Abstract
STUDY QUESTION
Is there a relationship between body mass index (BMI), body shape and endometriosis?
SUMMARY ANSWER
Endometriosis is inversely associated with early adult BMI and may correlate with a peripheral body fat distribution.
WHAT IS KNOWN ALREADY
The literature suggests an inverse relation between endometriosis and BMI, although few studies have specifically explored this association in depth.
STUDY DESIGN, SIZE, DURATION
Prospective cohort study using data collected from 116 430 female nurses from September 1989 to June 2011 as part of the Nurses' Health Study II cohort.
PARTICIPANTS/MATERIALS, METHODS AND SETTING
Cases were restricted to laparoscopically confirmed endometriosis. Weight at age 18 and height were reported at baseline, and current weight was updated every 2 years. Waist and hip measurements were first taken in 1993 and updated in 2005. Rate ratios (RR) and 95% confidence intervals (CI) were calculated using Cox proportional hazards models with time-varying covariates.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 5504 incident cases of endometriosis were reported during 1 299 349 woman-years (incidence rate = 385 per 100 000 woman-years). BMI at age 18 and current BMI were each significantly inversely associated with endometriosis (P-value, test for linear trend <0.0001). Both associations were stronger among infertile women. Obese infertile women with current BMIs of 35–39.9 kg/m2 and ≥40 kg/m2 had a 55% (95% CI 0.30–0.67) and a 62% (95% CI 0.23–0.62) lower risk of endometriosis, respectively, compared with the low-normal BMI referent (18.5–22.4 kg/m2). Rates of endometriosis were nearly 3-fold higher in women with waist-to-hip ratios <0.60 (RR = 2.78, 95% CI 1.38–5.60) compared with those with waist-to-hip ratios between 0.70 and 0.79, although the sample size for this category was very small.
LIMITATIONS AND REASONS FOR CAUTION
Although women with undiagnosed endometriosis certainly remain in the comparison population even in this prospective cohort study, the community prevalence of endometriosis in an asymptomatic population is very low. Moreover, the characteristics of this small proportion of undiagnosed cases are diluted among the >90 000 women accurately defined as being endometriosis-free and are, therefore, unlikely to impact on effect estimation. Although geographically diverse, the NHS II cohort is overwhelmingly Caucasian, which may limit generalizability to more ethnically diverse populations.
WIDER IMPLICATIONS OF THE STUDY
The results of this study suggest that endometriosis is inversely associated with early adult BMI and may correlate with a peripheral body fat distribution.
STUDY FUNDING/COMPETING INTEREST
This study was supported by research grants HD48544 and HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Nurses' Health Study II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services. None of the authors has a conflict of interest to disclose.
Keywords: body mass index, endometriosis, body fat
Introduction
Endometriosis is estimated to affect 1 in 10 reproductive aged women (Giudice, 2010). Accounting for health-care expenditures and loss of productivity, annual costs of endometriosis in the United States are thought to exceed $49 billion (Simoens et al., 2012). Despite its significant impact on the health-care system, many of the genetic, lifestyle and anthropometric factors that predispose a woman to endometriosis remain poorly elucidated. Reproductive factors such as early menarche (Darrow et al., 1993; Han et al., 1994; Signorello et al., 1997; Missmer et al., 2004a,b; Matalliotakis et al., 2008) and short menstrual cycle length (Cramer et al., 1986; Matorras et al., 1995; Sangi-Haghpeykar and Poindexter, 1995; Arumugam and Lim, 1997; Moen and Schei, 1997; Matalliotakis et al., 2008) have been associated more consistently with increased risk of the disease.
Among the most consistent relations in the literature is the inverse association between endometriosis and a woman's current body mass index (BMI) (Cramer et al., 1986; Darrow et al., 1993; Signorello et al., 1997; Missmer et al., 2004a,b; Parazzini et al., 2004; Ferrero et al., 2005; Hediger et al., 2005). This was first identified in small case–control studies (Darrow et al., 1993; McCann et al., 1993; Signorello et al., 1997) as well as by early data from the Nurses' Health Study II (NHS II), an ongoing prospective cohort study of American nurses that began in 1989. Analysis of the first 10 years of data as part of a broader study of demographic, anthropometric and lifestyle risk factors revealed an inverse relation between endometriosis and BMI at age 18 among all women as well as current BMI in the subset of infertile women (Missmer et al., 2004a,b). Since then, there have been additional case–control studies supporting this conclusion (Ferrero et al., 2005; Matalliotakis et al., 2008; Nagle et al., 2009), although few have been designed to specifically address the association between BMI and endometriosis, and none has been able to account for changes in an individual's weight and associated covariates over time. We now have over 20 years of data on >100 000 women from the NHS II, making it the largest prospective study to date and affording the power to conduct subgroup analyses that may better elucidate the nature of this association. We have, therefore, conducted an updated analysis within NHS II, designed specifically to explore the effect of BMI and other anthropometric characteristics on the rate of laparoscopically confirmed endometriosis.
Methods
Data were collected in the NHS II cohort from September 1989 to June 2011. A total of 116 430 female registered nurses aged 25–42 and residing in one of 14 states in the United States completed the baseline questionnaire in 1989. Questionnaires are updated and mailed biennially with over 90% follow-up of the cohort in each 2-year interval. The study was approved by the Institutional Review Boards of the Harvard School of Public Health and Brigham and Women's Hospital, Boston, Massachusetts.
Assessment of outcome
Women were first asked whether they ‘ever had physician-diagnosed endometriosis’ in 1993, and if so, when the diagnosis occurred and whether it was laparoscopically confirmed. The same question was asked in each subsequent cycle. Incident cases of endometriosis were restricted to women who reported laparoscopically confirmed disease.
To assess the validity of self-reported endometriosis, supplementary questionnaires were mailed to 200 women randomly selected from 1766 cases who had reported an incident diagnosis of endometriosis up to that time. These validation methods have been described in detail in prior publications (Missmer et al., 2004a,b). In brief, 100% of nurses who reported that they underwent a laparoscopy were indeed confirmed to have undergone this procedure. Among those reporting that endometriosis was visualized at the time of surgery, 96% were validated within the operative report with the other 4% all having evidence of receiving post-operative treatment for presumed endometriosis.
