML. Purine levels and metabolism in human follicular fluid. Hum Reprod. 1990;5:529–532. doi: 10.1093/oxfordjournals.humrep.a137136. [DOI] [PubMed] [Google Scholar]
- Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;4:302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- Luque-Ramirez M, Alvarez-Blasco F, Uriol Rivera MG, Escobar-Morreale HF. Serum uric acid concentration as non-classic cardiovascular risk factor in women with polycystic ovary syndrome: effect of treatment with ethinyl-estradiol plus cyproterone acetate versus metformin. Hum Reprod. 2008;23:1594–1601. doi: 10.1093/humrep/den095. [DOI] [PubMed] [Google Scholar]
- Macut D, Simic T, Lissounov A, Pljesa-Ercegovac M, Bozic I, Djukic T, Bjekic-Macut J, Matic M, Petakov M, Suvakov S, et al. Insulin resistance in non-obese women with polycystic ovary syndrome: relation to byproducts of oxidative stress. Exp Clin Endocrinol Diabetes. 2011;119:451–455. doi: 10.1055/s-0031-1279740. [DOI] [PubMed] [Google Scholar]
- Marinello E, Riario-Sforza G, Marcolongo R. Plasma follicle-stimulating hormone, luteinizing hormone, and sex hormones in patients with gout. Arthritis Rheum. 1985;28:127–131. doi: 10.1002/art.1780280203. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Siega-Riz AM, Browne RW, Gaskins AJ, Trevisan M, Steiner AZ, Daniels JL, Zhang C, Perkins NJ, et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the biocycle study. J Clin Endocrin Metabo. 2010;95:E80–E85. doi: 10.1210/jc.2010-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Gaskins AJ, Pollack AZ, Perkins NJ, Whitcomb BW, Ye A, Wactawski-Wende J. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol. 2011a;25:448–459. doi: 10.1111/j.1365-3016.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Schisterman EF, Siega-Riz AM, Gaskins AJ, Steiner AZ, Daniels JL, Olshan AF, Hediger ML, Hovey K, Wactawski-Wende J, et al. Cholesterol, endocrine and metabolic disturbances in sporadic anovulatory women with regular menstruation. Hum Reprod. 2011b;26:423–430. doi: 10.1093/humrep/deq322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, Takishita S. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–841. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–451. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucher GW, Griffith FR, Jr, Brownell KA, Klein JD, Carmer ME. Studies in human physiology. VI. Variations in blood chemistry over long periods of time, including those characteristic of menstruation. J Nutr. 1933;7:169–193. [Google Scholar]
- Puig JG, Mateos FA, Ramos TH, Capitan CG, Michan AA, Mantilla JM. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) Adv Exp Med Biol. 1986;195(Pt A):317–323. doi: 10.1007/978-1-4684-5104-7_54. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, Lehnert H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–841. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Gaskins AJ, Mumford SL, Browne RW, Yeung E, Trevisan M, Hediger M, Zhang C, Perkins NJ, Hovey K, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women. Am J Epidemiol. 2010;172:430–439. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354:650. doi: 10.1016/S0140-6736(99)92381-4. [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M BioCycle Study Group. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Perrett D, Jones N, Tozer AJ, Docherty SM, Iles RK. High follicular fluid adenosine levels may be pivotal in the metabolism and recycling of adenosine nucleotides in the human follicle. Metabolism. 2010;59:1145–1155. doi: 10.1016/j.metabol.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Wingrove CS, Walton C, Stevenson JC. The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism. 1998;47:435–438. doi: 10.1016/s0026-0495(98)90056-7. [DOI] [PubMed] [Google Scholar]
- Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, Martínez J, Gómez-Zumaquero JM, González I, Hernando V, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93:2230–2233. doi: 10.1210/jc.2007-2467. [DOI] [PubMed] [Google Scholar]
- Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: The BioCycle study. J Clin Endocrin Metabo. 2010;95:5435–5442. doi: 10.1210/jc.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]