This case definition results in a complex interplay between endometriosis and infertility status. The baseline prevalence of infertility (attempting pregnancy ≥1 year without success) was greater among women with laparoscopically confirmed endometriosis (20%) compared with those diagnosed without laparoscopy (4%). Many of these women may have been diagnosed only with endometriosis during an infertility workup. By contrast, women without infertility who were diagnosed with endometriosis presumably presented with pain that prompted surgical evaluation. Because endometriosis in infertile women is more often indicative of asymptomatic disease, the risk factors for endometriosis with infertility may differ from those for endometriosis without infertility. Analyses were therefore stratified by infertility history.
Assessment of anthropometric characteristics
Weight at age 18 and current weight and height were reported on the baseline questionnaire, and current weight was updated every 2 years. BMI was calculated as weight in kilograms over height in meters squared (kg/m2). The validity of self-reported height and weight at age 18 was evaluated using medical records at the time of a nurses' entry into college or nursing school. The validity of self-reported weight was reassessed at multiple time points in the NHSII cohort; correlations between reported and measured height and weight were 0.94 and 0.87, respectively (Troy et al., 1995).
The 1993 questionnaire instructed participants how to measure their waist and hip circumference. The validity of self-measurement has been previously confirmed using standardized measurement taken by study researchers. Pearson's correlations were 0.89 for waist and 0.84 for hip measurement (Rimm et al., 1990).
BMI was categorized based on the World Health Organization classification into underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). Epidemiologic studies examining the relation between obesity and coronary heart disease (Willett et al., 1995) and ovulatory infertility (Rich-Edwards et al., 2002) observe elevated risk starting at the upper range of normal BMI. The normal BMI range was, therefore, subdivided into lower (18.5–22.4 kg/m2) and upper (22.5–24.9 kg/m2) categories to assess for a similar effect with endometriosis. The low-normal BMI range (18.5–22.4 kg/m2) was used as the referent value. Change in BMI since age 18 (current BMI − BMI at age 18) was categorized into four groups: a BMI change of <−1 kg/m2, −1 to 1 kg/m2, >1 to 6 kg/m2 and >6 kg/m2. Waist circumference was broken into quartiles (22–29.9 cm, 30–32.9 cm, 33–37.9 cm and 38–65 cm) and height into quintiles (40–62 in, 63–64 in, 65–66 in, 67–68 in, 69–85 in). Waist-to-hip ratio was categorized into five groups (<0.60, 0.60–0.69, 0.70–0.79, 00.80–0.89, ≥0.90) based on the distribution of the data.
Assessment of covariates
Each questionnaire cycle collected data on factors thought to be potential confounders or modifiers of the relation between body size and endometriosis, including age at first birth (<20 years, 20–29 years, 30–39 years, ≥40 years), time since last birth (≤1 year, 1–5 years, 5–10 years, ≥10 years), parity (nulliparous, 1, 2, 3, ≥4 pregnancies), smoking status (never, past, current), alcohol use (none, 0–5 gram per day, 5.01–10 grams per day, >10 g per day), infertility history (yes/no) and oral contraceptive use (never, past, current). The 1993 questionnaire included specific questions regarding a participant's menstrual history, including current menstrual cycle length (<21 days, 21–25 days, 26–31 days, 32–29 days, ≥40 days) and current menstrual pattern (regular, usually irregular, always irregular, no menses). In the 1989 questionnaire, participants were asked to recall the length of their menstrual cycles at age 18–22, the pattern of their menstrual cycles both at age 18–22 and in high school and their age at menarche (≤11 years, 12–13 years, ≥14 years). Race (White, Black, Asian, other) was assessed in 1989, birthweight (pre-term, <5.5 lbs, 5.5–6.9 lbs, 7–8.4 lbs, >8.4 lbs) was assessed in 1991 and ethnicity (Hispanic, not-Hispanic) was assessed in 2005.
In 1989, participants were also asked to recall their body size at ages 5 and 10 years using a previously described 9-level figure drawing where the first category represents the most lean body shape and the ninth represents the most obese (Vitonis et al., 2010). The averages of each participant's figures at ages 5 and 10 years were used to obtain an estimate of childhood body size. As previous studies have identified an inverse association between childhood body size and incidence of laparoscopically confirmed endometriosis that is independent of adult BMI (Vitonis et al., 2010), we conducted a sub-analysis to evaluate the impact of childhood body size on the association between endometriosis and current BMI. Additional analyses were conducted using current BMI as the primary exposure while adjusting for BMI at age 18 and using BMI at age 18 as the primary exposure while adjusting for current BMI.
Inclusion and exclusion criteria
Women who reported a history of endometriosis before 1989 were excluded. Analyses were limited to premenopausal women with intact uteri, given the rarity of incident endometriosis after hysterectomy or menopause. Women were also censored upon report of malignancy other than non-melanoma skin cancer.
Statistical methods
Person-time expressed as woman-months was calculated from the time of entry into the cohort until the time of self-reported, laparoscopically confirmed endometriosis, fulfillment of a censoring criterion or until the end of the follow-up period.
Multivariable incidence rate ratios (RR) with 95% confidence intervals (CI) were calculated using time-varying Cox proportional hazards models, which are designed to simultaneously account for a participant's age as well as calendar time. Other potential risk factors for endometriosis were considered to be confounders if their inclusion in the model changed the RR of the main effect by >10% (Greenland, 1989). These potential confounders included: parity, race, ethnicity, birthweight, age at menarche, length of menstrual cycle (currently and at age 18–22 years), pattern of menstrual cycle (currently, at age 18–22, and in high school), age at first birth, time since last birth, current alcohol use, current smoking status, infertility, use of oral contraceptives, and perceived body size at ages 5 and 10 years (Vitonis et al., 2010). Based on these criteria, only infertility status and parity were adjusted for in the final models.
Missing anthropometric and co-variate data were handled using the missing indicator variable method (Miettinen, 1985) that retains full person-time contribution but also identified missingness that is associated with the rate of endometriosis diagnosis (i.e. not missing at random). No missing indicator for any of the main exposures evaluated was statistically significant or indicated a magnitude of effect inconsistent with the null.
Test for trend variables were set to the median value within a category and included continuously in the model. Two-sided Wald P-values <0.05 were considered statistically significant. Effect modification (by infertility status, parity, smoking status, early menarche and low birthweight) was assessed via the likelihood ratio test comparing the model with main effects only with the model with main effects and interaction terms.
Graphs were produced using restricted cubic regression splines with knots specified at 18.5, 22.4, 24.9, 29.9, 34.9 and 39.9, with a reference value of 21 (Durrleman and Simon, 1989). Ranges were restricted to BMI values between the 1st and 99th percentiles.
Results
A total of 116 430 female registered nurses completed the baseline questionnaire in 1989. Eight hundred and fifty-two women (<1%) were missing baseline height and weight data. The baseline prevalence of self-reported endometriosis was 6.5%. A total of 5504 incident cases of endometriosis were reported during 1 299 349 woman-years (incidence rate = 385 per 100 000 woman-years).
Table I presents the distribution of characteristics in the study population by BMI at baseline (1989). As the study design allows for time-varying changes in BMI, there are women missing BMI information in 1989 who subsequently re-enter the analytic population by providing current BMI data at other time points—one of the key strengths of using a time-varying covariate model. An overwhelming majority of participants (>90%) in the NHSII cohort were Caucasian. Women of higher current BMI were on average older, had a younger age at menarche, and a longer reported menstrual cycle length.
Table I.
Baseline characteristics of the Nurses' Health Study II cohort (n = 101 074 women) by current BMI (kg/m2).
| <18.5 | 18.5–22.4 | 22.5–24.9 | 25–29.9 | 30–34.9 | 35–39.9 | ≥40 | |
|---|---|---|---|---|---|---|---|
| n = 3461 | n = 45 153 | n = 22 592 | n = 18 606 | n = 6876 | n = 2854 | n = 1532 | |
| Agea | 33.0 (4.5) | 34.1 (4.6) | 34.7 (4.6) | 35.1 (4.6) | 35.4 (4.5) | 35.6 (4.4) | 36.0 (4.3) |
| Raceb | |||||||
| Caucasian | 91% | 93% | 93% | 92% | 92% | 92% | 93% |
| Other | 9% | 7% | 7% | 8% | 8% | 8% | 7% |
| Age at menarcheb | |||||||
| <11 years | 13% | 18% | 25% | 31% | 36% | 41% | 43% |
| 12–13 years | 55% | 59% | 59% | 56% | 53% | 50% | 49% |
| ≥ 14 years | 32% | 22% | 16% | 13% | 11% | 9% | 9% |
| Cycle lengthb | |||||||
| <26 days | 18% | 18% | 17% | 17% | 15% | 13% | 11% |
| 26–31 days | 68% | 69% | 69% | 67% | 63% | 62% | 57% |
| 32+ days | 14% | 13% | 14% | 16% | 21% | 25% | 32% |
| Parityb | |||||||
| Nulliparous | 38% | 31% | 28% | 27% | 31% | 36% | 45% |
| P1 | 19% | 19% | 19% | 20% | 20% | 20% | 17% |
| P2 | 29% | 33% | 34% | 33% | 31% | 29% | 25% |
| P3+ | 14% | 17% | 19% | 19% | 18% | 15% | 13% |
| Infertility statusb | |||||||
| No | 84% | 85% | 84% | 84% | 82% | 81% | 78% |
| Yes | 16% | 15% | 16% | 16% | 18% | 19% | 22% |
| OC useb | |||||||
| Never | 19% | 17% | 16% | 18% | 19% | 24% | 25% |
| Past | 66% | 69% | 70% | 70% | 70% | 68% | 65% |
| Current | 14% | 15% | 14% | 12% | 11% | 9% | 9% |
| Birthweightb,c | |||||||
| <5.5 lbs | 6% | 4% | 4% | 3% | 4% | 4% | 4% |
| 5.5–8.4 lbs | 84% | 83% | 81% | 81% | 79% | 78% | 78% |
| >8.4 lbs | 10% | 13% | 15% | 16% | 17% | 18% | 18% |
| Smokingb | |||||||
| Never | 69% | 66% | 65% | 65% | 66% | 67% | 67% |
| Past | 15% | 21% | 22% | 21% | 20% | 21% | 20% |
| Current | 16% | 12% | 13% | 13% | 14% | 12% | 13% |
| Alcoholb | |||||||
| 0 g/day | 39% | 33% | 36% | 42% | 47% | 51% | 56% |
| 0.01–5 g/day | 40% | 43% | 43% | 42% | 41% | 39% | 37% |
| 5.01–10 g/day | 11% | 13% | 11% | 9% | 6% | 5% | 3% |
| >10 g/day | 9% | 11% | 10% | 8% | 6% | 5% | 4% |
OC, oral contraceptive.
aResults expressed as mean (standard deviation).
bResults expressed as percentage. Some categories may not sum to 100 due to rounding.
cResults expressed only among full-term births.
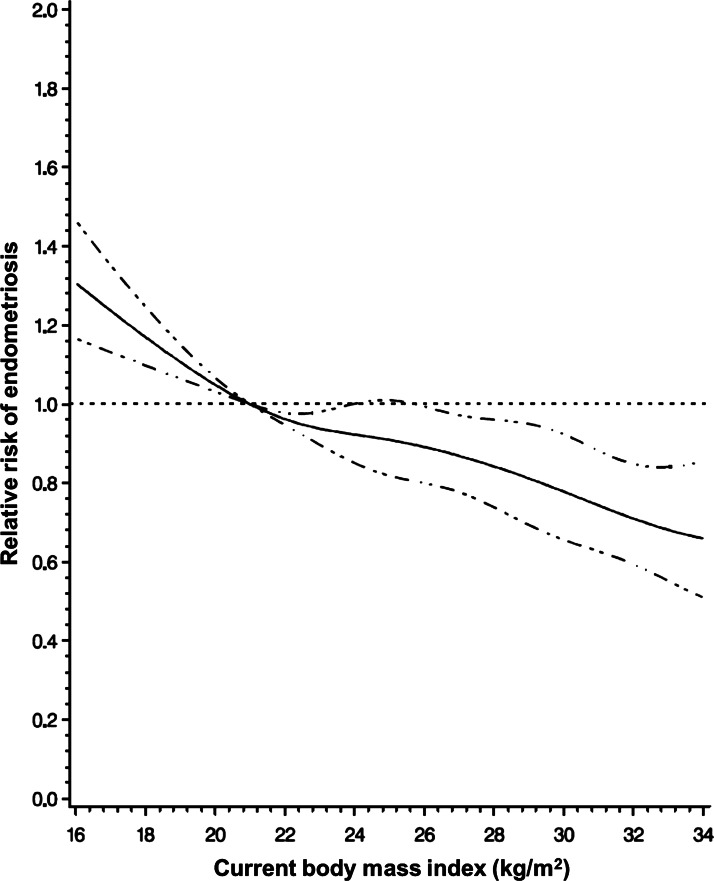
A significant inverse trend was observed between current BMI and risk of endometriosis (Fig. 1). Morbidly obese women with BMI>40 kg/m2 had a 39% lower rate of endometriosis compared with the low-normal referent (95% CI = 0.50–0.75) (Table II). When data were stratified by infertility status, the inverse association between BMI and endometriosis strengthened in the subset of infertile women (P-value, test for linear trend <0.0001). While a linear trend was not observed among women without reported infertility, the direction of the association remained evident with a significantly higher rate of endometriosis among underweight women (RR = 1.31, 95% CI = 1.07–1.60) and a significantly lower rate among morbidly obese women (RR = 0.70, 95% CI = 0.55–0.88) relative to women with low-normal BMI. These associations were not confounded by race, ethnicity, birthweight, age at menarche, length of menstrual cycle (current and at age 18–22 years) and pattern of menstrual cycle (current, at age 18–22 years, and in high school), nor by the time-varying covariates of age at first birth, time since last birth, current alcohol use, current smoking status, infertility or use of oral contraceptives. In addition, there were no differential effects when data were restricted to women with a normal cycle length or stratified by age (<35 versus >35 years), low birthweight (<5.5 versus >5.5 lbs among full-term births), smoking status (ever versus never smokers), early menarche (<11 versus >11 years) or nulliparity (data not shown). When adjusted for BMI at age 18, the association between current BMI and endometriosis risk was attenuated, although morbidly obese women with BMI ≥ 40 kg/m2 still had a 25% lower rate of endometriosis compared with the low-normal referent (95% CI = 0.60–0.95) (Table II). The inverse trend between current BMI and endometriosis risk remained statistically significant, but only among infertile women (P-value, test for linear trend = 0.0003).
Figure 1.
Relative risk of endometriosis by current BMI. Cubic regression spline of the relative risk of endometriosis by current BMI among 101 926 premenopausal women with current BMI data in the Nurses' Health Study II (1989–2009), adjusting for age, infertility status and parity. Dashed lines represent 95% confidence intervals.
Table II.
BMI (current and at age 18) and the incidence of laparoscopically confirmed endometriosis in the Nurse's Health Study cohort, 1989–2011a.
| All women |
Infertileb |
Not infertileb |
Pheterogeneityc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | No. of p-yrs | RR | 95% CI | No. of cases | No. of p-yrs | RR | 95% CI | No. of cases | No. of p-yrs | RR | 95% CI | ||
| Current BMI (kg/m2) | |||||||||||||
| <18.4 | 133 | 24884 | 1.16 | (0.97–1.38) | 30 | 1806 | 0.84 | (0.57–1.23) | 102 | 22719 | 1.31 | (1.07–1.60) | <0.0001 |
| 18.5–22.4 | 1733 | 418941 | 1.00 | Referent | 477 | 25116 | 1.00 | Referent | 1232 | 386002 | 1.00 | Referent | |
| 22.5–24.9 | 1097 | 280198 | 1.03 | (0.96–1.11) | 248 | 14588 | 0.95 | (0.81–1.12) | 836 | 259158 | 1.07 | (0.98–1.17) | |
| 25–29.9 | 1049 | 292774 | 0.99 | (0.91–1.06) | 173 | 14573 | 0.68 | (0.57–0.82) | 856 | 269590 | 1.09 | (1.00–1.19) | |
| 30–34.9 | 441 | 127949 | 0.94 | (0.85–1.05) | 58 | 6365 | 0.53 | (0.40–0.70) | 379 | 117333 | 1.10 | (0.98–1.24) | |
| 35–39.9 | 213 | 57117 | 0.96 | (0.83–1.11) | 26 | 3126 | 0.45 | (0.30–0.67) | 181 | 52002 | 1.15 | (0.98–1.34) | |
| >40 | 98 | 38269 | 0.61 | (0.50–0.75) | 17 | 2336 | 0.38 | (0.23–0.62) | 78 | 34544 | 0.70 | (0.55–0.88) | |
| Ptrend | <0.0001 | <0.0001 | 0.42 | ||||||||||
| BMI at age 18 (kg/m2) | |||||||||||||
| <18.4 | 861 | 183731 | 1.18 | (1.10–1.28) | 210 | 11099 | 1.13 | (0.96–1.32) | 627 | 165712 | 1.19 | (1.09–1.31) | 0.0005 |
| 18.5–22.4 | 2971 | 789239 | 1.00 | Referent | 648 | 40378 | 1.00 | Referent | 2237 | 719631 | 1.00 | Referent | |
| 22.5–24.9 | 670 | 183455 | 0.94 | (0.86–1.02) | 125 | 9400 | 0.81 | (0.66–0.99) | 526 | 167360 | 0.98 | (0.89–1.07) | |
| 25–29.9 | 345 | 98812 | 0.81 | (0.72–0.90) | 52 | 5860 | 0.52 | (0.39–0.69) | 278 | 89358 | 0.90 | (0.79–1.01) | |
| 30–34.9 | 88 | 23398 | 0.78 | (0.63–0.96) | 12 | 1562 | 0.39 | (0.22–0.70) | 70 | 20833 | 0.88 | (0.69–1.11) | |
| 35–39.9 | 23 | 5939 | 0.75 | (0.50–1.13) | 1 | 413 | 0.12 | (0.02–0.88) | 21 | 5319 | 0.97 | (0.63–1.49) | |
| >40 | 8 | 2316 | 0.59 | (0.30–1.19) | 1 | 198 | 0.23 | (0.03–1.70) | 7 | 2042 | 0.82 | (0.39–1.72) | |
| Ptrend | <0.0001 | <0.0001 | 0.0003 | ||||||||||
| Current BMI adjusted for BMI at age 18 (kg/m2) | |||||||||||||
| <18.4 | 133 | 24742 | 1.06 | (0.89–1.27) | 30 | 1799 | 0.80 | (0.54–1.17) | 102 | 22586 | 1.19 | (0.97–1.47) | <0.0001 |
| 18.5–22.4 | 1725 | 415862 | 1.00 | Referent | 472 | 24912 | 1.00 | Referent | 1229 | 383223 | 1.00 | Referent | |
| 22.5–24.9 | 1090 | 277877 | 1.08 | (1.00–1.17) | 247 | 14457 | 1.00 | (0.85–1.18) | 830 | 257032 | 1.11 | (1.02–1.22) | |
| 25–29.9 | 1047 | 290400 | 1.07 | (0.98–1.16) | 173 | 14463 | 0.75 | (0.62–0.91) | 854 | 267407 | 1.17 | (1.07–1.28) | |
| 30–34.9 | 434 | 126885 | 1.05 | (0.94–1.18) | 57 | 6321 | 0.63 | (0.46–0.85) | 373 | 116357 | 1.20 | (1.06–1.37) | |
| 35–39.9 | 212 | 56665 | 1.14 | (0.97–1.33) | 25 | 3104 | 0.60 | (0.38–0.93) | 181 | 51590 | 1.31 | (1.10–1.55) | |
| >40 | 97 | 37963 | 0.75 | (0.60–0.95) | 17 | 2319 | 0.62 | (0.36–1.09) | 77 | 34262 | 0.80 | (0.62–1.04) | |
| Ptrend | 0.74 | 0.0003 | 0.15 | ||||||||||
| BMI at age 18 adjusted for current BMI (kg/m2) | |||||||||||||
| <18.4 | 825 | 175288 | 1.21 | 1.12–1.31 | 206 | 10804 | 1.12 | (0.94–1.33) | 608 | 160169 | 1.24 | (1.12–1.36) | 0.0008 |
| 18.5–22.4 | 2832 | 754492 | 1.00 | Referent | 628 | 39522 | 1.00 | Referent | 2160 | 696132 | 1.00 | Referent | |
| 22.5–24.9 | 644 | 175644 | 0.94 | 0.86–1.02 | 124 | 9193 | 0.95 | (0.77–1.17) | 513 | 162064 | 0.94 | (0.85–1.04) | |
| 25–29.9 | 325 | 94666 | 0.80 | 0.71–0.91 | 50 | 5732 | 0.68 | (0.49–0.93) | 269 | 86687 | 0.86 | (0.74–0.98) | |
| 30–34.9 | 84 | 22394 | 0.82 | 0.65–1.04 | 11 | 1524 | 0.52 | (0.27–1.00) | 70 | 20249 | 0.90 | (0.70–1.16) | |
| 35–39.9 | 20 | 5703 | 0.76 | 0.48–1.19 | 1 | 404 | 0.19 | (0.03–1.42) | 19 | 5186 | 0.95 | (0.60–1.52) | |
| >40 | 8 | 2208 | 0.71 | 0.35–1.44 | 1 | 195 | 0.34 | (0.04–2.55) | 7 | 1969 | 0.93 | (0.44–1.98) | |
| Ptrend | <0.0001 | 0.0006 | 0.0004 | ||||||||||
aMultivariable incidence rate ratios (RR) with 95% confidence intervals (CI) calculated using time-varying Cox proportional hazards models, adjusting for parity and infertility status. Test for heterogeneity was assessed using the likelihood ratio test comparing the model with main effects only with the model with main effects and interaction terms. Number of cases among infertile and that among non-infertile women may not sum to number of cases for all women due to missing data. Total number of cases differs between exposures due to the number of women who contributed data toward a given exposure. p-yrs = person-years.
bInfertility is defined as attempting to conceive for >1 year without success. Infertility-stratified analyses are adjusted for parity.
cTest for heterogeneity comparing the effect of BMI on endometriosis risk between women with concurrent infertility and those without concurrent infertility.
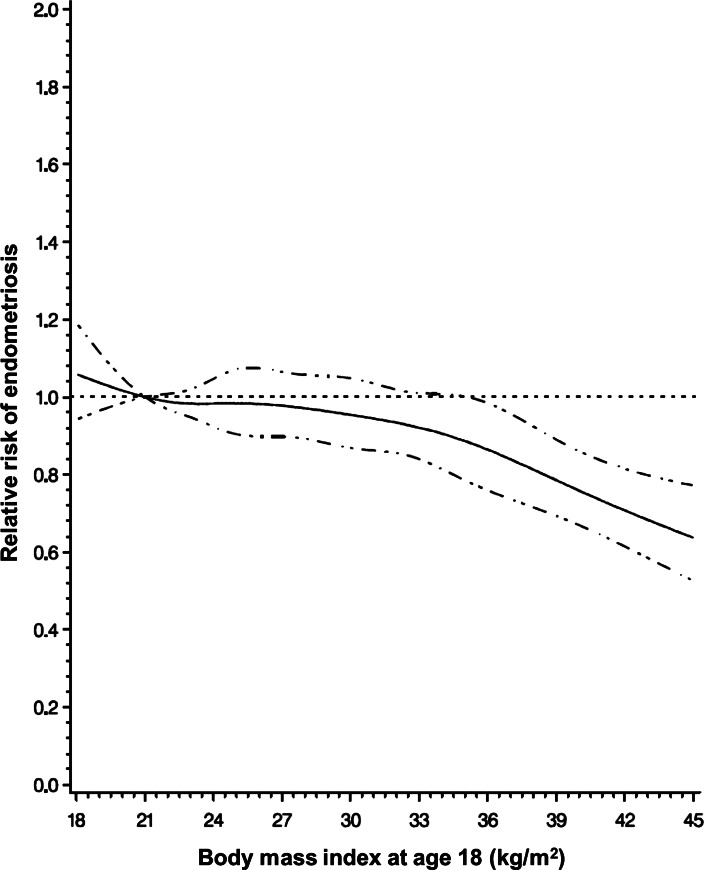
A similar inverse trend was noted between endometriosis risk and BMI at age 18 (P-value, test for linear trend < 0.0001; Fig. 2). The association persisted in both infertile women and those without infertility, but was again noted to be stronger among women with infertility (Table II). Although infertile women who were obese at age 18 had between a 61% (95% CI = 0.22–0.70) and an 88% (95% CI = 0.02–0.88) lower risk of endometriosis compared with the referent population, only small numbers of cases underlie these associations. Adjusting for current BMI attenuated the results, but the strong inverse trend between BMI at age 18 and endometriosis risk remained statistically significant for all populations (P-value, test for linear trend ≤ 0.0006; Table II). A sub-analysis restricted to women with a normal cycle length did not alter the stratified results (data not shown).
Figure 2.
Relative risk of endometriosis by BMI at age 18. Cubic regression spline of the relative risk of endometriosis by BMI at age 18 among 101 127 premenopausal women with BMI at age 18 data in the Nurses' Health Study II (1989–2009), adjusting for age, infertility status and parity. Dashed lines represent 95% confidence intervals.
Among women with infertility, there was an inverse trend between magnitude of weight change since age 18 and the rate of endometriosis (P-value, test for linear trend =0.0009). Greater waist circumference was similarly associated with a lower rate of endometriosis diagnosis only in the subgroup of women with infertility (P-value, test for linear trend =0.0003). There was no clear relation observed between height and endometriosis risk (P-value, test for linear trend =0.19, Table III).
Table III.
Change in BMI, height, waist circumference, waist-to-hip ratio and the incidence of laparoscopically confirmed endometriosis in the Nurse's Health Study cohort, 1989–2011a.
| All women |
Infertileb |
Not infertileb |
Pheterogeneityc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | No. of p-yrs | RR | 95% CI | No. of cases | No. of p-yrs | RR | 95% CI | No. of cases | No. of p-yrs | RR | 95% CI | ||
| Change in BMI (kg/m2) | |||||||||||||
| <−1 | 310 | 76625 | 0.94 | (0.82–1.07) | 67 | 4534 | 0.78 | (0.59–1.03) | 239 | 70924 | 0.98 | (0.85–1.14) | <0.0001 |
| −1 to 1 | 795 | 199176 | 1.00 | Referent | 200 | 11509 | 1.00 | Referent | 585 | 184210 | 1.00 | Referent | |
| >+1 to 6 | 2469 | 613706 | 1.09 | (1.01–1.18) | 584 | 34371 | 1.02 | (0.87–1.21) | 1851 | 565573 | 1.10 | (1.00–1.21) | |
| >+6 | 1164 | 340888 | 1.02 | (0.93–1.12) | 170 | 16960 | 0.66 | (0.53–0.82) | 971 | 311750 | 1.12 | (1.01–1.25) | |
| Ptrend | 0.69 | 0.0009 | 0.03 | ||||||||||
| Waist circumference (cm) | |||||||||||||
| 22–29.9 | 1260 | 293356 | 1.05 | (0.95–1.17) | 299 | 15343 | 1.06 | (0.84–1.34) | 951 | 272606 | 1.05 | (0.94–1.18) | 0.0002 |
| 30–32.9 | 525 | 141516 | 1.00 | Referent | 109 | 6799 | 1.00 | Referent | 408 | 132230 | 1.00 | Referent | |
| 33–37.9 | 408 | 115201 | 0.99 | (0.87–1.13) | 66 | 5445 | 0.85 | (0.61–1.17) | 342 | 107679 | 1.07 | (0.92–1.23) | |
| 38–65 | 262 | 71266 | 0.98 | (0.84–1.14) | 38 | 3797 | 0.57 | (0.39–0.84) | 221 | 66171 | 1.10 | (0.93–1.30) | |
| Ptrend | 0.19 | 0.0003 | 0.60 | ||||||||||
| Height (in) | |||||||||||||
| 40–62 | 921 | 242844 | 1.05 | (0.97–1.15) | 184 | 12949 | 1.02 | (0.84–1.24) | 702 | 220417 | 1.06 | (0.96–1.17) | 0.96 |
| 63–64 | 1303 | 352110 | 1.03 | (0.96–1.12) | 272 | 18279 | 1.07 | (0.90–1.27) | 993 | 320541 | 1.04 | (0.95–1.13) | |
| 65–66 | 1293 | 357655 | 1.00 | Referent | 273 | 18936 | 1.00 | Referent | 981 | 325793 | 1.00 | Referent | |
| 67–68 | 1057 | 242663 | 1.18 | (1.09–1.28) | 232 | 13334 | 1.22 | (1.02–1.47) | 795 | 220446 | 1.19 | (1.08–1.31) | |
| 69–85 | 418 | 102239 | 1.06 | (0.95–1.19) | 96 | 6008 | 1.06 | (0.83–1.35) | 313 | 92587 | 1.08 | (0.95–1.23) | |
| Ptrend | 0.19 | 0.43 | 0.23 | ||||||||||
| Waist/hip ratio | |||||||||||||
| <0.60 | 8 | 758 | 2.78 | (1.38–5.60) | 1 | 30 | 4.00 | (0.49–32.95) | 7 | 711 | 2.90 | (1.37–6.15) | 0.16 |
| 0.60–0.69 | 185 | 41423 | 0.99 | (0.85–1.16) | 50 | 2375 | 1.24 | (0.89–1.71) | 134 | 38269 | 0.94 | (0.79–1.13) | |
| 0.70–0.79 | 1376 | 334976 | 1.00 | Referent | 284 | 17123 | 1.00 | Referent | 1084 | 311726 | 1.00 | \Referent | |
| 0.80–0.89 | 698 | 193977 | 0.95 | (0.87–1.04) | 150 | 9307 | 1.05 | (0.85–1.30) | 539 | 181360 | 0.92 | (0.83–1.02) | |
| ≥0.90 | 178 | 47359 | 1.01 | (0.86–1.18) | 27 | 2409 | 0.73 | (0.48–1.11) | 149 | 43997 | 1.08 | (0.91–1.28) | |
| Ptrend | 0.41 | 0.13 | 0.76 | ||||||||||
aMultivariable incidence rate ratios (RR) with 95% confidence intervals (CI) calculated using time-varying Cox proportional hazards models, adjusting for parity and infertility status. Test for heterogeneity was assessed using the likelihood ratio test comparing the model with main effects only with the model with main effects and interaction terms. Number of cases among infertile and that among non-infertile women may not sum to number of cases for all women due to missing data. Total number of cases differs between exposures due to the number of women who contributed data toward a given exposure. p-yrs = person-years.
bInfertility is defined as attempting to conceive for >1 year without success. Infertility-stratified analyses are adjusted for parity.
cTest for heterogeneity comparing the effect of BMI on endometriosis risk between women with concurrent infertility and those without concurrent infertility.
Waist-to-hip ratio was not found to be consistently associated with the rate of endometriosis diagnosis, and the associations did not change when stratified by infertility status. Women with the smallest waist-to-hip ratio (<0.60) were noted to have a significant 3-fold increase in the rate of endometriosis diagnosis (RR = 2.78, 95% CI = 1.38–5.60), but the association was driven by only eight cases. Of note, 57 926 women had missing waist circumference measurements and were therefore excluded from analyses using waist circumference or waist-to-hip ratio as the primary exposure. However, women who did and who did not report waist circumference had similar distributions of BMI (24.5 versus 23.3 kg/m2, respectively) and rate of endometriosis diagnosis (5.5% versus 4.1%, respectively).
With all anthropometric exposures, adjustment for childhood body size had little effect on the association between BMI and endometriosis. The largest impact was seen with current BMI; adjusting for childhood body size attenuated the inverse association with endometriosis risk by 9.4%. None of the rate ratios for the primary exposures was changed by >10%.
Discussion
This is the largest prospective study specifically designed to address the relation between body size and endometriosis. It provides strong evidence that a woman's current BMI and BMI at age 18 are significantly inversely related to the rate of laparoscopically confirmed endometriosis, although the most robust association was observed with BMI at age 18. The magnitude of both relations was stronger in the subset of women with infertility. Waist circumference and weight change since age 18 were related to endometriosis risk only in the subset of infertile women. Waist-to-hip ratio was not linearly associated with endometriosis, although women with a waist-to-hip ratio of <0.60 may be at increased risk. It is important to note that despite the strength of evidence underlying the association between body weight and endometriosis, inferences regarding causation or the pathophysiologic processes underlying these relations cannot be made.
Although the literature has consistently demonstrated an inverse relation between endometriosis and body weight (Cramer et al., 1986; Darrow et al., 1993; McCann et al., 1993; Signorello et al., 1997; Missmer et al., 2004a,b; Parazzini et al., 2004; Ferrero et al., 2005; Hediger et al., 2005; Nagle et al., 2009), there is no consensus as to whether a lean body type is the cause of endometriosis or a result of the disease. A case–control study looking at self and mother-reported childhood BMI demonstrated increased risk of endometriosis in overweight girls (AOR = 2.8, 95% CI = 1.1–7.5) (Nagle et al., 2009), whereas prior investigation within the NHSII cohort suggested a persistent inverse relation between childhood body size and endometriosis risk (Vitonis et al., 2010). In the present study, it appears that the inverse association between current BMI and endometriosis is largely driven by a woman's BMI at age 18, suggesting that there is an etiologically relevant ‘early window of exposure’ during which higher body size reduces an individual's subsequent risk of developing endometriosis. A similar risk profile has been noted with disease states such as premenopausal breast cancer that also exhibit an inverse association with childhood and adolescent body size (Trentham-Dietz et al., 1997; Harris et al., 2011). Given the prospective design of the NHSII cohort, one can reasonably conclude based on the results of the present study that the impact of BMI on endometriosis risk precedes the diagnosis of the disease. It is harder to confirm that the impact of body size on endometriosis truly precedes the onset of disease—given the inherent impossibility of identifying the precise time point at which endometriosis first appears.
Among the most interesting findings of this study was the consistently stronger association between body size and the rate of endometriosis diagnosis in the subset of infertile women and the comparative absence of such associations in women without reported infertility. The NHSII had previously identified infertility status as an effect modifier for endometriosis risk (Missmer et al., 2004a,b), but this has not been addressed by other investigators since then. One possible explanation for this phenomenon is that the prevalence of polycystic ovary syndrome (PCOS) is likely to be higher among obese infertile women compared with normal-weight infertile women or obese women without infertility. The original diagnostic criteria for PCOS adopted by the National Institutes of Health in 1990 included oligo- or anovulation as well as clinical or biochemical evidence of hyperandrogenism. Although lean variants exist, the characteristic PCOS phenotype is manifested by an obese, oligoovulatory, infertile woman. The observed negative association between PCOS and endometriosis is thought to be a consequence of both anovulation and the hyperandrogenic state (Barbieri, 1990). Women with PCOS may be ‘protected’ against endometriosis via multiple mechanisms; persistent anovulation may decrease volume of retrograde menstruation, and a hyperandrogenic environment may retard lesion growth (Selak et al., 2001). Unfortunately, PCOS was poorly quantified in the NHSII cohort due to ambiguity in its clinical definition across time, precluding us from definitively addressing this hypothesis in the present study. As a proxy for the presence of PCOS, however, we did conduct subanalyses restricted to women with a normal cycle length, the results of which did not alter the original stratified results. It has also been suggested that the inverse relation between endometriosis and obesity is due to diagnostic bias—physicians may be less likely to recommend operative intervention to obese women with pelvic pain, reducing the likelihood that these patients would obtain a laparoscopic diagnosis of endometriosis. If this were the case, one would expect to see a stronger inverse relation between BMI and endometriosis among women presenting with pain rather than infertility. The strength of the association among infertile women in our data, however, does not support this hypothesis. An alternative explanation is that increased body weight masks pain symptoms, therefore resulting in less frequent diagnosis of endometriosis. Again, the strength of the inverse association in the subset of infertile women refutes this hypothesis. Moreover, although there are isolated reports of reduced post-operative pain in obese women (Cadish et al., 2010), the literature generally supports a strong relation between obesity and chronic pain conditions, including osteoarthritis and low back pain (Stevens-Lapsley and Kohrt, 2010; Wright et al., 2010).
A principal strength of the present study is the prospective design of the NHSII cohort, which avoids many of the limitations of case–control studies with respect to appropriate control selection (Missmer and Cramer, 2003; Missmer et al., 2004a,b). Given the invasive nature of endometriosis diagnosis, research controls are often selected from women undergoing laparoscopy for other indications such as tubal ligation, a group that is unlikely to represent the general population at risk of the disease. The prospective cohort design avoids this selection bias by following a group of people without the disease of interest who differ from each other in terms of the exposure in question. Although women with undiagnosed endometriosis certainly remain in the comparison population even in a prospective study, the community prevalence of endometriosis in an asymptomatic population is thought to be <2% and this group is, therefore, unlikely to contain substantial numbers of undiagnosed cases (Zondervan et al., 2002). Moreover, the characteristics of this small proportion of undiagnosed cases are diluted among the >90 000 women accurately defined as being endometriosis-free and are, therefore, unlikely to impact on effect estimation. A second benefit of the prospective cohort is the ability to account for exposures that vary with time in a given individual. For example, as a woman's weight changes, she is able to contribute person-time to more than one exposure category.
Epidemiologic studies examining the relation between obesity and coronary heart disease (Willett et al., 1995) and ovulatory infertility (Rich-Edwards et al., 2002) have demonstrated changes in risk profile across the full range of BMI, with increased risk evident at a BMI of 22.5 kg/m2. The large sample size of the NHSII cohort afforded the opportunity to subdivide the normal BMI range into lower (18.5–22.4 kg/m2) and upper (22.5–24.9 kg/m2) categories. The present data suggest that a similarly nuanced relationship may exist with endometriosis, as the inverse relationship between BMI and endometriosis is evident even at the upper end of the normal BMI range.
The NHSII cohort does not collect information regarding stage of endometriosis. As disease severity has not been shown to correlate with symptoms (Porpora et al., 1999) or prognosis, there is little reason to believe that it is significantly correlated with body size. Although it is possible that the requisite laparoscopic confirmation preferentially selects more ‘severe’ cases of endometriosis by overlooking women with asymptomatic or medically controlled disease, studies have failed to demonstrate increased severity of endometriosis among women with laparoscopically confirmed disease (Sangi-Haghpeykar and Poindexter, 1995). Indeed, the prevalence of stage I/II (minimal/mild) disease within the NHSII has been estimated to be 61% based on surgical record abstraction.
Like any epidemiologic or clinical study, data from the NHSII are limited by any inaccuracy in its collection. All data are self-reported. However, per several published validation studies, the exposures, outcome and covariates have been demonstrated to have a very low proportion of misclassification. Many of these concerns are in part obviated by the relative accuracy and reliability of nurses in measurement and reporting.
With over 20 years of follow-up among >100 000 women, this study confirms the robust inverse association between body size and endometriosis, taking into account potential confounders and effect modifiers. Further work will need to focus on elucidating underlying biologic relations that contribute directly to the initiation and promotion of endometriosis.
Authors' roles
All the authors have made substantial contributions to the conception and design of this data, as well as its analysis and interpretation. D.K.S. has drafted the article and all the other authors have revised it critically for intellectual content. All the authors have approved the final version for publication.
Funding
This study was supported by research grants HD48544 and HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Nurses' Health Study II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services.
Conflict of interest
None declared.
References
- Arumugam K, Lim JM. Menstrual characteristics associated with endometriosis. Br J Obstet Gynaecol. 1997;104:948–950. doi: 10.1111/j.1471-0528.1997.tb14357.x. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Endometriosis 1990. Current treatment approaches. Drugs. 1990;39:502–510. doi: 10.2165/00003495-199039040-00003. [DOI] [PubMed] [Google Scholar]
- Cadish LA, Hacker MR, Dodge LE, Dramitinos P, Hota LS, Elkadry EA. Association of body mass index with hip and thigh pain following transobturator midurethral sling placement. Am J Obstet Gynecol. 2010;203:508 e501–508 e505. doi: 10.1016/j.ajog.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–1908. [PubMed] [Google Scholar]
- Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4:135–142. doi: 10.1097/00001648-199303000-00009. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121:94–98. doi: 10.1016/j.ejogrb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Pan L, Wu B, Bian X. A case-control epidemiologic study of endometriosis. Chin Med Sci J. 1994;9:114–118. [PubMed] [Google Scholar]
- Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174:909–918. doi: 10.1093/aje/kwr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84:1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalliotakis IM, Arici A, Cakmak H, Goumenou AG, Koumantakis G, Mahutte NG. Familial aggregation of endometriosis in the Yale Series. Arch Gynecol Obstet. 2008;278:507–511. doi: 10.1007/s00404-008-0644-1. [DOI] [PubMed] [Google Scholar]
- Matorras R, Rodiquez F, Pijoan JI, Ramon O, Gutierrez de Teran G, Rodriguez-Escudero F. Epidemiology of endometriosis in infertile women. Fertil Steril. 1995;63:34–38. doi: 10.1016/s0015-0282(16)57293-8. [DOI] [PubMed] [Google Scholar]
- McCann SE, Freudenheim JL, Darrow SL, Batt RE, Zielezny MA. Endometriosis and body fat distribution. Obstet Gynecol. 1993;82(4 Pt 1):545–549. [PubMed] [Google Scholar]
- Miettinen OS. New-York: John Wiley & Sons; 1985. Theoretical epidemiology: principles of occurrence research in medicine. [Google Scholar]
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. doi: 10.1016/s0889-8545(02)00050-5. vii. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004a;104(5 Pt 1):965–974. doi: 10.1097/01.AOG.0000142714.54857.f8. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004b;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand. 1997;76:559–562. doi: 10.3109/00016349709024584. [DOI] [PubMed] [Google Scholar]
- Nagle CM, Bell TA, Purdie DM, Treloar SA, Olsen CM, Grover S, Green AC. Relative weight at ages 10 and 16 years and risk of endometriosis: a case-control analysis. Hum Reprod. 2009;24:1501–1506. doi: 10.1093/humrep/dep048. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod. 2004;19:1755–1759. doi: 10.1093/humrep/deh395. [DOI] [PubMed] [Google Scholar]
- Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429–434. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, Willett WC, Wand H, Manson JE. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Sangi-Haghpeykar H, Poindexter AN., III Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85:983–992. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2001:CD000068. doi: 10.1002/14651858.CD000068. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7:267–741. doi: 10.1016/s1047-2797(97)00017-3. [DOI] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, Kohrt WM. Osteoarthritis in women: effects of estrogen, obesity and physical activity. Womens Health (Lond Engl) 2010;6:601–615. doi: 10.2217/whe.10.38. [DOI] [PubMed] [Google Scholar]
- Trentham-Dietz A, Newcomb PA, Storer BE, Longnecker MP, Baron J, Greenberg ER, Willett WC. Body size and risk of breast cancer. Am J Epidemiol. 1997;145:1011–1019. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod. 2010;25:1325–1334. doi: 10.1093/humrep/deq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, Hennekens CH. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, Afari N. Chronic pain, overweight, and obesity: findings from a community-based twin registry. J Pain. 2010;11:628–635. doi: 10.1016/j.jpain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17:1415–1423. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]